Abstract
Objective
Septic arthritis is commonly caused by Staphylococcus aureus and is a medical emergency requiring antibiotics and joint irrigation. The bacteria produce α-toxin causing rapid cartilage cell (chondrocyte) death. Saline (0.9%NaCl) lavage is normally used to remove bacteria and toxins, however, its composition might be suboptimal to suppress the lethal effects of α-toxin. We utilized rabbit erythrocyte hemolysis as a sensitive, biologically relevant assay of α-toxin levels to determine if changes to osmolarity, temperature, pH, and divalent cation (Mg2+, Ca2+) concentration were protective.
Design
Erythrocytes were incubated in the various conditions and then exposed to α-toxin (“chronic” challenge) or incubated with α-toxin and then exposed to experimental conditions (“acute” challenge).
Results
Raising osmolarity from 300 mOsm (0.9%NaCl) to 400, 600, or 900 mOsm (sucrose addition) when applied chronically, significantly reduced hemolysis linearly. As an acute challenge, osmotic protection was significant and similar over 400 to 900 mOsm. Reducing temperature chronically from 37°C to 25°C and 4°C significantly reduced hemolysis, however, when applied as an acute challenge although significant, was less marked. Divalent cations (Mg2+, Ca2+ at 5mM) reduced hemolysis. Varying pH (6.5, 7.2, 8.0) applied chronically marginally reduced hemolysis. The optimized saline (0.9% NaCl; 900 mOsm with sucrose, 5 mM MgCl2 (37°C)) rapidly and significantly reduced hemolysis compared with saline and Hank’s buffered saline solution applied either chronically or acutely.
Conclusions
These results on the effect of S. aureus α-toxin on erythrocytes showed that optimizing saline could markedly reduce the potency of S. aureus α-toxin. Such modifications to saline could be of benefit during joint irrigation for septic arthritis.
Keywords: septic arthritis, Staphylococcus aureus α-toxin, cartilage, chondroprotection, osmotic pressure
Introduction
Septic arthritis, a destructive joint disease leading to permanent cartilage damage and disability, affects all ages with an incidence in Western Europe of 4 to 10 cases/100,000 persons/year.1,2 Staphylococcus aureus (S. aureus) accounts for 40% to 70% of all cases of septic arthritis1,3,4 and the incidence is rising due to factors including an aging population, increased use of immunosuppressive agents, musculoskeletal prosthetics, and surgical procedures.5 Management of septic arthritis is to rapidly eliminate the bacteria and associated toxins through intravenous antibiotics and copious joint irrigation.5 Despite eradication of the bacteria, the damage caused to articular cartilage persists in >50% of cases and may lead to osteoarthritis (OA).1,2,6
S. aureus produces an array of toxins, including exotoxins (including alpha [α], beta [β], gamma [γ], and delta [δ] hemolysin),7 which are potent virulence factors.8-11 Recent work using in vitro bovine cartilage and an in vivo murine model of septic arthritis with isogenic mutants of S. aureus, has identified α-toxin as the primary agent causing the rapid death of cartilage cells (chondrocytes).12-14 Chondrocytes are the only cell type capable of maintaining the tissue’s resilience through the turnover of extracellular matrix proteins and their loss, through the action of α-toxin, will result in cartilage degradation. S. aureus α-toxin also has longer term damaging effects on chondrocytes, for example, it may increase expression of catabolic factors, including matrix metalloproteinases (MMPs) and inducible nitric oxide synthase (iNOS) leading to deleterious changes to cartilage metabolism.15-18 While there has been considerable attention given to the development of antibacterials for treating S. aureus infection, the protection of chondrocytes against the deleterious effects of α-toxin has not been as intensively investigated.
S. aureus α-toxin binds to the A Disintegrin And Metalloproteinase 10 (ADAM10) receptor present on animal and human articular chondrocytes and rabbit erythrocytes.19-21 This leads to the formation of a heptameric pore and rapid influx of Na+ and water, causing cell swelling and lysis, leading to the release of intracellular components resulting in inflammation.22 Rabbit erythrocytes show only low sensitivity to other hemolysins23 (in contrast to human erythrocytes24) and are therefore an extremely flexible and sensitive model system for studying the interaction between this α-toxin and cell lysis.24 Additionally, the release of hemoglobin can easily be measured spectrophotometrically, allowing the dynamic effects of biologically relevant activity of α-toxin on cell viability to be assessed.24
The fluid used for joint irrigation is normally isotonic saline (0.9% NaCl; 300 mOsm), which is hypo-osmotic compared with normal synovial fluid (400 mOsm).25 Previous work has shown that the sensitivity of chondrocytes to other forms of injury may be markedly reduced when the osmolarity of isotonic saline or culture medium (typically 300 mOsm) is increased.26,27 This raised the possibility that the saline currently used for irrigation might be suboptimal and that altering some of its properties might reduce the injurious effects of α-toxin and thus be chondroprotective against α-toxin. Accordingly, we have tested the effects of osmolarity (300, 400, 600, 900 mOsm), temperature (4°C, 25°C, 37°C), divalent cations (Ca2+ and Mg2+) and pH (6.5, 7.2, 8.0) on the potency of S. aureus α-toxin using the sensitive rabbit erythrocyte hemolysis assay. The aim of this study therefore was to determine if these relatively simple alterations to the properties of standard saline could reduce the damaging effect of S. aureus α-toxin.
Materials and Methods
Biological Materials, Tissue Culture, Reagents
Saline (0.9%; 300 mOsm) used clinically for irrigation was obtained from Baxter Healthcare Ltd., Norfolk, UK. Hank’s buffered saline solution (HBSS; 300 mOsm) was purchased from Invitrogen Ltd., Paisley, UK. HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer was obtained from Sigma-Aldrich Chemical Co., Gillingham, UK. TSA (tryptone soya agar), TSB (tryptone soya broth), and skimmed milk were obtained from Oxoid Ltd., Basingstoke, UK.
