Abstract
Objective
Chondrospheres represent a variant of tissue spheroids biofabricated from chondrocytes. They are already being used in clinical trials for cartilage repair; however, their biomechanical properties have not been systematically investigated yet. The aim of our study was to characterize chondrospheres in long-term in vitro culture conditions for morphometric changes, biomechanical integrity, and their fusion and spreading kinetics.
Results
It has been demonstrated that the increase in chondrospheres secant modulus of elasticity is strongly associated with the synthesis and accumulation of extracellular matrix. Additionally, significant interplay has been found between biomechanical properties of tissue spheroids and their fusion kinetics in contrast to their spreading kinetics.
Conclusions
Extracellular matrix is one of the main structural determinants of chondrospheres biomechanical properties during chondrogenic maturation in vitro. The estimation of tissue spheroids’ physical behavior in vitro prior to operative treatment can be used to predict and potentially control fusogenic self-assembly process after implantation in vivo.
Keywords: cartilage, chondrospheres, tissue spheroids, extracellular matrix, biomechanical properties
Introduction
Cartilage injuries and diseases are essential clinical problems for which a solution has yet to be found.1 The cartilage tissue is capable of effective regeneration during embryonic stage in comparison with an adult organism where self-repair of cartilage is completely lost. Cartilage injuries often lead to the development of osteoarthritis.2 Tissue engineering, an extensively evolving field of biomedical science, was proposed to enable biofabrication of artificial cartilage tissue for sufficient repair of cartilage defects.3 To date, 2 main strategies in cartilage tissue engineering exist. The first one employs synthetic and natural scaffolds as a temporal biodegradable support for seeding chondrocytes with sequential formation of cartilaginous tissue, usually in special bioreactors.4 The second strategy represents scaffold-free approach, based on the use of self-assembling living building blocks, also known as tissue spheroids (TS).5,6 Chondrospheres (CS) are densely packed TS biofabricated from chondrocytes or stem cells capable of differentiation into chondrogenic lineage.7,8 Both approaches have certain advantages and disadvantages. In support of the scaffold-based approach, better biomechanical properties of engineered constructs provided by employment of solid scaffolds can be observed. At the same time, a scaffold-free method has been developed to overcome the possibility of contamination, inflammation, and body reaction by renouncing any scaffolds obtained from both natural and synthetic sources.
In this study, we have focused on the second, that is, scaffold-free approach. It has become widely used in cartilage tissue engineering over the past decade.9,10 Growing interest in the scaffold-free approach is at least partly due to the successful results of 3-dimensional autologous chondrocyte transplantation technology.11,12 The results showed significant improvement in both quality of life and health of patients suffering from traumatic or degenerative injuries of articular cartilage. Despite the current progress of translation CS into clinical practice, their fundamental characteristics are still poorly understood. Neither biomechanical properties of CS nor structural determinants of their maturation were characterized systematically in the prior studies. This work describes, for the first time, the correlation between biomechanical properties of CS and their capacity of spreading and fusion.
The study of fusion kinetics in vitro is a powerful tool for prediction of TS capacity to form integrated mature tissue. The fusion of TS is controlled by tissue maturation factors, and this process can be quantified by changing the total length and/or the contact area of interacting TS, which has been seen previously.13,14 These studies have demonstrated that the increased production of type I collagen reduces the rate of fusion and spreading of TS biofabricated from fibroblasts. TS fusion and spreading therefore appear to be indispensable features enabling optimal integration with the host tissue after implantation in vivo.15
Biomechanical properties of TS are a topic of interest for biomedical research for their ability to estimate maturity level and functional properties of the cells in 3D cultures. Various techniques for determining the TS biomechanical properties exist. A classic one is an atomic force microscopy method used to study the rigidity of component cells within the cancer TS and to assess their ability of migration and invasion.16,17 The development of new measurement methods, such as force-sensing micro-tweezers as well as micro-scale parallel-plate compression testing systems, has made it possible to apply a tensiometry method for TS, whereby they achieved significantly higher values of modulus of elasticity compared with individual cells.18,19 The modulus of elasticity is related to the cell cohesion within the TS. The cell cohesion is caused by the interactions of cells that occur when cohesive cells are placed contiguously on the nonadhesive surface and leads to the self-assembly process and subsequent TS formation. Numerous studies have shown the importance of biomechanical properties in the prediction of interactions among various types of embryonic cells and biomaterials, and in developing new methods to modify the intercellular cohesion through cadherin-based mechanisms and extracellular matrix (ECM) molecules.20-22 Additionally, the rigidity of the microenvironment in TS biofabricated from mesenchymal stem cells affects their differentiation and can be enhanced by the addition of gelatin microparticles.23 In this approach, the measurement of TS biomechanical properties by the parallel-plate compression method is proposed as one of the crucial steps for tissue engineering of bone, adipose, and cartilaginous tissue intended for use in regenerative medicine. Thus, the tensiometry method opens a unique opportunity to estimate the biomechanical properties of TS and to establish the correlation between biomechanical properties of TS and their fusion kinetics. However, no comprehensive studies on the effect of constituent cells and ECM molecules on the biomechanical properties of CS have been carried out previously.
