Abstract
Background
C-terminal cross-linked telopeptides of type II collagen (CTX-II) are one of the most frequently assessed markers for osteoarthritis (OA) diagnosis. The aim of this meta-analysis was to confirm the diagnostic value of urinary CTX-II in knee OA.
Materials and Methods
PubMed, ScienceDirect, and EMBASE were searched for studies measured urinary CTX-II in patients with knee OA and in healthy controls. Urinary CTX-II levels were compared between knee OA patients and controls. Differences between groups were expressed as standardized mean differences (SMD) when individual outcomes were measured with different scales. Otherwise, outcomes were presented as mean differences (MD). Subgroup analyses were also conducted to compare efficiency of urinary CTX-II between Kellgren-Lawrence (KL) classification, genders, ethnicities, and study size.
Results
Thirteen studies involved a total of 2856 participants were included. Pooled SMD showed that urinary CTX-II levels were significantly elevated in knee OA group compared to controls (SMD 0.82; 95% CI 0.41-1.24; P < 0.0001). For KL 3 to 4 versus KL 2, higher urinary CTX-II levels were found in severe knee OA patients. Subgroup analyses revealed that urinary CTX-II performed better in females as compared with males and in European subjects as compared with Asian population. Also, study size did not influence the statistic results.
Conclusion
This is the largest scale meta-analysis assessing the diagnostic performance of urinary CTX-II levels as biomarker for knee OA. According to our findings, urinary CTX-II levels have a potential to distinguish knee OA patients from healthy controls which can serve as biomarker for knee OA.
Keywords: knee osteoarthritis, CTX-II, biomarker, meta-analysis
Introduction
Osteoarthritis (OA) is a chronic and progressive disease characterized by degeneration of articular cartilage. It is the most common joint disease among the elderly, causing disability and affecting patients’ quality of lives. Because of the high loads borne by the knee joint during daily activities and mobility, the knees are the most frequent site of OA. Knee OA affects more than 27 million people in the United States alone.1 The number of patients is estimated to be more than 50 million by the year 2020.2 Although countless efforts have been made to diagnose knee OA, clinical assessment of OA is still hampered by the lack of precise measures of the incidence and progression of the disease, especially during the early stage.
To date, the most well-established method for assessing knee OA is joint space width (JSW) measurement using plain radiographs. However, irreversible joint damage has often occurred at the time radiographic evidence of OA is established. In addition, since changes in JSW are relatively small compared with the precision error of plain X-ray measurement, it usually needs at least 1 year or probably 2 years to accurately estimate the progression or the reduction of joint damage induced by treatment.3 Magnetic resonance imaging (MRI) is currently an optimal method for monitoring knee OA, but its reproducibility is not yet fully validated. Arthroscopy is considered as the gold standard for the evaluation of cartilage lesions as it can provide a direct and magnified view of the cartilage surface.4 However, with its invasive characteristics and requirement of well-trained professional investigators, arthroscopy cannot be routinely applied to all patients. Therefore, it is of critical importance to obtain a noninvasive, inexpensive and more precise method for the evaluation of knee OA that is applicable to most patients.
The measurement of body fluid markers of cartilage metabolism is developed for such purpose and has received much research attention. Articular cartilage structure is maintained when chondrocyte metabolism of extracellular matrix components is balanced. When degradation exceeds synthesis, articular cartilage structure degenerates.5 The assessment of biomarkers can potentially reveal articular cartilage metabolism. A variety of biomarkers have been used to diagnose knee OA. C-terminal cross-linked telopeptides of type II collagen (CTX-II), a byproduct of articular cartilage breakdown, is one of the most assessable biomarkers. CTX-II has been detected in both urine and synovial fluid. An advantage of urine evaluation is that urine can be obtained more easily than synovial fluid, which facilitates a stronger study design because samples can be collected from a comparable control group. Several previous studies have already shown that the levels of urinary CTX-II are significantly elevated in patients with knee OA as compared with controls,4,6,7 and the concentrations increase with disease severity.8-10 However, a single study cannot conclusively confirm the usefulness of urinary CTX-II for diagnosing knee OA. Thus, by combining all the available publications, the present meta-analysis is conducted to assess the diagnostic and prognostic value of urinary CTX-II as biomarker for knee OA.