Rabbit Red Blood Cells
The rabbit red blood cell (RBC) hemolysis assay was used to semiquantitatively determine biologically relevant α-toxin activity.24 Fresh, heparinized RBCs from Orygen Ltd., Penicuik, UK, were prepared at ~5% hematocrit in saline (0.9% NaCl) and kept at 5°C until required. Fresh blood was obtained weekly and was suitable for up to 5 days of experimentation after receipt.
Preparation of Bacterial Pellets and Supernatant Samples
S. aureus strain 8325-4 was kindly provided by Prof. T. J. Foster and stored at −80°C in 10% v/w skimmed milk. This strain is a well-characterized prophage-cured derivative of strain NCTC8325 that produces large amounts of α-toxin28 and has comparable potency to clinical strains of S. aureus in terms of chondrocyte-damaging potential.12 When required, bacteria were thawed and streaked onto TSA plates and cultured (24 hours; 37°C). TSB plates containing 2 µg/mL tetracycline were then prepared and inoculated with several single bacterial colonies from the TSA plates and incubated (24 hours; 37°C) with shaking. From this culture, serial dilutions were performed in saline to 10−6, spread on TSA plates and incubated (24 hours; 37°C). The number of colony forming units/mL (CFU/mL) in TSB was typically ~1 × 109. α-toxin-containing supernatants were obtained by centrifugation (800 × g; 10 minutes) of the TSB cultures, which were then filter-sterilized and stored (4°C) until required, which was within 1 week. To establish an appropriate time course at the beginning of a week’s experiments, the sensitivity of rabbit RBCs to α-toxin were assessed by adding a small volume of toxin to a 5% RBC suspension and incubating at 37°C for 60 minutes. Samples were taken every 10 minutes, centrifuged (10,000 × g; 10 seconds) and hemolysis determined by the absorbance of hemoglobin at 540 nm (Abs540) on a Nanodrop spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Maximum (100%) hemolysis was determined by freeze/thawing a sample of the RBC suspension. Percent hemolysis (%H) was then calculated ((Abs540 of sample − Abs540 of negative control)/(Abs540 of 100% hemolysis − Abs540 of negative control) × 100%) to give a measure of α-toxin activity. The sensitivity of erythrocyte samples to α-toxin and the amount and potency of α-toxin produced from each S. aureus culture, were variable. Rabbit erythrocytes which were relatively insensitive to toxin requiring >1 hour of incubation before hemolysis was detectable, were not studied further. A rabbit blood sample that produced ~50% hemolysis after about 15 minutes was considered acceptable for experimentation.
Chronic or Acute Exposure of α-Toxin-Treated Erythrocytes to Various Solutions and Temperatures
Chronic Exposure
Suspensions of rabbit erythrocytes were initially exposed to the experimental conditions of osmolarity, temperature, pH, or divalent cations for 10 minutes before an aliquot of α-toxin was added, the cell suspension mixed quickly, and the time course of percent hemolysis (%H) measurements commenced.
Acute Exposure
The α-toxin-treated erythrocyte suspensions were initially incubated under control conditions and hemoglobin release measured until this reached 20% to 30% hemolysis. The cell suspension was then challenged with the various experimental conditions, and the extent of hemolysis determined until the end of the time course. The rate of change in %H/10 min for the chronic challenge was measured over 10 to 20 minutes and for the acute challenge, the time course was measured over 20 to 30 minutes after the start of the experiment. Data were shown as the change in %H/10 min. For the control condition in the “acute exposure” experiments for osmolarity and divalent cations, an identical volume of saline was added at the same time point to correct for α-toxin dilution. For the pH experiments, HEPES (10 mM) was present and pH adjusted using HCl or NaOH. For some experiments, erythrocytes were suspended in HBSS (pH 7.2).
Data Analysis and Statistics
Data are shown as means ± standard error of the mean (SEM) for N independent experiments and n replicates for each experiment (N(n)) and analyzed using GraphPad Prism Version 7.0b (GraphPad, San Diego, CA, USA). Nonparametric t tests and analyses of variance were performed as indicated and significant differences accepted when P < 0.05.
Results
Suppression of α-Toxin Damage by Raising Saline Osmolarity
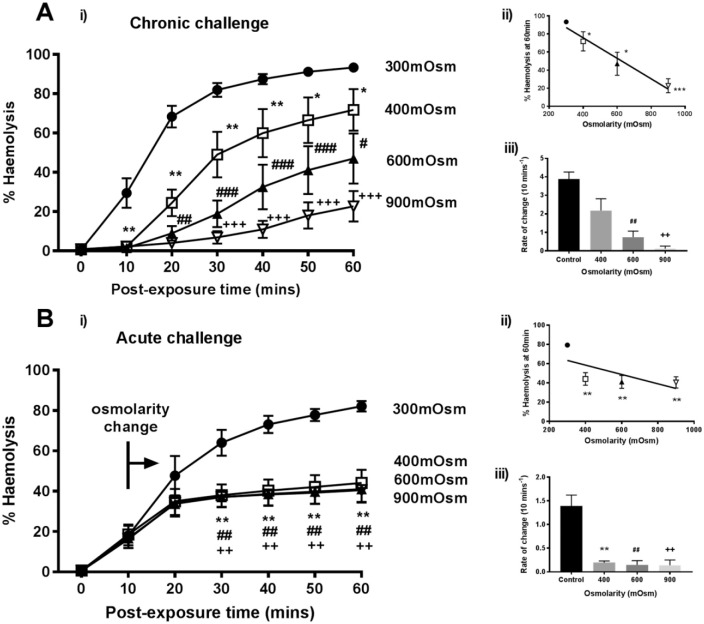
To assess the effects of raising saline osmolarity (300 mOsm) above that of synovial fluid (i.e., ⩾400 mOsm) on the damaging effect of α-toxin on rabbit erythrocytes, 2 types of experiments were performed: (1) chronic challenge - where erythrocytes were exposed to the various osmotic conditions, including α-toxin throughout (Fig. 1A) or (2) acute challenge - where erythrocytes were exposed to α-toxin until approximately 20% to 30% hemolysis had occurred, before the hyperosmotic challenge was delivered (Fig. 1B). For the chronic challenge, by the end of the time course, control %H (300 mOsm; ~93%) was significantly greater than 400 mOsm (72%; P = 0.0008), 600 mOsm (47%; P < 0.0001), and 900 mOsm (21%; P < 0.0001) (Fig. 1A(i)). An inverse linear relationship was evident between osmolarity and %H induced by α-toxin (R2 = 0.9626; P = 0.0189; Fig. 1A(ii)). The change in %H/10 min was calculated after toxin exposure and was 3.9% for the control and although reduced at 400 mOsm, was not significant (2.2%; P = 0.506). However, a reduction was observed at 600 mOsm (0.7%; P < 0.0001) and 900 mOsm (0.1%; P < 0.0001; Fig. 1A(iii)).