As a follow-up to our ongoing studies on the systematic characterization of different types of TS,24,25 we elaborate on CS in this work. For that purpose, we evaluated their biomechanical properties using a tensiometry method combined with routine histological analysis of structural determinants to predict the most important properties of TS, spreading on adhesive substrates and fusion. We hypothesize that CS biomechanical properties are determined mainly by ECM accumulation, which is directly linked to the time of maturation. We strongly believe that such estimation will be useful in the prediction of CS integrative capacity after implantation in vivo as well as for determination of their maturity level in clinical trials.
Materials and Methods
Cell Culture
The specimens of male sheep articular hyaline cartilage were obtained at the N.N. Priorov National Medical Research Center of Traumatology and Orthopedics (Moscow, Russia) immediately after animals’ euthanasia. The tissue was isolated from the femur condyles of knee joints by tangential cutting of articular cartilages with a sterile scalpel; thus, it included all layers but calcified cartilage. Tissue specimens were minced to fragments of 1 to 2 mm3 and placed in a 0.1% solution of collagenase type II in DMEM medium (PanEco, Russia) and incubated for 24 hours at 37°C. After filtration through 100-µm cell strainers (Corning, USA), single-cell suspensions were transferred to 75 cm2 flasks in a complete growth medium, including DMEM (Gibco, USA) supplemented with 10% sheep serum (Sigma Aldrich, USA), 2 mM L-glutamine (Paneco, Russia), and 1% antibiotic/antimycotic (Gibco, USA). Isolated chondrocytes were cultured under standard conditions (37°C, 5% CO2) with change of medium twice a week and routinely split at 85% to 95% confluence using mild enzymatic dissociation with a 0.25% trypsin/0.53 mM EDTA solution (Gibco, USA). Cells were free of mycoplasmal contamination as verified using Hoechst 33258 (Sigma Aldrich, USA) staining protocol.
Preparation of Chondrospheres
TS were formed using 96-well Corning Spheroid Microplates according to the manufacturer’s protocol. Briefly, chondrocytes from 3 to 5 passages were detached from plastic and suspended in complete growth medium at the concentration of 8 × 104 cells per milliliter. A total of 100 µL of cell suspensions were dispensed to the wells of spheroid microplates. Spheroid microplates were then incubated at 37°C in a humidified atmosphere with 5% CO2 for 21 days with change of medium every 3 days. For immunohistochemical analysis we prepared TS using MicroTissues 3D Petri Dish micromolds (Sigma Aldrich, USA) in the following manner: 190 µL of suspension with concentration 3.4 × 106 cells per milliliter was placed into each 81-well nonadhesive agarose mold, and molds were placed in 12-well culture plates (Nunc, USA) and covered with complete growth media for 1 hour. In both methods, the resultant TS contained 8 × 103 chondrocytes. The variations in average diameters of CS during their prolonged incubation (after 1, 7, 14, 21 days in culture) were estimated using light microscopy (Nikon Eclipse Ti-E, Japan) equipped with optical accessories for differential interference contrast (DIC) and original NIS-Elements imaging software. Diameters of at least 96 TS for each time point were measured.
Fabrication of Electrospun Polyurethane Matrix
Biocompatible polyurethane EG-85A (Lubrizol, USA) has been dissolved to concentration of 17% in a solvent containing 40% N,N-dimethylformamide and 60% tetrahydrofuran. Electrospinning of microfibrous polyurethane matrix has been performed using Professional Electrospinning Lab Device (Yflow, Spain) under a voltage of 17 kV. The distance between the needle (diameter 0.84 mm) and collector was 20 cm; the speed of polymer movement was 1.3 mL/h. Biofabricated CS were placed on an electrospun polyurethane matrix for scanning electron microscopy (SEM) and allowed to attach for 6 hours. Samples were subsequently fixed with 2.5% glutaraldehyde/phosphate-buffered saline (PBS), dehydrated through ethanol series, and dried in a critical point dryer (HCP-2, Hitachi Koki Co. Ltd., Japan).
Electron Microscopy
The samples were transferred on a metal stub with adhesive surface, coated with gold using ion coater (IB-3, EIKO, Japan) and then observed under scanning electron microscope JSM-6510 LV (JEOL, Japan). For transmission electron microscopic (TEM) examination, CS were fixed in a 1% solution of glutaraldehyde in 0.1 M phosphate buffer, then treated with 1% OsO4 solution in 0.1 M phosphate buffer, dehydrated in descending alcohols, and finally in propylene oxide. After dehydration, TS were placed in a mixture of propylene oxide and araldite and then into araldite. Semi-thin and ultra-thin sections were obtained using ultramicrotome Leica EM UC7 (Leica, Germany). Semi-thin sections were stained with hematoxylin-eosin and toluidine blue dyes (BioVitrum, Russia) and then examined by light microscopy. Ultra-thin sections were contrasted with lead citrate and uranyl acetate and observed using scanning electron microscope JEM 1400 (JEOL, Japan).
Estimation of Tissue Spheroids Fusion and Spreading Kinetics
TS fusion assay was performed using Corning Spheroid Microplates. Pairs of CS after 1 day, 7 days, 14 days, and 21 days of maturation in culture were placed in close proximity where TS surfaces are touching each other and incubated for another 5 days. Bright-field images of TS doublets were obtained at points 0 day, 1 day, 2 days, 3 days, 4 days, and 5 days using light microscopy (Nikon Eclipse Ti-E, Japan). Twelve pairs of CS were measured for each time point. Doublet length was measured using ImageJ 1.48v software (NIH, Bethesda, MD). TS spreading assay was performed using adhesive plastic culture plates (Corning, USA). CS from the same time points were placed on the bottom of 24-well plates and incubated for 32 hours. Bright-field images of TS spreading were obtained at points 0 hours, 1 hour, 2 hours, 4 hours, 8 hours, 16 hours, 24 hours, and 32 hours using light microscopy (Nikon Eclipse Ti-E, Japan). Eight groups of 3 TS were measured for each time point. Spreading area was also measured using ImageJ 1.48v software. All results obtained from fusion and spreading assays were plotted as a function of time using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Measurements of doublet length and spreading area were reported as mean ± SEM.