Materials and Methods
Search Strategy
Databases of PubMed, EMBASE, and ScienceDirect were used to retrieve potential articles from inception time to February 2018. The search terms used were “osteoarthritis,” “C-terminal cross-linking telopeptide of type II collagen,” “levels,” “biomarkers,” and “diagnosis.” We screened all abstracts obtained from the initial search. If the abstracts did not contain sufficient information to include or exclude the study from the analysis, the study was carried out on full-text review. All reference lists of the included articles and other systematic reviews were also hand searched in case of omissive studies. There was no restriction regarding published date, study design, and patients’ ethnic group except that all studies must be published in English.
Eligibility Criteria
The most crucial aim of the relevant studies has to be the assessment of urinary CTX-II levels as biomarker for knee OA. All the eligible studies need to fulfill the following criteria: (1) all studies included an knee OA patients group and a comparable healthy control group; (2) patients in OA group with clinically or radiographically diagnosis of knee OA based on the American College of Rheumatology (ACR) criteria for diagnosing knee OA, Kellgren-Lawrence (KL) grade classification, or other criteria; and (3) the levels of urinary CTX-II were provided and available in both groups. Studies that did not satisfy the above requirements were all excluded.
Data Extraction
Two reviewers independently extracted all the necessary data according to a predefined form. For each included study, study characteristic, including sample size, gender, age, body mass index, ethnicities, quantify methods, manufacture of the quantify kits, diagnostic criteria of knee OA, and urinary CTX-II concentration in OA and control group were extracted. The level of urinary CTX-II was the variable of interest for this study. In the majority of included studies, the measured levels of urinary CTX-II are corrected by the urine creatinine concentration and conveyed as ng/mmol Cr. In addition, one study expressed urinary CTX-II in unit of measure pg/mL11 and one in ng/mL.12 For this analysis, urinary CTX-II concentration in different units of measure were all extracted and data were combined using a standardized mean differences (SMD) model.
Statistical Analysis
All statistical analyses were processed using Review Manager (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Intergroup analysis of urinary CTX-II was based on the difference in the levels of CTX-II between different subgroups. Comparisons were performed using chi-square test and presented as forest plot. When considerable heterogeneity existed among studies (I2 > 50%), a random-effects model was used for data calculation. On the contrary, a fixed effects model was applied when I2 < 50%. Differences between subgroups were expressed as SMD with 95% confidence intervals (CIs) when individual outcomes were measured with different scales. A positive SMD indicated higher urinary CTX-II levels in OA patients as compared with controls. Otherwise, statistic outcomes were presented as mean differences (MD) with 95% CI. Sensitivity analysis was performed by removing one study result at a time to explore the influence of each study have on the overall statistic result as well as verify the stability of the pooled effect. Subgroup analyses were conducted comparing urinary CTX-II between disease severity based on KL grade classification, large and small studies, genders (male and female), and ethnicities (Asian, European, and African) to confirm the actual clinical performance of this biomarker.
Results
Study Selection
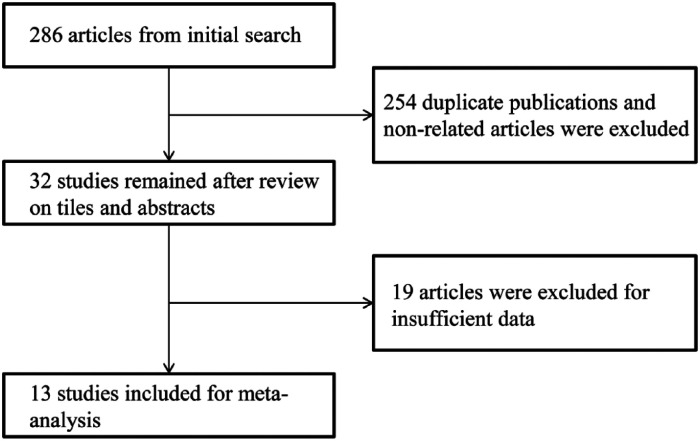
The search of electronic databases and manual cross-checking of reference lists initially obtained 286 articles. All titles and abstracts from the original search were screened. After excluding the duplicate publications and non-related articles, 32 studies were reviewed in their entirety. Eventually, 13 studies that met all our selection criteria were included in the meta-analysis.6-9,11-19 Figure 1 presented the flow diagram of study selection.