Figure 1.
Raised osmolarity reduced rabbit erythrocyte hemolysis induced by Staphylococcus aureus α-toxin. The percent hemolysis (%H) was measured over 60 minutes under either (A) chronic or (B) acute challenge with various osmolarities as follows; 0.9% saline (control, 300 mOsm (•)) and saline osmolarity raised by sucrose addition to 400 mOsm (□), 600 mOsm (▲) or 900 mOsm (∇) either before toxin addition (chronic) or at approximately 20% to 30% hemolysis (indicated by the bar and arrow – “osmolarity change”). The panels labeled (ii) show the %H data at t = 60 minutes plotted as a function of osmolarity, and the panels labeled (iii) show the rate of change of %H/10 min plotted as a function of osmolarity. In this and subsequent figures, significant differences are indicated as follows: * P < 0.05; ** P < 0.01; *** P < 0.001). Results are means ± SEM from 5(2), that is, from 5 independent experiments and 2 replicates for each experiment.
When osmolarity was raised >300 mOsm, approximately 10 minutes after α-toxin addition (“acute challenge”), there was rapid and almost complete protection of erythrocytes (Fig. 1B(i)). There was a significant decrease in hemolysis between the control (300 mOsm; 82%) and hyperosmotic solutions (44%, 41%, 41% for 400, 600, 900 mOsm, respectively) by the end of the experiment (P < 0.0001 for all osmolarities compared with 300 mOsm). Interestingly, in contrast to the chronic challenge, the effects of 400 mOsm and greater were not significantly different when compared with each other (P = 0.327; Fig. 1B(ii)). The rates of change in %H were significantly decreased for osmolarities of ⩾400 mOsm (Fig. 1B(iii)). Raising osmolarity using sucrose was thus strongly protective particularly after α-toxin damage to erythrocytes had been initiated.
The osmotic protection conferred by sucrose was compared with that of a different osmolyte (NaCl) to the same osmotic pressure. At 40 minutes, NaCl (600 mOsm) reduced %H from the control (300 mOsm) value of 66% ± 6% to 56% ± 8%, whereas with sucrose this was decreased further to 20% ± 3%. In additional experiments at the same time point, when the osmolarity was raised to 900 mOsm, the %H for the control was 68% ± 8% and reduced to 21% ± 7% in the presence of NaCl but was only 4% ± 2% with sucrose (data are means ± SD, N = 2). Although NaCl protected erythrocytes against α-toxin, it appeared less effective when present at the same osmolarity as sucrose.
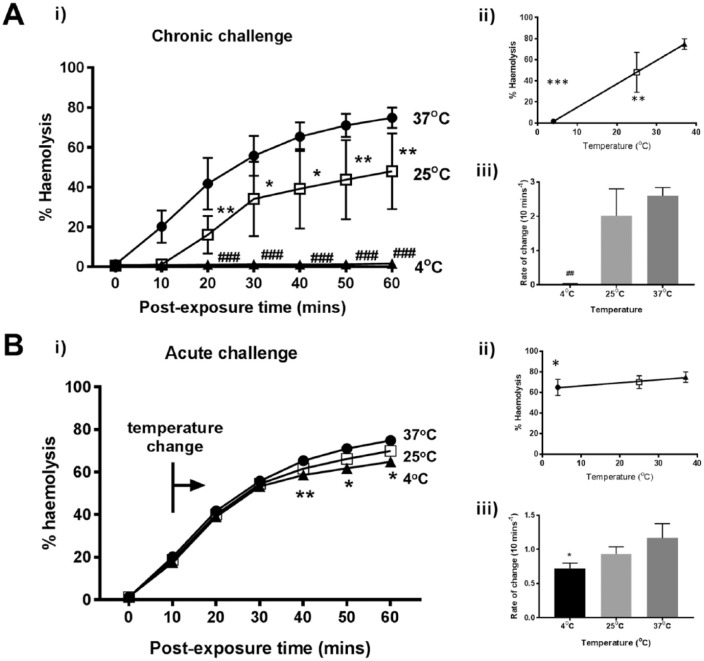
Reducing Temperature of Saline Conferred Protection against α-Toxin-Induced Hemolysis
RBC suspensions were incubated for 10 minutes at the various temperatures, treated with α-toxin and then hemolysis determined. The chronic exposure to reduced temperature protected erythrocytes treated with α-toxin (Fig. 2A(i)). After 60 minutes at 25°C, the %H was 48% and significantly less compared with 37°C (75%; P = 0.0056). However, when the α-toxin-treated RBCs were incubated at 4°C, hemolysis was virtually abolished over the time course studied (P < 0.0001; Fig. 2A(i)). An inverse linear regression was observed between decreasing temperature and hemolysis (R2 = 1.00; P = 0.002; Fig. 2A(ii)). The rate of change in hemolysis (%H/10 min) for 4°C, 25°C, and 37°C was 0.02%, 2.0%, and 2.6%, respectively (4°C vs. 37°C, P = 0.0115; 4°C vs. 25°C, P = 0.0451) (Fig. 2A(iii)). When temperature was changed quickly during the time course (“temperature switch protocol”), no significant difference in hemolysis was observed between 37°C and 25°C until the 50-minute time point (71% vs. 66%; P = 0.0232; Fig. 2B(i)). A significant difference was also found between 37°C and 4°C after 40 minutes (65% vs. 59%; P = 0.001). Although a linear relationship was evident, there was no significant deviation from a gradient of zero (R2 = 0.979; P = 0.0923; Fig. 2B(iii)). Therefore, reducing temperature was more protective before α-toxin addition. However, after its addition, reducing the temperature from 37°C to 4°C reduced hemolysis by ~10% (Fig. 2B(i)).