Estimation of Chondrospheres Biomechanical Properties
TS biomechanical properties were measured using a micro-scale parallel-plate compression testing system Microsquisher (CellScale, Canada) and corresponding SquisherJoy software. TS were formed using Corning Spheroid Microplates as described previously. For mechanical testing TS were placed in a PBS-filled bath at 37°C and compressed to 50% deformation for 20 seconds. The microbeam with diameter 304.8 µm (recommended max force 917 mN) was employed with relation to the rigidity and sensitivity required to measure TS from different times of maturation. The stress was calculated based on the SquisherJoy software as the force divided by the nondeformed initial cross-section area of TS. The strain of TS at the vertical compression was calculated as ε = (D0 − D)/D0, where D0 is an initial diameter of TS, and D is the diameter of TS during deformation. The secant modulus of elasticity was calculated on the initial portion of the stress-strain curve (0% to 20% strain) at ε = 0.2 for each TS. CS were measured after 1 day, 7 days, 14 days, and 21 days in culture in 4 groups of 12 samples, one group for each time point.
Immunohistochemical Analysis
CS in molds were covered with molten agarose, then fixed in 10% buffered formalin (pH 7.4) for 24 hours and embedded in paraffin (Biovitrum, Russia). Serial sections with a thickness of 5 µm were cut with Microtome HMS 740 (Thermo Fisher Scientific, USA) and mounted on poly-L-lysine coated glass. Dewaxing was carried out in xylene and a battery of downstream alcohols, and the antigen retrieval was performed using proteinase K (Sigma-Aldrich, USA) for 20 minutes. Further procedures were performed automatically in Autostainer 360 system (Thermo Fisher Scientific, USA). The protocol included 10 minutes H2O2, 10 minutes protein block, 30 minutes primary antibody, 10 minutes secondary antibody, and 5 minutes 3,3′-diaminobenzidine (DAB) treatment. Washing was carried out in Tris buffer (pH 6.0) with Tween 20. Primary polyclonal rabbit antibodies to sheep aggrecan, type I collagen, and type II collagen (all Abbiotec, USA) were used in dilutions of 1:100. Nuclei were counterstained with Mayer’s hematoxylin. Finally, sections were dehydrated and enclosed in Bio-Mount (Bio Optica Milano SPA, Italy).
Estimation of Chondrospheres Viability
Cell viability within TS was monitored using the Live/Dead Cell Double Staining Kit (Sigma-Aldrich, USA) according to the manufacturer’s protocol. This assay was used to visually determine if cells within CS remained viable after TS formation and cultivation. Briefly, after the desired maturation period, 1-day-old, 7-day-old, 14-day-old, and 21-day-old CS were incubated with a solution containing Calcein AM and propidium iodide (PI) at 37°C for 30 minutes. After washing with PBS, TS were imaged by fluorescent microscopy (Nikon Eclipse Ti-E, Japan). Cell viability was assessed in 4 groups of 12 samples, one group for the each time point.
Statistical Analysis
Statistical analysis was performed using analysis of variance (ANOVA) followed by Tukey HSD post hoc test. All statistical data were analyzed using GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA) and reported as mean ± SEM. Statistical significance was determined at P < 0.05. Each experiment was repeated independently at least 3 times.
Results
Morphology and Viability of Chondrospheres
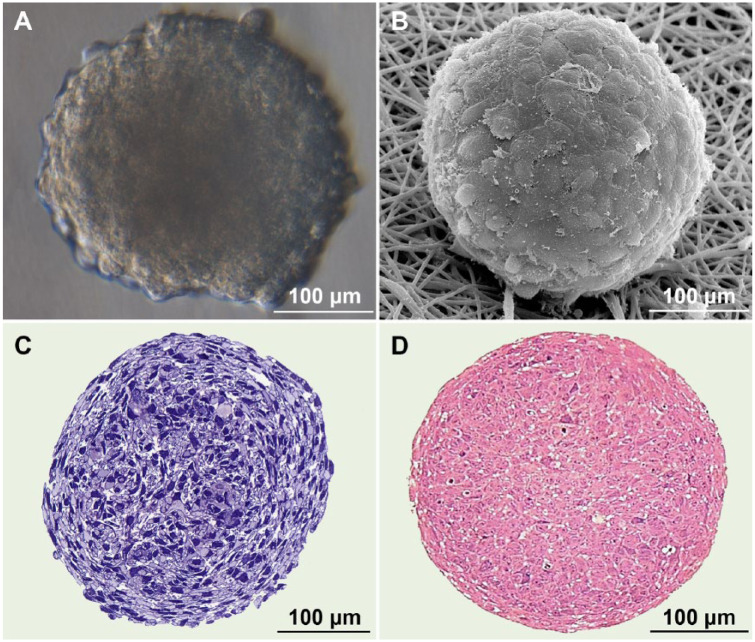
CS exhibited rather smooth surfaces formed by intimately contacting compact polygonal cells (Fig. 1A and B). Semi-thin sections demonstrated the initial absence of any ECM components and extremely high cell density within the TS (Fig. 1C and D). CS diameter decreased nearly twice from 413.7 ± 42.5 to 208.5 ± 38.1 µm, which translated to even greater reduction in CS volume. This suggests possible cell-shape changes and compaction to be the most realistic mechanism of CS size reduction at later stages of maturation.