Figure 1.
Flowchart of literature search.
Study Description
The descriptive characteristics of the enrolled studies and study participants are presented in Table 1 . This analysis involved a total of 2,856 participants of whom 1,341 were knee OA patients. We believe that it is by far the largest scale of meta-analysis assessing the efficiency of urinary CTX-II as biomarker for knee OA. Study design such as joint and type of OA and source of CTX-II extraction was restricted in our study. Of the 13 studies, sample size ranged from 54 to 627. Studies selected patients with age ranging from 20 to 80 years. Ten studies reported choosing an age-matched control group. Another 3 studies did not provide information regarding the relationship of baseline characteristics between knee OA patients and controls. Eight studies conducted within the European area, 4 studies researched in the Asian population, and 1 study was performed in Africa. As for diagnostic criteria for the enrolled knee OA patients, 5 studies applied the ACR criteria for knee OA, another 5 studies only included patients with a KL score 2 or higher, the remaining 2 used other criteria such as Research Diagnostic Criteria for Temporomandibular Disorders or International Cartilage Repair Society grade. Other characteristics in terms of patients’ age, number of male and female participants, body mass index (BMI), and CTX-II quantified method were varied from study to study.
Table 1.
Characteristics of Included Studies for CTX-II.
| First Author | Year | Group | n | Females (n) | Age (Years) | BMI (kg/m2) | Diagnostic Criteria | Region | ELISA |
|---|---|---|---|---|---|---|---|---|---|
| Christgau13 | 2004 | Control | 415 | 444 | 61.4 ± 7.6 | NA | Healthy control | Belgium | CartiLaps ELISA assay |
| Knee OA | 212 | 66 | NA | ACR criteria | |||||
| Dam7 | 2009 | Control | 145 | 138 | 48 ± 16.52 | 24.9 ± 4.06 | Healthy control | Denmark | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 142 | 64 ± 10.44 | 27.65 ± 4.1 | KL grade | |||||
| Garnero6 | 2001 | Control | 67 | 79 | 62.6 ± 8.1 | NA | Healthy control | France | CartiLaps ELISA assay (Osteometer Biotech, Herlev, Denmark) |
| Knee OA | 67 | 63.6 ± 9.1 | NA | ACR criteria | |||||
| Jordan8 | 2005 | Control | 97 | 0 | 64.5 ± 2.6 | 27.1 ± 1.2 | Healthy control | UK | CartiLaps ELISA assay |
| Knee OA | 79 | 0 | 65.05 ± 2.6 | 27.75 ± 1.1 | KL grade | ||||
| Jung14 | 2004 | Control | 48 | 29 | 64.67 ± 12.77 | NA | Gymnastics groups for elderly persons | Denmark | CartiLaps ELISA assay |
| Knee OA | 37 | 24 | 67.7 ± 8.2 | NA | Radiological and clinical manifestation of knee OA | ||||
| Kalai15 | 2012 | Control | 57 | 57 | 53.51 ± 8.54 | 30.73 ± 7.9 | Healthy control | Tunisia | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 125 | 125 | 53.6 ± 7.6 | 32.79 ± 5.61 | ACR criteria | ||||