Figure 2.
Decreasing temperature suppressed rabbit erythrocyte hemolysis induced by Staphylococcus aureus α-toxin. The percent hemolysis (%H) was measured over 60 minutes under either (A) chronic or (B) acute challenge at different temperatures as follows; 37°C (control) (•), 25°C (□), 4°C (▲) either before toxin addition (chronic) or at about 20% hemolysis (indicated by the bar and arrow – “temperature change”). The panels labeled (ii) show the %H data at t = 60 minutes plotted as a function of temperature, and the panels labeled (iii) show the rate of change of %H/10 min plotted as a function of temperature. Results are means ± SEM from (4(2)), that is, from 4 independent experiments and 2 replicates for each experiment.
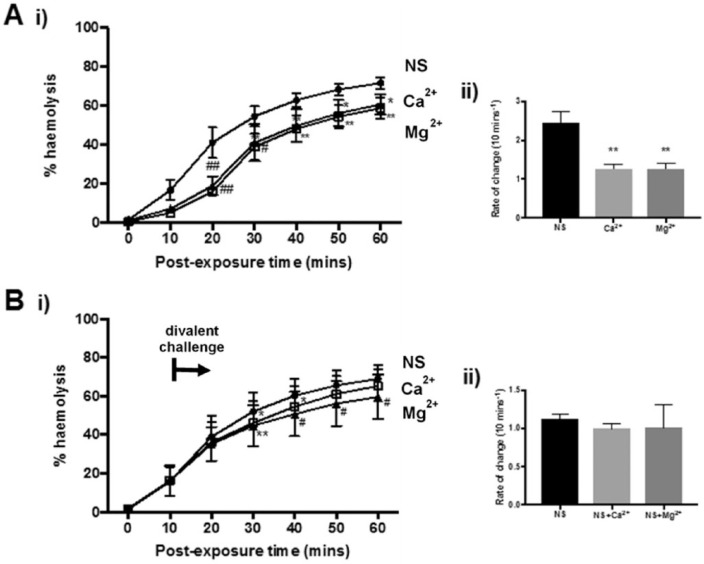
Protective Effect of Divalent Cations against α-Toxin-Induced Erythrocyte Hemolysis
For the chronic exposure experiments, divalent cations (Ca2+ or Mg2+ at 5 mM) produced a modest decrease in hemolysis with significant protection observed as early as 10 minutes with CaCl2 (P = 0.0149) and 20 mins with MgCl2 (P < 0.01; Fig. 3A(i)). This was maintained throughout the exposure protocol. By the end of the experiment, lower hemolysis levels resulted from cell suspensions containing Ca2+ (58.8%, P = 0.0063) or Mg2+ (60.7%, P = 0.0231) compared with the control (71.7%; Fig. 3A(i)). The protective effects of the divalent cations were indistinguishable (P > 0.99; Fig. 3A(i, ii)). The addition of Mg2+ after α-toxin exposure (“acute exposure”) gave significant protection (P = 0.02) against hemolysis; however, no difference was recorded with Ca2+ (P = 0.12) at 60 minutes (Fig. 3B(i)). Furthermore, the rate of change showed no differences between the control and divalent ions (P > 0.05; Fig. 3B(ii)). Thus, divalent ions provided only minor protection against hemolysis induced by α-toxin.
Figure 3.
Divalent cations inhibited rabbit erythrocyte hemolysis induced by Staphylococcus aureus α-toxin. The percent hemolysis (%H) was measured over 60 minutes under either (A) chronic or (B) acute challenge in the presence of Ca2+ or Mg2+ normal saline (NS; 0.9% NaCl control) (•), Ca2+ (5 mM) or Mg2+ (5 mM) (▲) either before toxin addition (chronic) or at about 20% hemolysis (indicated by the bar and arrow – divalent challenge).The panels labeled (ii) show the %H data at t = 60 minutes plotted as a function of temperature, and the panels labeled (iii) show the rate of change of %H/10 min plotted as a function of temperature. Results are means ± SEM from (4(2)), that is, from 4 independent experiments and 2 replicates for each experiment.
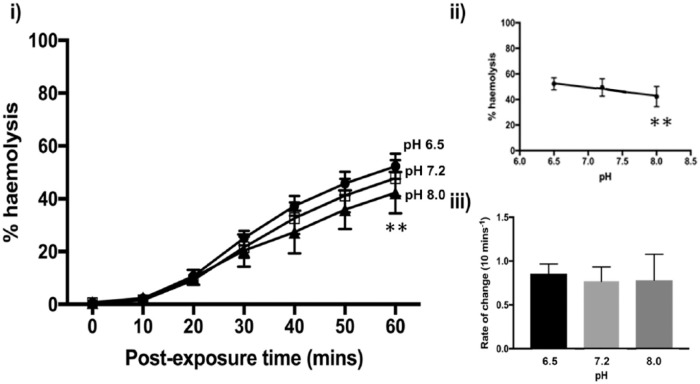
Influence of pH on α-Toxin-Induced Erythrocyte Hemolysis
Altering saline pH might provide some protection against the damaging effects of α-toxin. A significant decrease in %H occurred between pH 6.5 and 8.0 (2-way analysis of variance; P = 0.0015) at 60 minutes (Fig. 4(i)). However, there were no significant differences between normal pH (pH 7.2) and pH 6.5 (P = 0.1995) or pH 8.0 (P = 0.0892). The data points fitted a linear regression, but the slope was not significantly different from zero (R2 = 0.9685, P = 0.1136; Fig. 4(ii)). The rate of change of %H between 10 and 20 minutes of toxin exposure showed little variation across the pH values (P > 0.99) (Fig. 4(ii)) suggesting that increasing saline pH may only offer marginal protection.