Figure 1.
Seven-day-old chondrospheres visualized by different microscopy methods: DIC (differential interference contrast) (A), SEM (scanning electron microscopy) (B), toluidine blue (C), and azure-eosin (D) staining of semi-thin sections (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
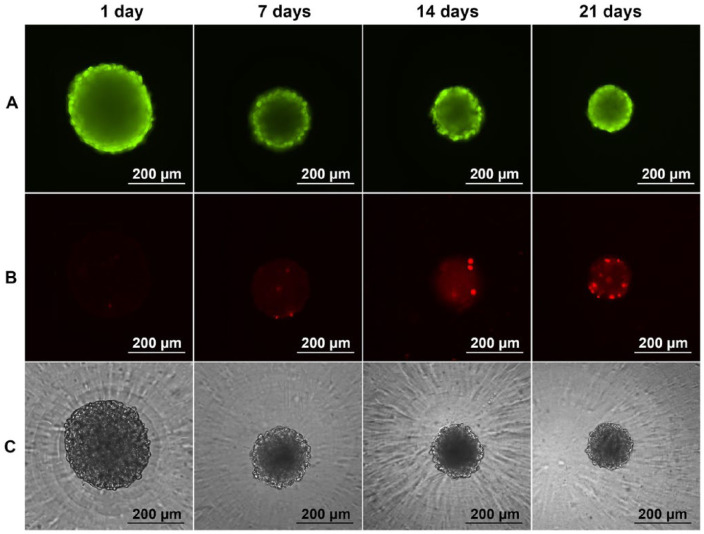
We estimated the viability of cells within CS using Live/Dead assay. Qualitative estimation of chondrocytes viability revealed a sufficient amount of living cells (Fig. 2A, green) during 21 days in culture. Population of nonviable cells increased significantly at the latest stage of cultivation (Fig. 2B, red). The localization of dead cells was not specific. Correlative phase-contrast microscopy demonstrated intact CS morphology after 1 day, 7 days, 14 days, and 21 days in culture (Fig. 2C).
Figure 2.
Viability of chondrospheres (CS) during the culture. Live/Dead assay: calcein AM, green (A); propidium iodide, red (B); phase contrast (C). cS possessed intact morphology and sufficient number of viable cells during the 21-day cultivation (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
Ultrastructure of Chondrospheres and Specific ECM Components Accumulation
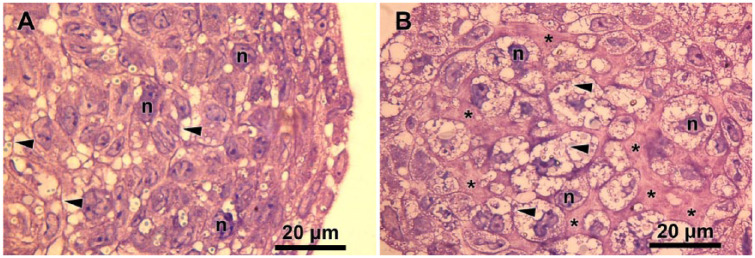
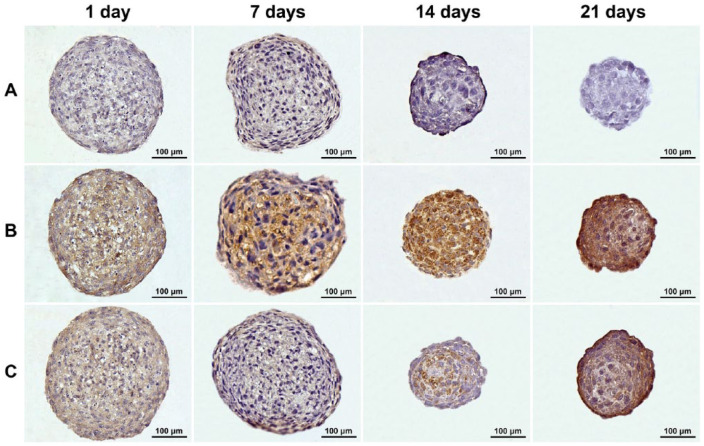
The histological sections of CS revealed extensive accumulation of ECM, which closely correlated with the time of maturation (Fig. 3). The immunohistochemistry analysis confirmed the absence of type I collagen and gradual increase in levels of type II collagen and aggrecan, the typical markers of cartilage tissue (Fig. 4). The growing production of such ECM molecules was the evidence of constant tissue organization processes during long-term cultivation in vitro. After 14 and 21 days of incubation, the chondrogenic phenotype of cells in TS became more evident.
Figure 3.
Increase in extracellular matrix (ECM) content during chondrospheres (CS) maturation: Hematoxylin-eosin staining of 1-day-old CS (A) and 21-day-old CS (B) semi-thin sections. ECM accumulation (stars), cytoplasm (arrowheads), and nuclei (n) are indicated in the figure.
Figure 4.