| Meulenbelt16 | 2006 | Control | 183 | 252 | 60.3 ± 7.5 | 27.0 ± 4.7 | Healthy control | Netherlands | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 118 | 60.3 ± 7.5 | 27.0 ± 4.7 | ACR criteria + KL grade | |||||
| Ok17 | 2017 | Control | 36 | 15 | 29.6 ± 8.16 | NA | Healthy control | South Korea | Ann Arbor, MI, USA (IDS Nordic Bioscience, Herlev, Denmark) |
| Knee OA | 31 | 23 | 26.65 ± 4.13 | NA | Research Diagnostic Criteria for Temporomandibular Disorders | ||||
| Røtterud18 | 2014 | Control | 6 | 1 | 35.7 ± 2.3 | NA | Healthy control | Norway | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 48 | 12 | 33.4 ± 9.0 | 27.3 ± 4.9 | International Cartilage Repair Society grade 3/4 | ||||
| Sowers19 | 2009 | Control | 36 | 36 | 47.5 ± 2.6 | 29.7 ± 6.2 | Healthy control | France | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 36 | 36 | 47.5 ± 2.6 | 39.15 ± 8.22 | KL grade | ||||
| Tanishi9 | 2009 | Control | 106 | 59 | 60 ± 14.7 | NA | Healthy control | Japan | CartiLaps ELISA assay (Nordic Bioscience Diagnostics, Herlev, Denmark) |
| Knee OA | 190 | 112 | 72.07 ± 8.11 | NA | KL grade | ||||
| Xin11 | 2017 | Control | 20 | 12 | 57.6 ± 6.1 | NA | Healthy control | China | In house |
| Knee OA | 82 | 44 | 59.1 ± 6 | NA | KL grade | ||||
| Xu12 | 2010 | Control | 120 | 120 | 63.0 ± 12.8 | 25.6 ± 2.8 | Healthy control | China | ELISA assay (Shanghai Xitang Bio-Tech Company, China) |
| Knee OA | 120 | 120 | 65.3 ± 10.2 | 26.3 ± 3.1 | ACR criteria |
OA = osteoarthritis; CTX-II = C-terminal cross-linking telopeptide of type II collagen; ELISA = enzyme-linked immunosorbent assay; ACR = American College of Rheumatology; KL = Kellgren-Lawrence grade; BMI = body mass index; NA = not available.
The Levels of CTX-II as a Biomarker for Knee OA
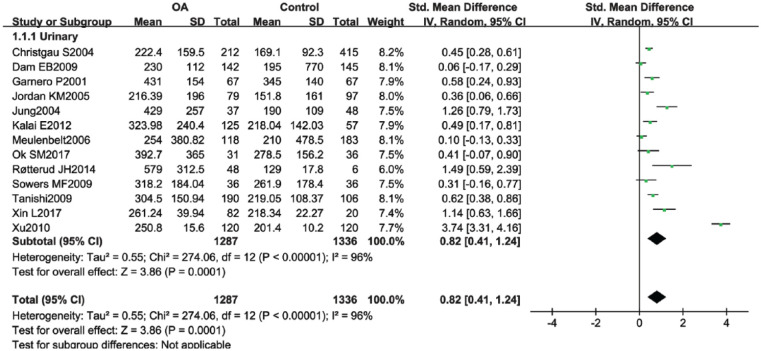
Pooled statistic of 13 studies showed a significant elevation of urinary CTX-II in knee OA group when compared to controls (SMD 0.82; 95% CI, 0.41-1.24; P < 0.0001) ( Fig. 2 ). Remove-one-factor-at-a-time sensitivity analysis was performed. There were no significant changes on the SMD and I2 value when omitting each study except the study of Xu et al.12 We found that the study of Xu et al.12 affected both the SMD value and I2. When removing the outcome, the pooled SMD changed from 0.82 (95% CI, 0.41-1.24; P < 0.0001) to 0.52 (95% CI, 0.33, 0.71, P < 0.0001), and the I2 value dropped from 95% to 76% while still remained statistical significance.
Figure 2.
Forest plot for the comparison of urinary C-terminal cross-linking telopeptide of type II collagen (CTX-II) between knee osteoarthritis (OA) patients and healthy controls.