Figure 4.
Effect of varying pH on rabbit erythrocyte hemolysis induced by Staphylococcus aureus α-toxin. The percent hemolysis (%H) was measured over 60 minutes under chronic challenge at pH values of 7.2 (control) (□), 6.5 (•), and 8.0 (▲) in Hank’s buffered saline solution (HBSS) containing the buffer HEPES (10 mM) with pH altered using HCl or NaOH (all at 300 mOsm). The panel labeled (ii) shows the %H data at t = 60 minutes plotted as a function of temperature, and the panel labeled (iii) shows the rate of change of %H/10 min plotted as a function of temperature. Results are means ± SEM from (3(2)), that is, from 3 independent experiments and 2 replicates for each experiment.
Effect of Optimized Saline on α-Toxin-Induced Erythrocyte Hemolysis
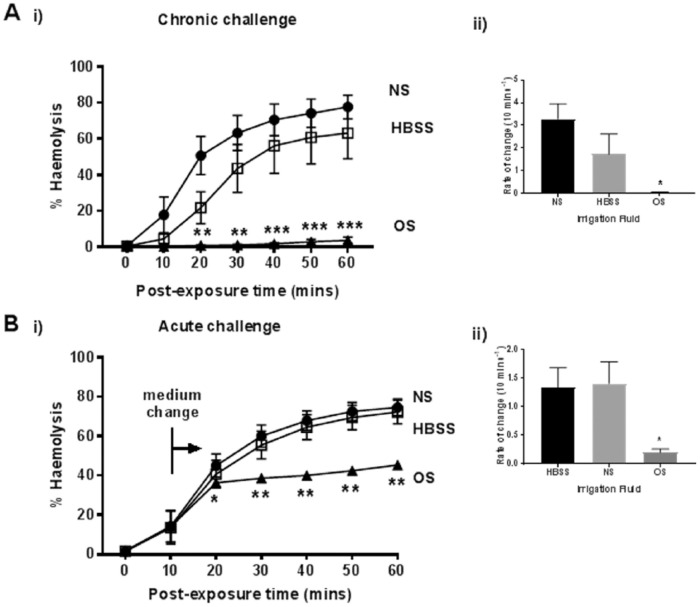
On the basis of the previous results, an optimized saline solution (0.9% NaCl, 900 mOsm, 5 mM MgCl2, 37°C) was prepared. This was compared with normal saline (0.9% NaCl) and HBSS (which contains Ca2+) in its ability to protect erythrocytes against α-toxin (Fig. 5A(i)). When the RBCs were preincubated in these solutions for 10 minutes before addition of α-toxin, the optimized saline abolished subsequent hemolysis in contrast to both normal saline and HBSS (both P < 0.0001), where levels of hemolysis were 78% (normal saline), 63% (HBSS), 4% (optimized saline) at 60 minutes (Fig. 5A(i)). The rate of change of hemolysis was greatest for normal saline (3.3%) followed by HBSS (1.7%) then optimized saline (0.05%; Fig. 5A(ii)). The addition of optimized saline after toxin exposure also significantly suppressed hemolysis compared with the other solutions. With optimized saline, hemolysis showed a small increase from 36% to 46% by the end of the experiment, whereas these levels rose considerably more with the use of normal saline (75%) and HBSS (72%; Fig. 5B(i)). Likewise, the rate of change was much reduced with optimized saline (0.2% H/10 min) compared with normal saline (1.3% H/10 min, P = 0.0374) and HBSS (1.4% H/10 min, P = 0.03; Fig. 5B(ii)).
Figure 5.
The protective effect of optimized saline on rabbit erythrocyte hemolysis induced by Staphylococcus aureus α-toxin. The percent hemolysis (%H) was measured over 60 minutes under either (A) chronic or (B) acute challenge in the presence normal saline (NS; 0.9% NaCl control) (•), HBSS (□), or optimized saline (OS; 0.9% NaCl, 900 mOsm, 5 mM MgCl2, 37°C) (▲) either before toxin addition (chronic) or at about 20% hemolysis (indicated by the bar and arrow – medium change). The panels labeled (ii) show the rate of change of %H/10 min for chronic and acute challenges, respectively, in the various solutions. Results are means ± SEM from (5(2)), that is, from 5 independent experiments and 2 replicates for each experiment.
Discussion
During treatment for S. aureus septic arthritis, it is important that the synovial fluid and infected tissues of the joint are rinsed quickly with a benign solution to remove bacteria and associated toxins. Traditionally, saline (0.9% NaCl) is used; however, this might be suboptimal for suppressing α-toxin activity and there may be opportunities for its composition and other properties to be modified to protect chondrocytes. To assess the protective effects of these modified solutions, the release of hemoglobin from rabbit erythrocytes was used as a sensitive and biologically relevant measure of S. aureus α-toxin activity. The results suggested that increased osmolarity, reduced temperature, divalent cations, and to a lesser extent alkaline pH, could significantly reduce the damaging effect of α-toxin suggesting that relatively simple modifications to saline could be of benefit during joint irrigation for septic arthritis.
It could be considered that the rabbit hemolysis assay for determining methods for protecting cells against the damaging effects of α-toxin would not be an appropriate model. However, rabbit erythrocytes contain the S. aureus α-toxin receptor ADAM10, which is also present on chondrocytes of animals and normal and degenerate human cartilage.19-21 The measurement of hemoglobin release following the interaction between α-toxin and erythrocytes provided a dynamic and sensitive assay for the lethal effects of α-toxin and was highly flexible experimentally and reproducible. While it would be possible to test these conditions on cartilage explants, interpreting the results obtained under these various conditions might not be as straightforward, as for example access to the receptor in cartilage zones could be delayed and/or restricted. While studies on isolated chondrocytes could be of benefit, the receptor may be damaged or its sensitivity altered as a result of the enzymic treatment of cartilage, which is required for the release of chondrocytes. Our previous work has demonstrated that S. aureus α-toxin is the key damaging agent to chondrocytes in a cartilage model of septic arthritis.12,14 Thus, although the rabbit erythrocyte model could be considered a limitation in this study, it nevertheless yielded valuable information about whether protection against α-toxin was possible and identified alterations to the irrigation fluid which could potentially be extended to detailed in vivo and clinical studies on S. aureus septic arthritis.