Accumulation of specific extracellular matrix components within chondrospheres (CS) during maturation in vitro: type I collagen (A), type II collagen (B), and aggrecan (C) were dyed brown in 1-day-old, 7-day-old, 14-day-old, and 21-day-old CS sections via immunohistochemical staining. The immunohistochemistry analysis confirmed the absence of type I collagen and gradual increase in levels of hyaline cartilage-specific type II collagen and aggrecan during long-term in vitro incubation.
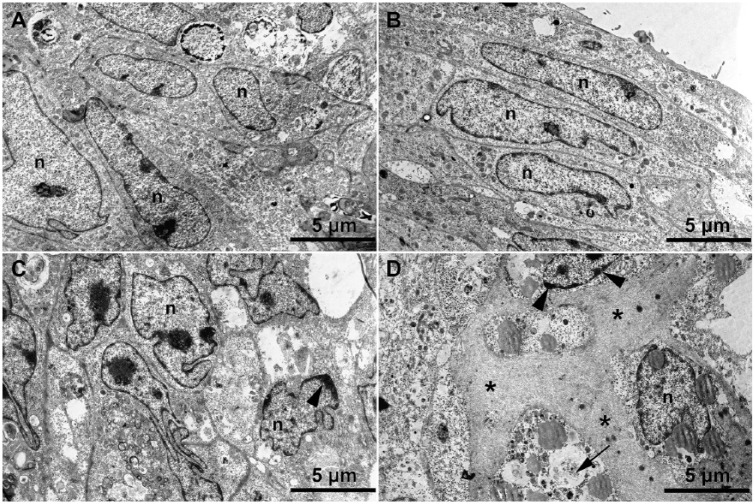
To estimate the preservation of cellular ultrastructure in CS and to register the earliest stages of ECM synthesis and accumulation, transmission electron microscopic study of their ultra-thin sections was performed (Fig. 5), which showed that CS consisted of tightly packed chondrocytes with well-developed intracellular ultrastructure that retained nuclei and cellular membranes at early stages of incubation. No signs of ECM accumulation were initially observed (Fig. 5A and B). However, there was a growing pattern of cellular degeneration such as defects in cell membrane, clumping of nuclear chromatin, and so on (Fig. 5C and D). At later stages of incubation, the cells’ ultrastructure was rather irregular, and at the same time, the accumulation of ECM reached its highest level. Thus, electron microscopic observation confirmed data on gradual accumulation of ECM according to histological and immunohistochemical analyses.
Figure 5.
Changes to cellular microstructure during chondrospheres (CS) maturation in vitro: 1-day-old (A), 7-day-old (B), 14-day-old (C), and 21-day-old CS (D). Transmission electron microscopic. Cellular degeneration is indicated as defects in cell membrane (arrow) and clumping of nuclear chromatin (arrowheads). The increased accumulation of extracellular matrix in CS during their long-term in vitro incubation (stars) and nuclei (n) are shown.
Correlation between Biomechanical Properties of Chondrospheres and Their Ability to Fusion and Spreading
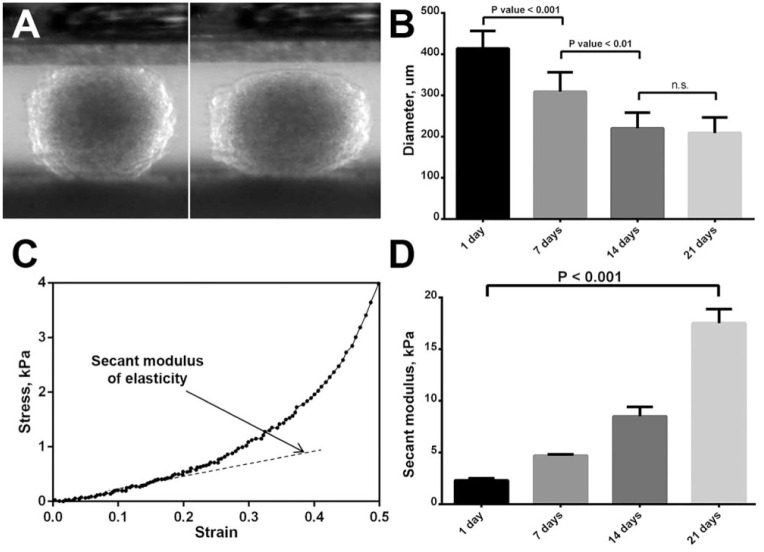
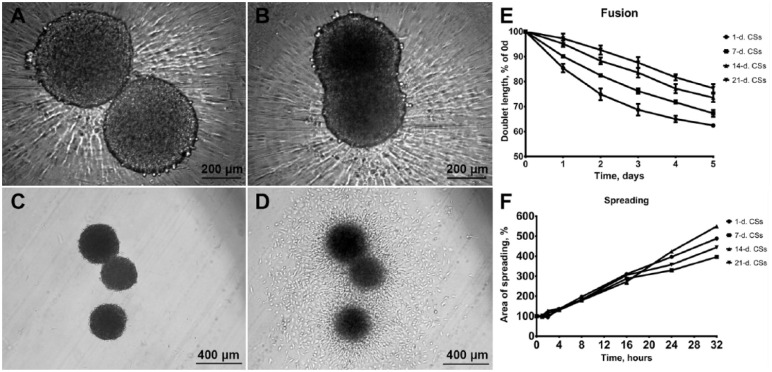
To assess the biomechanical properties of CS we have calculated the secant moduli of elasticity, which have been gradually increasing, reaching maximum values after 21 days of incubation (17.5 ± 1.2 kPa) (Fig. 6). Conversely, gradual decrease of CS diameters during incubation in vitro can de observed (Fig. 6B). There was no correlation between spreading on adhesive plastic surface and biomechanical properties of CS (Fig. 7C, D, and F). However, significant correlation has been found between CS biomechanical properties and their fusion kinetics during long-term cultivation in vitro (Fig. 7A, B, and E).