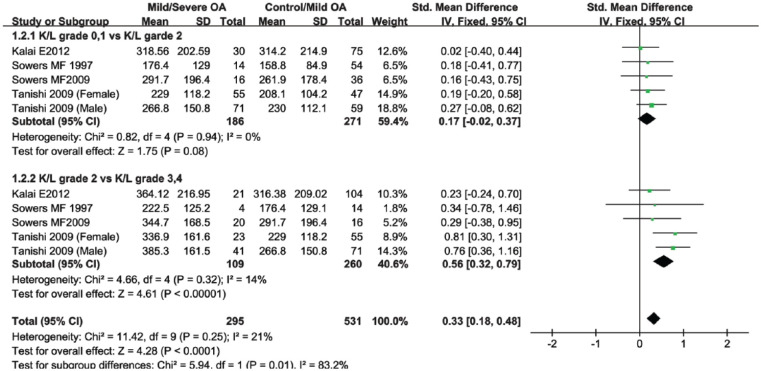
To gain more insight into the mechanism of urinary CTX-II concentrations in patients with knee OA, subgroup analysis based on OA severity was performed. Studies selected in this subgroup were categorized by the KL grade. We classified knee OA patients with KL grade 0 and 1 as healthy controls, with KL grade 2 as mild knee OA, and with KL grade 3 and 4 as severe knee OA. The study of Sowers et al.19 provided 2 groups of data that were performed in 1997 and 2009 with the same cohort of patients. The study by Tanishi et al.9 provided separate male and female CTX-II outcomes for different knee OA severity. Concerning that it might decrease the statistical significance by merging these data, experimental data of the 2 studies were divided into 4 groups. According to the subgroup results, patients with mild knee OA had a higher urinary CTX-II levels than patients in the control group, although it did not reach statistical significance (SMD 0.17; 95% CI, −0.02 to 0.37; P = 0.08) ( Fig. 3 ). Comparison between KL grade 2 versus KL grade 3 and 4 revealed that the levels of urinary CTX-II increased significantly in severe knee OA group (SMD 0.56; 95% CI, 0.32-0.79; P < 0.0001) ( Fig. 3 )
Figure 3.
Forest plot for the comparison of urinary C-terminal cross-linking telopeptide of type II collagen (CTX-II) between knee patients with disease severity.
Subgroup Analysis Based on Gender, Ethnic Groups, and Study Size
Some studies argued that gender difference might influence the expression of urinary CTX-II in knee OA patients. To investigate whether the levels of urinary CTX-II should be treated differently as a biomarker in male and female patients with knee OA, a subgroup analysis based on sex were also done. Results demonstrated that urinary CTX-II were significantly higher in both male (SMD 0.42; 95% CI, 0.13-0.71; P = 0.004) and female (SMD 0.96; 95% CI, 0.07-1.85; P = 0.03) OA patients compared with controls ( Table 2 ). We observed that the SMD in female were higher than in male. However, when removing the data of Xu et al.,12 the result of the female comparison became 0.40 (95% CI, 0.20-0.60; P = 0.0001), and the I2 value also dropped from 95% to 58%. These finding further proved that the study of Xu et al.12 might be the source of heterogeneity. Notably, in the comparison of CTX-II levels between OA male patients and female patients, women patients did have a higher urinary CTX-II than man (MD −28.62; 95% CI, −56.71 to −0.53; P = 0.05) ( Table 2 ).
Table 2.
Subgroup Analyses of Urinary CTX-II with Different Genders, Ethnic Groups, and Study Size.
| Subgroups | Number of Studies | SMD/MD | 95% CI | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 4 | 0.42 | 0.13-0.71 | 0.004 |
| Female | 6 | 0.96 | 0.07-1.85 | 0.03 |
| Male vs Female in OA | 3 | −28.62 | −56.71 to −0.53 | 0.05 |
| Ethnicities | ||||
| Asia | 4 | 1.48 | −0.02 to 2.98 | 0.05 |
| Europe | 8 | 0.47 | 0.22-0.71 | 0.0002 |
| Africa | 1 | 0.49 | 0.17-0.81 | 0.002 |
| Study size | ||||
| OA < 100 | 7 | 0.72 | 0.41-1.04 | <0.001 |
| OA > 100 | 6 | 0.89 | 0.18-1.61 | 0.01 |
CTX-II = C-terminal cross-linked telopeptides of type II collagen; OA = osteoarthritis; SMD = standardized mean difference; MD = mean differences; CI = confidence interval.
Small size of the study sample was another reason threatening the validity of meta-analysis. Therefore, we divided the included studies into 2 subgroups. Studies categorized as small study size group had less than 100 knee OA population. Large sample size study had more than 100 OA patients. Both small and large sample size groups showed a significant elevated urinary CTX-II concentration in knee OA patients compared with controls ( Table 2 ).
We hypothesized that patients in different ethnicities, with different body structure and life styles, might have different response in knee OA. Interestingly, our data showed that urinary CTX-II did not differ significantly between OA patients and healthy controls in Asian population (SMD 1.48; 95% CI, −0.02 to 2.98; P = 0.05). Whereas the CTX-II elevation in European OA population remained statistically significance (SMD 0.47; 95% CI, 0.22-0.71; P = 0.0002) (Table 2).