Two protocols were used, which would broadly correspond to different stages of α-toxin action on the cell membrane, which is time-dependent with the binding and pore formation occurring within 2 to 3 minutes.24 For the chronic challenge, erythrocytes were equilibrated with the various conditions (osmolarity, temperature, pH, divalent cations), before toxin was added and the hemolysis time course commenced. For the acute challenge, the time course was started by α-toxin addition to the RBC suspension, and when there was 20% to 30% hemolysis, the erythrocytes were exposed to the experimental conditions. Thus, the chronic exposure would mainly represent the effect of experimental conditions on early steps of toxin action but for the acute exposure, pore formation would be complete and the pathological changes (i.e., increased ion permeability, cell swelling) would be underway with hemolysis following. The acute exposure would be closer to the clinical situation where the majority of cells in the joint would already have been exposed to prevailing levels of α-toxin, and cell injury/death would be proceeding. While the overall effects of chronic and acute challenges of osmolarity, reduced temperature and divalent cation concentration (Figs. 1-4) were similar and gave significant protection, the time courses appeared different.
Raising osmolarity prior to toxin addition (chronic osmotic challenge) reduced the rate of hemolysis in a dose-dependent manner (Fig. 1A(i)-(iii)). This suggested that erythrocyte shrinkage could have interfered with early events of toxin action i.e. monomeric α-toxin binding to the cell membrane and pore formation. However, studies by Cooper et al.,24 where an osmolyte (polyethylene glycol) was added after α-toxin addition, suggested that binding and pore formation were unaffected and that pore permeability instead was more sensitive to osmolarity. An acute hyperosmotic challenge to erythrocytes in which α-toxin pores would already have formed, was rapidly (within 10 minutes) effective over the range studied (400-900 mOsm). However, there was no difference between the osmolarities (Fig. 1B(i)-(iii)). This protective effect may be different compared to the chronic challenge, with the acute hyperosmotic medium causing rapid erythrocyte shrinkage thereby conferring protection against the cell swelling induced by α-toxin. The raised osmolarity might simply shrink the cells rapidly meaning that it would take longer for the cells to swell to a critical volume. It was noted that NaCl was less effective at protecting erythrocytes compared to sucrose to the same osmolarity. This may be because Na+ can enter via Na+ channels and Na+/K+/2Cl− cotransporter29 effectively reducing the osmolarity compared to sucrose. Thus, sucrose would be the preferred osmolyte for the optimized irrigation fluid as it is impermeable and metabolically inert (see Amin et al.26).
Preincubating erythrocytes at 25°C delayed the damaging action following α-toxin addition. However, once established, the rate of increase in hemolysis was the same as for cells equilibrated at a physiological temperature (Fig. 2A(iii)). This suggested that the early steps of pore formation were sensitive to reduced temperature. However, preequilibration at 4°C completely protected erythrocytes against α-toxin (Fig. 2A(i)). This may accord with Reichwein et al.,30 who demonstrated a temperature-dependent transition from toxin monomers to a functional heptameric pore. They preincubated rabbit erythrocytes with α-toxin (0°C; 30 minutes), and then washed and maintained the erythrocytes at either 0°C or 37°C. Enzyme-linked immunosorbent assays (ELISA) showed that there were no α-toxin oligomers on the cell membrane nor any hemolysis at the lower temperature. In contrast, the cells that were incubated at 37°C experienced hemolysis, suggesting that α-toxin binding/pore formation was suppressed at low temperature. This is supported by Freer31 who showed that α-toxin binding did not necessarily lead to erythrocyte destruction and that lysis (i.e., functional pore formation) did not occur until temperatures were >12°C. Notably, the effects of reduced temperature in the acute challenge experiments (Fig. 2B(i)) were less marked than for the chronic challenge as there was a delay before the inhibition occurred (Fig. 2A and B). This could be because the pores had formed, and reduced temperature had little effect on the cation flow and subsequent erythrocyte swelling. Clinically, the acute challenge methodology would represent the situation where irrigation fluid was introduced into the infected joint during treatment. The toxin would already be present, bound to the cell membranes and acting on chondrocytes and other cells in the joint to cause its damaging effects.
There was a mild but significant protection of erythrocytes when pH was increased from 6.5 to 8.0 (Fig. 4(i)). The pH of 0.9% NaCl should be ~7.0 but the true value often oscillates around pH = 5.5 due to varying levels of dissolved CO232). Work by others33 suggested that acidity converted α-toxin from an amphipathic form into a more hydrophobic molecule, thus accelerating pore formation. It has also been proposed that acidic pH enhanced H+ binding to histidine residues on the toxin molecule vital for polymerization, accelerating pore formation.34 There may therefore be benefit in introducing a benign pH buffer to stabilize irrigation fluid pH, perhaps at a slightly alkaline level, to provide some protection against α-toxin and also dampen any elevated pCO2 levels present in the clinical environment.