Figure 6.
Determination of biomechanical properties of chondrospheres (CS): compression stages during the test of 7-day-old CS using the compression system (A), the reduction of CS diameter as a result of their maturation process (B), CS strain-stress graph (C), increase of secant modulus of elasticity during the maturation of CS (D). Data of the graphs (B, D) are represented as mean ± SEM.
Figure 7.
Increase of chondrospheres (CS) biomechanical properties affects their fusion kinetics, while CS spreading kinetics on adhesive substrate is likely to remain stable. Phase-contrast microscopy of 2 CS fusion: starting point (A), 24 hours later (B). Phase-contrast microscopy of 3 CS spreading: starting point (C), 24 hours later (D). Fusion dynamics of CS at different maturation stages, data are represented as mean ± SEM (E). Spreading dynamics of CS at different maturation stages, data are represented as mean ± SEM (F).
Discussion
The main outcome of the study is the phenotyping characterization of CS during their maturation in vitro. Although we used differentiated cells from primary culture of articular cartilage for TS biofabrication, CS initially have no ECM; therefore, they could not be considered as a mature cartilage on a tissue level.
Several events took place during in vitro chondrogenic maturation of TS fabricated from differentiated chondrocytes. The CS volume is reduced consistently over time. TS have been generated with initial diameters of 350 to 400 µm, which corresponds to the physiological limits of nutrients and oxygen diffusion.26 However, after 3 weeks of incubation CS diameter has dramatically decreased by half, which means the volume went approximately 8 times down, according to the sphere volume formula. It can be assumed that changes in TS volume were directly related to cell shape changes and compaction.
As demonstrated by Live/Dead assay, the ratio of living and dead cells gradually decreased throughout the entire incubation process. According to Malcolm Steinberg’s differential adhesion hypothesis, the sorting-out behavior of nonviable cells due to the loss or shedding of cell adhesion receptors during apoptosis cannot be completely excluded.27 This could partly explain why non-viable cells were pushed out at TS peripheral zone. The ultrastructural studies confirmed the presence of cellular degeneration. After 3 weeks of incubation, it was difficult to find unaltered cells according TEM.
The accumulation of ECM is likely to cause reduced diffusion of oxygen and nutrients, and therefore biofabrication of TS with smaller diameter could guarantee their better viability. However, the femur condyle cartilage in adult humans and in some mammals reaches 2 to 3 mm in thickness, which is at least 12-fold higher than the diameter of TS employed in our study and it does not lead to any problems with chondrocytes viability in vivo.28 The physiological explanation of this discrepancy is a mechanical stimulation that induces avascular movement of synovial fluid inside the cartilage. As is well known, mechanical stimulation of scaffold-free constructs considerably initiates production of basic cartilage matrix components (proteoglycans and type II collagen).29 A growing body of evidence suggests that mechanical conditioning in specially designed bioreactors could improve both cell viability and the efficiency of chondrogenic differentiation in engineered cartilage constructs.30-32
In addition to biomechanical stimulation, the use of bioreactors makes it possible to culture cartilaginous tissue constructs under desirable environment conditions through controlled perfusion of the nutrient medium enriched by optimal quantities of growth factors, metabolic agents, and gases.33,34 The possibility of using versatile bioreactors for individual cellular aggregates has recently been demonstrated for TS fabricated from cancer cells.35 The utilization of ultralow shear dynamic stress has promoted intercellular connection, increased TS size and number of cycling cells, and reduced DNA damage. Thus, controlled in vitro culture conditions would improve the cell viability, tissue organization, the intensive accumulation of ECM components, and ultimately control the biomechanical properties of CS intended to replace articular cartilage defects. Our morphological observations are consistent with the data of previous studies, where vacuolization and shrinkage of cells were associated with long-term cultivation of cartilage constructs without a bioreactor system performing dynamic shear and compressive loading on developing cartilage tissues to mimic the rolling and squeezing action of articular joints.36,37
The increased accumulation of ECM in CS during their in vitro incubation was confirmed in our study by histological, immunohistochemical, and electron microscopic studies. Furthermore, sheep chondrocytes in CS did not produce type I collagen, whereas the levels of type II collagen and aggrecan, the typical markers of cartilage tissue, were gradually increased. This observation was consistent with other studies comparing the cultivation of chondrocytes in 2D and 3D cultures.38,39
Our study also demonstrated gradual increase in CS rigidity during maturation in vitro. Since changes in CS rigidity were accompanied by the accumulation of type II collagen and aggrecan, it is reasonable to postulate that CS ECM is one of the main structural determinants of their biomechanical properties. The results of our work provide well-established correlation between CS biomechanical properties and the level of ECM accumulation, consistent with studies where TS were fabricated from human cartilage progenitor cells and other cartilage models.40,41
CS ability to spread on adhesive substrates did not relate to their biomechanical properties. Nevertheless, we observed the well-established connection between CS biomechanical properties and their fusion kinetics, significantly decreasing during maturation process, which seems valuable for prediction of TS behavior after implantation in vivo.