Discussion
Biomarkers, which is associated with the subtle changes in cartilage, bone, and synovial tissues, is believed to be a potential alternative for the earlier diagnosis of asymptomatic OA to prevent exacerbation.20 Many structural molecules or fragments have been considered as available candidates for diagnosing OA, such as serum cartilage oligomeric matrix protein (COMP), urinary CTX-II, matrix metalloproteinases (MMPs), and so on. A systematic review using the “BIPED” classification to assess the current status of available biomarkers suggested that urinary CTX-II and serum COMP had the best performance.21 The study also indicated that serum COMP seemed to perform less than urinary CTX-II within most “BIPED” categories. Although the authors pointed out that different patient cohorts and study design might be the reasons causing the discrepant results, we could not ignore the possibility that urinary CTX-II might be more preferable in the prediction of OA. Thus, the present meta-analysis was designed to further proved the diagnostic value of urinary CTX-II as biomarkers for knee OA.
In our analysis, pooled results of 13 studies showed significant elevation of urinary CTX-II in OA group compared with controls. This finding was in accordance with 2 previous meta-analyses that also evaluated the potential of urinary CTX-II as knee OA biomarker. Although the 2 studies applied different statistical methods for their calculation, they both concluded that CTX-II could be valuable diagnostic and prognostic tools for knee OA.22,23 The sensitivity analysis confirmed that our result was credible and also found that the study of Xu might be the source of heterogeneity. When omitting the outcome of Xu et al.,12 although CTX-II levels in knee OA group were still significantly higher than controls, both the SMD and I2 values dropped. We further looked into the reasons causing this phenomenon. The basic patient characteristics and study design of Xu et al.12 did not differ much from the other included studies. Notably, while most of the study applied a CartiLaps ELISA assay manufactured by Nordic Bioscience, Herlev, Denmark, the study of Xu et al. did not mention the type of ELISA assay used in the CTX-II quantification and the kits were supplied by Shanghai Xitang Bio-Tech Company, China. Also, the CTX-II concentration were expressed in the units of ng/mL, which was different from other studies. The aforementioned 2 conditions might be the reasons inducing the discrepancy across the results. Thus, the use of the study of Xu et al.12 in future research need careful deliberation.
Whether the levels of urinary CTX-II increase with knee OA severity is another issue that received much attention. Therefore, we performed subgroup analysis based on K/L classification for knee OA. Our study together with other research studies revealed that urinary CTX-II levels were consistently increased in severe versus mild radiographic knee OA.22,24 Although all 3 studies only involved no more than 5 groups of data for comparisons, but the same findings indicated that the use of urinary CTX-II as biomarker could be promising. To enhance the credibility of our study, comparison between small and large sample size study was performed. Outcomes showed that urinary CTX-II concentration in OA patients did not differ between small and large size study.
Several conditions can affect the expression of biomarkers. Some evidence suggests that medication, normal activity, hormone levels in the body, weight, gender, age, and ethnicity all can lead to fluctuations in biomarkers levels. According to the patients’ characteristics retrieved from the included literature, we conducted 2 subgroup analyses regarding different gender and ethnic groups. For the subgroup of sex, results showed a significant difference in CTX-II levels between female and male patients with knee OA compared with controls, which implied that the diagnostic use of urinary CTX-II was effective in both male and female. Our meta-analysis also showed higher urinary CTX-II levels in female knee OA patients while compared with male patients, which implied that urinary CTX-II might have a better performance in females. This finding was also supported by another publication, which found the same result.24 However, our subgroup analysis only included 3 pairs of data, and it might be insufficient to draw absolute conclusion. Researchers argued that the more elevated urinary CTX-II in female patients might be a result of other factors such as sample size. We noticed that this group of study contained 509 female patients and only 288 male patients. The mismatched sample size might be one reason causing higher urinary CTX-II in females. Moreover, evidence showed that bone marker levels were increased in postmenopausal women.25 Also, data suggested that urinary CTX-II had unique correlations with bone marker as compared with other cartilage markers and reflected bone as well as cartilage metabolism.25,26 Thus, the urinary CTX-II in women might be confounded by bone remodeling, which resulted in a higher CTX-II levels.