Ca2+ and Mg2+ produced small but significant protection against α-toxin (Fig. 3A and B) with no differences between these ions. Previous studies on Ca2+ suggested that it reduced the lateral movement of monomeric α-toxin in the plane of the membrane thereby reducing the rate of pore formation.35 Depletion of Ca2+ was sufficient to remove the protection suggesting a reversible effect. However, it was unclear if this was Ca2+-specific or whether due to the osmolarity of the CaCl2, which would contribute ~90 mOsm.35 Apart from the protective effects of divalents on the action of α-toxin on cells, antibacterial roles for Ca2+ and Mg2+ have been reported.36 Raised divalent concentrations disrupted S. aureus membranes possibly by forming complexes with cardiolipin which introduced membrane bending and destabilized its integrity. Stationary-phase bacteria, which are resilient against environmental pressures, were subjected to either divalent ion for 40 minutes and ~60% of the bacterial culture did not survive. A threshold of 10 mM Ca2+ and 20 mM Mg2+ to destroy S. aureus was established.36 This study used concentrations greater than the present work, and therefore it may be interesting to further investigate increasing Ca2+ and Mg2+ concentrations on S. aureus survivability and α-toxin potency.
On the basis of these experiments, we tested an optimized saline applied as either a chronic or an acute challenge and observed substantial protection of erythrocytes against α-toxin (Fig. 5A and B). That there was little difference between normal saline and HBSS suggested that the majority of the protection was due to the raised osmolarity. With the acute challenge (Fig. 5B), the protection was very rapid indicating the quick suppression of the damaging effect of α-toxin, which could be considered potentially clinically relevant for joint irrigation. There was still, however, a small increase in %H (Fig. 5B) possibly because further optimization may be required, and/or there are other toxic elements produced by S. aureus, which could have a relatively minor damaging effect on rabbit erythrocytes. A modified irrigation solution may also have benefits beyond those of protecting cells against α-toxin. For example, cooled irrigation fluid could offer pain relief and anti-inflammatory effects. A study involving patients who underwent total knee arthroplasty found that saline administered at 4°C alleviated pain, localized swelling, and decreased analgesia intake, as well as improving the quality of postoperative recovery.37 Furthermore, a hyperosmolar irrigation saline, in addition to rapid protection against α-toxin, could be beneficial as Chan and Foster38 found that addition of 20 mM sucrose in growth media suppressed α-toxin gene (hla) expression by ~98% of the control.
While the present results were obtained using the rabbit RBC model, some caution should be exercised when extrapolating these results to the protection of chondrocytes within the cartilage matrix. Previous studies using a bovine osteochondral explant model have shown that S. aureus α-toxin can rapidly penetrate the matrix and cause chondrocyte death.12 We have also shown that chondrocyte volume changes very quickly (within minutes) following alterations to extracellular osmolarity.39 Thus there is the expectation that by raising osmolarity, protection of in situ chondrocytes against α-toxin should be achieved in the same way this has been demonstrated with rabbit erythrocytes. With these observations in mind and taking the results from the present study together with previous observations, the beneficial effects of modifying the irrigation saline used during joint lavage should be considered in further in vivo animal and/or clinical research. The use of a relatively benign, inexpensive, drug-free and rapidly effective modified saline as part of the normal lavage process is potentially an attractive novel method for limiting the damaging action of S. aureus α-toxin during septic arthritis.
S. aureus infections have been treated with β-lactams (e.g., penicillin) for decades, but the appearance and rapid spread of methicillin-resistant S. aureus (MRSA) have all but eliminated these antibacterials for treatment.40 Non-antibiotic treatment is therefore an area of important research interest since suppressing activity of bacterial toxins either by influencing toxin production, or blocking their action would not only make the bacteria less pathogenic, but may also increase their susceptibility to host immune defence.41 For example, inhibition of S. aureus pathogenesis by interfering with the signal transduction pathways for virulence using the RNA III inhibiting peptide has been described.42 This peptide reduced the pathology and delayed the onset of disease symptoms in models of S. aureus infection, including septic arthritis.42 Other methods include an α-toxin antibody,43 cyclodextrin-lipid complexes to suppress the damaging effect of S. aureus α-toxin44 and nanoparticle-based α-toxin entrapment to deliver the nondisrupted pore-forming toxin for immune processing.45 These methods could be particularly important for cells with high levels of the ADAM10 receptor,21 which would render them particularly sensitive to S. aureus α-toxin. In summary, the development of the optimized irrigation saline described here potentially offers a cheap, very rapid (within minutes) and relatively benign method to suppress the damaging effects of α-toxin and may be of benefit during joint irrigation for septic arthritis caused by S. aureus.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank Prof. T. J. Foster, Trinity College, Dublin for providing Staphylococcus aureus strain 8325-4.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Kaandorp CJ, Dinant HJ, van de, Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56(8):470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldenberg DL. Septic arthritis. Lancet. 1998;351:197-202. [DOI] [PubMed] [Google Scholar]
- 3. Kaandorp CJ, Krijnen P, Moens HJ, Habbema JD, van Schaardenburg D. The outcome of bacterial arthritis: a prospective community-based study. Arthritis Rheum. 1997;40:884-92. [DOI] [PubMed] [Google Scholar]
- 4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375:846-55. [DOI] [PubMed] [Google Scholar]
- 6. Krieg AM. A possible cause of joint destruction in septic arthritis. Arthritis Res. 1999;1:3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham R, Cockayne A, Humphreys H. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol. 1996;44:157-64. [DOI] [PubMed] [Google Scholar]
- 8. O’Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, et al. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65(5):1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitz FJ, Veldkamp KE, Van Kessel KP, Verhoef J, Van Strijp JA. Delta-toxin from Staphylococcus aureus as a costimulator of human neutrophil oxidative burst. J Infect Dis. 1997;176(6):1531-7. [DOI] [PubMed] [Google Scholar]