Conclusions
CS secant modulus of elasticity directly correlates with ECM synthesis and accumulation. Thus, ECM could be considered as one of the main structural determinants of their biomechanical properties. The change in CS rigidity does not affect their spreading kinetics on adhesive substrates. Conversely, the well-established connection between the CS biomechanical properties and their fusion kinetics opens unique opportunities for modelling a self-assembly process after implantation in vivo.
The long-term in vitro cultivation of TS consisting of articular chondrocytes is associated with gradual accumulation of ECM and near 2-fold reduction of CS diameter. This phenomenon points to the need for either using CS for in vivo implantation after the short period of in vitro incubation, not exceeding 2 weeks, or optimizing the existing culturing protocol by using specially designed chondrogenic cell medium, bioreactors, and mechanical stimulation.
The results presented here define biomechanical properties and fusogenic behavior of biofabricated CS, which could be extremely useful in their translation into clinical practice for the treatment of cartilage defects.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was conducted with the support of the RUDN University Program 5-100.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the local Ethical Committee of the N.N. Priorov National Medical Research Center of Traumatology and Orthopedics (Approval Number: 21).
Animal Welfare: Sheep were raised and treated according to the principles of laboratory animal care of the institute’s animal ethical committee.
ORCID iDs: Elena A. Bulanova  https://orcid.org/0000-0001-8460-3113
https://orcid.org/0000-0001-8460-3113
Vladislav A. Parfenov  https://orcid.org/0000-0003-1234-0208
https://orcid.org/0000-0003-1234-0208
Anna A. Gryadunova  https://orcid.org/0000-0002-5189-8530
https://orcid.org/0000-0002-5189-8530
References
- 1. Christensen BB. Autologous tissue transplantations for osteochondral repair. Dan Med J. 2016;63(4):B5236. [PubMed] [Google Scholar]
- 2. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917-21. doi: 10.1126/science.1222454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920-6. [DOI] [PubMed] [Google Scholar]
- 4. Ma PX, Elisseeff J. Scaffolding in tissue engineering. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 5. Lee JK, Link JM, Hu JCY, Athanasiou KA. The self-assembling process and applications in tissue engineering. Cold Spring Harb Perspect Med. 2017;7(11):a025668. doi: 10.1101/cshperspect.a025668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasui Y, Ando W, Shimomura K, Koizumi K, Ryota C, Hamamoto S, et al. Scaffold free, stem cell-based cartilage repair. J Clin Orthop Trauma. 2016;7(3):157-63. doi: 10.1016/j.jcot.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer U, Wiesmann HP, Libera J, Depprich R, Naujoks C, Handschel J. Cartilage defect regeneration by ex vivo engineered autologous microtissue—preliminary results. In Vivo. 2012;26(2):251-7. [PubMed] [Google Scholar]
- 8. Schubert T, Anders S, Neumann E, Schölmerich J, Hofstädter F, Grifka J, et al. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int J Mol Med. 2009;23(4):455-60. [DOI] [PubMed] [Google Scholar]
- 9. Baptista LS, Silva KR, Pedrosa CS, Amaral RJ, Belizário JV, Borojevic R, et al. Bioengineered cartilage in a scaffold-free method by human cartilage-derived progenitor cells: a comparison with human adipose-derived mesenchymal stromal cells. Artif Organs. 2013;37(12):1068-75. doi: 10.1111/aor.12121 [DOI] [PubMed] [Google Scholar]
- 10. Silva KR, Rezende RA, Pereira FD, Gruber P, Stuart MP, Ovsianikov A, et al. Delivery of human adipose stem cells spheroids into lockyballs. PLoS One. 2016;11(11):e0166073. doi: 10.1371/journal.pone.0166073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fickert S, Gerwien P, Helmert B, Schattenberg T, Weckbach S, Kaszkin-Bettag M, et al. One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage. 2012;3(1):27-42. doi: 10.1177/1947603511417616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siebold R, Suezer F, Schmitt B, Trattnig S, Essig M. Good clinical and MRI outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):831-9. doi: 10.1007/s00167-017-4491-0 [DOI] [PubMed] [Google Scholar]
- 13. Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101(3):695-711. doi: 10.1002/jcb.21224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hajdu Z, Mironov V, Mehesz AN, Norris RA, Markwald RR, Visconti RP. Tissue spheroid fusion-based in vitro screening assays for analysis of tissue maturation. J Tissue Eng Regen Med. 2010;4(8):659-64. doi: 10.1002/term.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartz C, Meixner M, Giesemann P, Roël G, Bulwin GC, Smink JJ. An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. J Transl Med. 2016;14(1):317. doi: 10.1186/s12967-016-1065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lekka M, Gil D, Pogoda K, Dulińska-Litewka J, Jach R, Gostek J, et al. Cancer cell detection in tissue sections using AFM. Arch Biochem Biophys. 2012;518(2):151-6. doi: 10.1016/j.abb.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 17. Cross SE, Jin YS, Tondre J, Wong R, Rao J, Gimzewski JK. AFM-based analysis of human metastatic cancer cells. Nanotechnology. 2008;19(38):384003. doi: 10.1088/0957-4484/19/38/384003 [DOI] [PubMed] [Google Scholar]