The influence of race on the expression of biomarker were investigated mostly in serum COMP researches. Several studies have compared COMP levels in different ethnic groups and found that serum COMP levels vary by ethnicity.27,28 We believe that the present analysis is the first study that has probed into whether ethnicity affects the urinary CTX-II levels in patients. In the analysis based on different ethnic groups, the diagnostic effectiveness of urinary CTX-II seemed to disappear in Asian population. However, our research only collected data from 4 studies that conducted research in Asia, absolute conclusion could not be drawn based on these limited data.
Nevertheless, our study has some limitations that must be taken into account. Although we found higher urinary CTX-II levels in female OA patients, we did not eliminate the influence by age. A study disclosed that even in healthy people, urinary CTX-II levels were affected by age. High values were discovered in children and adolescents where skeletal growth is occurring. Stable levels were detected in adults between 30 and 50 years and a slightly increase values were found in individuals older than 50 years.29 A study also showed that the levels of urinary CTX-II significantly differed between premenopausal and postmenopausal women.30 Therefore, the increased levels of urinary CTX-II in female patients might be the results of menopause. Yet, of all the enrolled studies, only the study of Tanishi et al.9 provided separated data of urinary CTX-II in different age group and majority of studies included with age ranging from 40 to 80 years, which prevented us from conducting a subgroup analysis to reveal the impact of age on the biomarker levels.
The results of previous trails suggested that CTX-II concentration is associated with BMI in the knee OA group.15 Other researches also argued that medication can alter biomarker levels. A study put forward that the use of bisphosphonates might lead to a decrease in urinary CTX-II levels.9 However, because of the limited information available in the included literature, we cannot perform subgroup analyses in terms of weight and drug to test this hypothesis. Moreover, our study investigated the correlation between urinary CTX-II and knee OA, the results of this study cannot be extrapolated to other biomarkers in different joints of OA.
Conclusion
A significant elevation of urinary CTX-II levels in knee OA patients was observed in our analysis. It appeared that urinary CTX-II levels have a potential to distinguish knee OA patients from healthy controls. Furthermore, the levels of urinary CTX-II increased more rapidly in severe compared to moderate knee OA. Subgroup analyses revealed that urinary CTX-II might have a better performance in female gender and European ethnicity. Yet, the results of our study still need further validation.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Muraki S, Oka H, Akune T, Mabuchi A, En-yo Y, Yoshida M, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17:1137-43. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravaud P, Giraudeau B, Auleley GR, Drape JL, Rousselin B, Paolozzi L, et al. Variability in knee radiographing: implication for definition of radiological progression in medial knee osteoarthritis. Ann Rheum Dis. 1998;57:624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46:2613-24. [DOI] [PubMed] [Google Scholar]
- 5. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626-34. [DOI] [PubMed] [Google Scholar]
- 6. Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dam EB, Loog M, Christiansen C, Byrjalsen I, Folkesson J, Nielsen M, et al. Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther. 2009;11:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jordan KM, Syddall HE, Garnero P, Gineyts E, Dennison EM, Sayer AA, et al. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis. 2006;65:871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanishi N, Yamagiwa H, Hayami T, Mera H, Koga Y, Omori G, et al. Relationship between radiological knee osteoarthritis and biochemical markers of cartilage and bone degradation (urine CTX-II and NTX-I): the Matsudai Knee Osteoarthritis Survey. J Bone Miner Metab. 2009;27:605-12. [DOI] [PubMed] [Google Scholar]