- 10. Dajcs JJ, Thibodeaux BA, Girgis DO, O’Callaghan RJ. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002;21(5-6):375-82. [DOI] [PubMed] [Google Scholar]
- 11. Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol. 2009;174(2):509-18. doi: 10.2353/ajpath.2009.080394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith ID, Winstanley JP, Milto KM, Doherty CJ, Czarniak E, Amyes SG, et al. Rapid in situ chondrocyte death induced by Staphylococcus aureus toxins in a bovine cartilage explant model of septic arthritis. Osteoarthritis Cartilage. 2013;21(11):1755-65. [DOI] [PubMed] [Google Scholar]
- 13. Clement R, Hall AC, Howie SEM, Simpson AHRW. A murine model of septic arthritis demonstrates that infection with an α toxin-producing strain of S. aureus leads to significantly elevated levels of chondrocyte death within 48 hours of infection when compared with infection with an alpha toxin-deficient mutant strain. Orthop Proc. 2016;98-B (Suppl. 16):20. [Google Scholar]
- 14. Smith IDM, Milto KM, Doherty CJ, Amyes SGB, Simpson AHRW, Hall AC. A potential key role of α-haemolysin of Staphylococcus aureus in mediating chondrocyte death in septic arthritis. Bone Joint Res.2018;7(7):457-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hultgren OH, Stenson M, Tarkowski A. Role of IL-12 in Staphylococcus aureus–triggered arthritis and sepsis. Arthritis Res 2001;3:41-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang SG, Yu SS, Poo H, Chun JS. c-Jun/activator protein-1 mediates interleukin-1β-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J Biol Chem. 2005;280:29780-7. [DOI] [PubMed] [Google Scholar]
- 17. Guo Y, Li J, Hagström E, Ny T. Protective effects of plasmin(ogen) in a mouse model of Staphylococcus aureus induced arthritis. Arthritis Rheum. 2008;58:764-72. [DOI] [PubMed] [Google Scholar]
- 18. Hong EH, Yun HS, Kim J, Um HD, Lee KH, Kang CM, et al. Nicotinamide phosphoribosyltransferase is essential for interleukin-1β-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J Biol Chem. 2011;286:28619-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chubinskaya S, Cs-Szabo G, Kuettner KE. ADAM-10 message is expressed in human articular cartilage. J Histochem Cytochem. 1998;46(6):723-9. [DOI] [PubMed] [Google Scholar]
- 20. Chubinskaya S, Mikhail R, Deutsch A, Tindal MH. ADAM-10 protein is present in human articular cartilage primarily in the membrane-bound form and is upregulated in osteoarthritis and in response to IL-1alpha in bovine nasal cartilage. J Histochem Cytochem. 2001;49:1165-76. [DOI] [PubMed] [Google Scholar]
- 21. Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 2010;107(30):13473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55(4):733-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cooper LZ, Madoff MA, Weinstein L. Hemolysis of rabbit erythrocytes by purified Staphylococcal alpha-toxin. I. Kinetics of the lytic reaction. J Bacteriol. 1964;87:127-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baumgarten M, Bloebaum RD, Ross SD, Campbell P, Sarmiento A. Normal human synovial fluid: osmolality and exercise-induced changes. J Bone Joint Surg Am. 1985;67(9):1336-9. [PubMed] [Google Scholar]
- 26. Amin AK, Huntley JS, Simpson AH, Hall AC. Increasing the osmolarity of joint irrigation solutions may avoid injury to cartilage: a pilot study. Clin Orthop Relat Res. 2010;468(3):875-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amin AK, Simpson AHRW, Hall AC. Iatrogenic articular cartilage injury: the elephant in the operating theatre: the surgeon’s role in chondroprotection. Bone Joint J. 2017;99-B:1555-6. [DOI] [PubMed] [Google Scholar]
- 28. Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13(1):16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423-93. [DOI] [PubMed] [Google Scholar]
- 30. Reichwein J, Hugo F, Roth M, Sinner A, Bhakdi S. Quantitative analysis of the binding and oligomerization of staphylococcal α-toxin in target erythrocyte membranes. Infect Immun. 1987;55(12):2940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freer JH. Cytolytic toxins and surface activity. Toxicon. 1982;20(1):217-21. [DOI] [PubMed] [Google Scholar]
- 32. Reddi BA. Why is saline so acidic (and does it really matter?). Int J Med Sci. 2013;10(6):747-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harshman S, Boquet P, Duflot E, Alouf JE, Montecucco C, Papini E. Staphylococcal α-toxin: a study of membrane penetration and pore formation. J Biol Chem. 1989;264(25):14978-84. [PubMed] [Google Scholar]
- 34. Bortoleto RK, Ward RJ. A stability transition at mildly acidic pH in the α-hemolysin (α-toxin) from Staphylococcus aureus. FEBS Lett. 1999;459:438-42. [DOI] [PubMed] [Google Scholar]
- 35. Harshman S, Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985;47(1):37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Y, Yang L. Calcium and magnesium ions are membrane-active against stationary-phase Staphylococcus aureus with high specificity. Sci Rep. 2016;6(1):20628. doi: 10.1038/srep20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Z, Liu D, Dong J, Gong L, Wang Y, Tang P, et al. Effects of cold irrigation on early results after total knee arthroplasty: a randomised, double-blind controlled study. Medicine (Baltimore). 2016;95(24):e3563. doi: 10.1097/MD.0000000000003563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan PF, Foster SJ. The role of environmental factors in the regulation of virulence-determinant expression of Staphylococcus aureus 8325-4. Microbiology. 1998;144(Pt 9):2469-79. [DOI] [PubMed] [Google Scholar]
- 39. Bush PG, Hall AC. The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J Orthop Res. 2001;19(5):768-78. [DOI] [PubMed] [Google Scholar]
- 40. DeLeo FR, Chambers HF. Re-emergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77(2):173-207. doi: 10.1128/MMBR.00052-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balaban N, Collins LV, Cullor JS, Hume EB, Medina-Acosta E, Viera da, Motta O, et al. Prevention of diseases caused by Staphylococcus aureus using the peptide RIP. Peptides 2000;21:1301-11. [DOI] [PubMed] [Google Scholar]
- 43. Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J Mol Biol 2013;425(10):1641-54. doi: 10.1016/j.jmb.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 44. Weeks AC, Balzli CL, Caballero A, Tang A, O’Callaghan R. Identification and potency of cyclodextrin-lipid inhibitors of Staphylococcus aureus α-toxin. Curr Eye Res. 2012;37:87-93. [DOI] [PubMed] [Google Scholar]
- 45. Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8(12):933-8. doi: 10.1038/nnano.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]