- 18. Jaiswal D, Cowley N, Bian Z, Zheng G, Claffey KP, Hoshino K. Stiffness analysis of 3D spheroids using microtweezers. PLoS One. 2017;12(11):e0188346. doi: 10.1371/journal.pone.0188346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler CM, Foty RA. Measurement of aggregate cohesion by tissue surface tensiometry. J Vis Exp. 2011;(50):2739. doi: 10.3791/2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122(5):1611-20. [DOI] [PubMed] [Google Scholar]
- 21. Ryan PL, Foty RA, Kohn J, Steinberg MS. Tissue spreading on implantable substrates is a competitive outcome of cell-cell vs. cell-substratum adhesivity. Proc Nat Acad Sci U S A. 2001;98(8):4323-27. doi: 10.1073/pnas.071615398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5(7):e11830. doi: 10.1371/journal.pone.0011830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baraniak PR, Cooke MT, Saeed R, Kinney MA, Fridley KM, McDevitt TC. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J Mech Behav Biomed Mater. 2012;11:63-71. doi: 10.1016/j.jmbbm.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koudan EV, Bulanova EA, Pereira FDAS, Parfenov VA, Kasyanov VA, Hesuani UJ, et al. Spreading of tissue spheroids on an electrospun polyurethane matrix. Biomed Eng. 2016;50(1):1-4. doi:0.1007/s10527-016-9575-5 [Google Scholar]
- 25. Koudan EV, Korneva JV, Karalkin PA, Gladkaya IS, Gryadunova AA, Mironov VA, et al. The scalable standardized biofabrication of tissue spheroids from different cell types using nonadhesive technology. 3D Printing Addit Manuf. 2017;4(1):53-60. doi: 10.1089/3dp.2016.0044 [DOI] [Google Scholar]
- 26. Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9-10):1172-84. doi: 10.1002/biot.200700228 [DOI] [PubMed] [Google Scholar]
- 27. Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278(1):255-63. doi: 10.1016/j.ydbio.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 28. Malda J, de Grauw JC, Benders KE, Kik MJL, van de Lest CH, Creemers LB, et al. Of mice, men and elephants: the relation between articular cartilage thickness and body mass. PLoS One. 2013;8(2):e57683. doi: 10.1371/journal.pone.0057683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ponomarev IV, Kochneva LM, Barnewitz D. Effect of 3D chondrocyte culturing conditions on the formation of extracellular matrix in cartilage tissue-engineering constructs. Bull Exp Biol Med. 2014;156(4):548-55. doi: 10.1007/s10517-014-2394-3 [DOI] [PubMed] [Google Scholar]
- 30. DiFederico E, Shelton JC, Bader DL. Complex mechanical conditioning of cell-seeded agarose constructs can influence chondrocyte biosynthetic activity. Biotechnol Bioeng. 2017;114(7):1614-25. doi: 10.1002/bit.26273 [DOI] [PubMed] [Google Scholar]
- 31. Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469(10):2764-72. doi: 10.1007/s11999-011-1819-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yusoff N, Osman NAA, Pingguan-Murphy B. Design and validation of a bi-axial loading bioreactor for mechanical stimulation of engineered cartilage. Med Eng Phys. 2011;33(6):782-8. doi: 10.1016/j.medengphy.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 33. Obradovic B, Carrier RL, Vunjak-Novakovic G, Freed LE. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol Bioeng. 1999;63(2):197-205. [DOI] [PubMed] [Google Scholar]
- 34. Mabvuure N, Hindocha S, Khan WS. The role of bioreactors in cartilage tissue engineering. Curr Stem Cell Res Ther. 2012;7(4):287-92. [DOI] [PubMed] [Google Scholar]
- 35. Massai D, Isu G, Madeddu D, Cerino G, Falco A, Frati C, et al. A versatile bioreactor for dynamic suspension cell culture. Application to the culture of cancer cell spheroids. PLoS One. 2016;11(5):e0154610. doi: 10.1371/journal.pone.0154610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130-8. doi: 10.1002/jor.1100170119 [DOI] [PubMed] [Google Scholar]
- 37. Shahin K, Doran PM. Shear and compression bioreactor for cartilage synthesis. Methods Mol Biol. 2015;1340:221-33. doi: 10.1007/978-1-4939-2938-2_16 [DOI] [PubMed] [Google Scholar]
- 38. Shi Y, Ma J, Zhang X, Li H, Jiang L, Qin J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr Biol (Camb). 2015;7(3):289-97. doi: 10.1039/c4ib00273c [DOI] [PubMed] [Google Scholar]
- 39. Tew SR, Murdoch AD, Rauchenberg RP, Hardingham TE. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods. 2008;45(1):2-9. doi: 10.1016/j.ymeth.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 40. Khoshgoftar M, Wilson W, Ito K, van Donkelaar CC. Influence of tissue- and cell-scale extracellular matrix distribution on the mechanical properties of tissue-engineered cartilage. Biomech Model Mechanobiol. 2013;12(5):901-13. doi: 10.1007/s10237-012-0452-1 [DOI] [PubMed] [Google Scholar]
- 41. Stuart MP, Matsui RAM, Santos MFS, Côrtes I, Azevedo MS, Silva KR, et al. Successful low-cost scaffold-free cartilage tissue engineering using human cartilage progenitor cell spheroids formed by micromolded nonadhesive hydrogel. Stem Cells Int. 2017;2017:7053465. doi: 10.1155/2017/7053465 [DOI] [PMC free article] [PubMed] [Google Scholar]