- 10. Karsdal MA, Byrjalsen I, Bay-Jensen AC, Henriksen K, Riis BJ, Christiansen C. Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis—the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation. BMC Musculoskelet Disord. 2010;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin L, Wu Z, Qu Q, Wang R, Tang J, Chen L. Comparative study of CTX-II, Zn2+, and Ca2+ from the urine for knee osteoarthritis patients and healthy individuals. Medicine (Baltimore). 2017;96:e7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu P, Yao J, Hou W. Relationships between COL2A1 gene polymorphisms and knee osteoarthritis in Han Chinese women. Mol Biol Rep. 2011;38:2377-81. [DOI] [PubMed] [Google Scholar]
- 13. Christgau S, Henrotin Y, Tankó LB, Rovati LC, Collette J, Bruyere O, et al. Osteoarthritic patients with high cartilage turnover show increased responsiveness to the cartilage protecting effects of glucosamine sulphate. Clin Exp Rheumatol. 2004;22:36-42. [PubMed] [Google Scholar]
- 14. Jung M, Christgau S, Lukoschek M, Henriksen D, Richter W. Increased urinary concentration of collagen type II C-telopeptide fragments in patients with osteoarthritis. Pathobiology. 2004;71:70-6. [DOI] [PubMed] [Google Scholar]
- 15. Kalai E, Bahlous A, Charni N, Bouzid K, Sahli H, Chelly M, et al. Increased urinary type II collagen C-telopeptide levels in Tunisian patients with knee osteoarthritis. Clin Lab. 2012;58:209-15. [PubMed] [Google Scholar]
- 16. Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellilo Le, Graverand MP, et al. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dis. 2006;65:360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ok SM, Lee SM, Park HR, Jeong SH, Ko CC, Kim YI. Concentrations of CTX I, CTX II, DPD, and PYD in the urine as a biomarker for the diagnosis of temporomandibular joint osteoarthritis: a preliminary study. Cranio. 2017;1:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Røtterud JH, Reinholt FP, Beckstrøm KJ, Risberg MA, Arøen A. Relationship between CTX-II and patient characteristics, patient-reported outcome, muscle strength, and rehabilitation in patients with a focal cartilage lesion of the knee: a prospective exploratory cohort study of 48 patients. BMC Musculoskelet Disord. 2014;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sowers MF, Karvonen-Gutierrez CA, Yosef M, Jannausch M, Jiang Y, Garnero P, et al. Longitudinal changes of serum COMP and urinary CTX-II predict x-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17:1609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poonpet T, Honsawek S. Adipokines: biomarkers for osteoarthritis? World J Orthop. 2014;5:319-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18:605-12. [DOI] [PubMed] [Google Scholar]
- 22. Huang M, Zhao J, Huang Y, Dai L, Zhang X. Meta-analysis of urinary C-terminal telopeptide of type II collagen as a biomarker in osteoarthritis diagnosis. J Orthop Translat. 2017;13:50-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valdes AM, Meulenbelt I, Chassaing E, Arden NK, Bierma-Zeinstra S, Hart D, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22:683-9. [DOI] [PubMed] [Google Scholar]
- 24. Jeremiasse B, Lafeber FP, Spil WV. The performance of urinary collagen type II C-telopeptide (UCTX-II) in knee osteoarthritis: a meta-analysis. Osteoarthritis Cartilage. 2017;25:S91-S92. [Google Scholar]
- 25. van Spil WE, Bijlsma JW, Mastbergen SC, Lafeber FP. Associations of CTX-II with biochemical markers of bone turnover raise questions on its tissue origin: data from check, a cohort study of early osteoarthritis. Osteoarthritis Cartilage. 2012;20:S78. [DOI] [PubMed] [Google Scholar]
- 26. van Spil WE, Vincken KL, Lems WF, Lafeber FP. CTX-II levels are associated with peripheral bone density, suggesting CTX-II epitope release from bone: data from Cohort Hip and Cohort Knee (CHECK). Osteoarthritis Cartilage 2013;21:S77-S78. [Google Scholar]
- 27. Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48:675-81. [DOI] [PubMed] [Google Scholar]
- 28. Das Gupta E, Ng WR, Wong SF, Bhurhanudeen AK, Yeap SS. Correlation of serum cartilage oligometric matrix protein (COMP) and interleukin-16 (IL-16) levels with disease severity in primary knee osteoarthritis: a pilot study in a Malaysian population. PLoS One. 2017;12:e0184802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mouritzen U, Christgau S, Lehmann HJ, Tankó LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bay-Jensen AC, Tabassi NC, Sondergaard LV, Andersen TL, Dagnaes-Hansen F, Garnero P, et al. The response to oestrogen deprivation of the cartilage collagen degradation marker, CTX-II, is unique compared with other markers of collagen turnover. Arthritis Res Ther. 2009;11:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]