Abstract
Interest is growing in measurement of novel biomarkers for the diagnosis of acute kidney injury. Multiplex assays may provide a rapid and cost-effective way of measurement; however, sparse information is published regarding their use in dogs. We aimed to validate a commercial magnetic bead–based assay for 5 biomarkers: clusterin (Clus), cystatin C (CysC), kidney injury molecule 1 (KIM-1), monocyte chemoattractant protein 1 (MCP-1), and neutrophil gelatinase–associated lipocalin (NGAL). Intra- and inter-assay imprecision, linearity under dilution (LUD), spike recovery (S-R), and hemoglobin interference were evaluated using serum from healthy and diseased dogs. Additionally, the effect of sample type (serum vs. plasma) was investigated. All values for Clus and MCP-1 were outside the assay’s measurable range. Intra- and inter-assay precision were acceptable for NGAL (CVs 8.8% and 13.2%, respectively). Regression analysis of LUD and S-R indicated good linearity for CysC and NGAL. Hemolysis did not affect measurement of any biomarker. Measured concentrations of CysC (p = 0.018) and NGAL (p = 0.015) were significantly lower in sodium citrate plasma compared to serum. We conclude that this magnetic bead–based assay is precise and accurate for NGAL measurement in canine serum. Inappropriate standards for MCP-1 and Clus, and poor accuracy for KIM-1 measurement, suggest that this assay cannot reliably quantify those biomarkers in canine blood. Measurements of CysC in canine blood using this assay must be interpreted with caution given inter-assay imprecision.
Keywords: acute kidney injury, biomarkers, dogs, immunoassay
Introduction
Acute kidney injury (AKI) is associated with significant morbidity and mortality.20,22,37 Although early interventions to treat AKI improve outcomes, conventional diagnosis relies on markers of renal function, such as serum creatinine concentration, which may not rise above the reference interval until 3 d or more after initiation of the syndrome.3,4,20 Investigation of novel biomarkers for AKI has been of great interest to the veterinary community since ~ 2010; a reliable early marker of AKI would offer a clear benefit in a clinical setting.9,17,29 Additionally, early biomarkers for AKI represent a valuable tool for research into treatment and prevention modalities for AKI, and have a potential role as outcome markers for nephrotoxicity in pre-clinical drug trials.3,24,40,43 Despite the identification of many promising AKI biomarkers, clinical development has been slow.31 Unfortunately, although a large amount of work has been done at the discovery phase of biomarker development, targeted and quantitative evaluation to assess the usefulness of biomarkers in specific settings, and development and validation of reliable assays, is lacking.33
Many potential AKI biomarkers are released or expressed in the kidney during structural renal damage; thus, alterations in their urinary concentration are expected to reflect renal pathology.9 As well, the presence of some biomarkers in systemic circulation reflects the presence and severity of AKI in dogs and people.24,28,36 The ability to measure these biomarkers in blood allows scrutiny of the relationship between their systemic and urinary concentrations, helping to further researchers’ understanding of the origins and production of these biomarkers during AKI.
Unfortunately, few of the renal biomarkers identified to date can be measured in canine samples, and then only by expensive and time-consuming single-analyte immunoassays (Table 1). Multiplexed assays utilizing bead-based technologies may provide a cheaper, simpler, and more rapid way to measure these biomarkers. At the time of writing, 2 companies market multiplex assay kits for the measurement of kidney biomarkers in canine samples (Bio-Plex Pro RBM canine kidney toxicity assays; Bio-Rad Laboratories, and MILLIPLEX MAP canine kidney toxicity panels 1 and 2; MilliporeSigma). Before these assays are utilized in a research or clinical setting, it is important that they are validated for the species and body fluid for which they are intended.6,12
Table 1.
Renal biomarkers for which commercial single-analyte immunoassays have been validated for use with canine serum or urine.
| Biomarker | Assay(s) | Published canine validation data |
|---|---|---|
| Clus | Canine clusterin ELISA (BioVendor) | 14 |
| CysC | Canine cystatin C ELISA (BioVendor) | Urine15; serum25 |
| KIM-1 | Dog KIM-1 ELISA (ICL) | 21 |
| MCP-1 | Quantikine ELISA canine CCL2/MCP-1 (R&D Systems) | Urine35; serum30 |
| NGAL | Dog NGAL ELISA (Bioporto Diagnostics) | Urine26; serum1 |
| Retinol-binding protein | Human RBP ELISA (Immundiagnostick); Human RBP ELISA kit (ICL); Canine RBP ELISA kit (ICL; not yet independently validated) | Urine23; serum32 |
| Symmetric dimethylarginine | SDMA ELISA (DLD Diagnostika); SDMA test (IDEXX) | Serum11 |
Clus = clusterin; CysC = cystatin C; KIM-1 = kidney injury molecule 1; MCP-1 = monocyte chemoattractant protein 1; NGAL = neutrophil gelatinase–associated lipocalin.
Our aims were: 1) to validate a commercial canine kidney toxicity assay (MILLIPLEX MAP canine toxicity panel 1) for use with canine serum, 2) to evaluate the impact of hemoglobin interference on the assay, and 3) to determine whether plasma prepared with 3 different anticoagulants varied from serum-measured biomarker concentration. The investigated assay is available for purchase with any combination of up to 7 analytes: clusterin (Clus), cystatin C (CysC), kidney injury molecule 1 (KIM-1), interleukin 8, neutrophil gelatinase–associated lipocalin (NGAL), monocyte chemoattractant protein 1 (MCP-1), and osteopontin. The assay that we purchased for use in our validation study included 5 analytes: Clus, CysC, KIM-1, NGAL, and MCP-1; interleukin 8 and osteopontin were not measured.
Materials and methods
Animals and samples
We used blood collected from 2 client-owned dogs and 4 Greyhounds that were part of a separate experimental study.8 Our study was approved by the Murdoch University Animal Ethics Committee (permits R2729/15 and R2726/15), and the dogs cared for as per the “Australian code for the care and use of animals for scientific purposes.”2
The 2 client-owned dogs were scheduled to undergo surgical sterilization in a veterinary college teaching laboratory. Informed owner consent was obtained for involvement of each dog in the study. Client-owned dogs were determined to be healthy based on history and physical examination, serum creatinine measurement, and urinalysis that included specific gravity, dipstick analysis, and sediment microscopy performed by a specialist veterinary clinical pathologist (G. Rossi). A sample of jugular venous blood (4 mL) was obtained from one bitch (sample A) and one male dog (sample B) prior to administration of any anesthetic drugs or surgery. Blood was collected into serum tubes (4.0-mL Vacutainer plastic serum tube with clot activator; Becton, Dickinson) and immediately centrifuged at 1,358 × g for 10 min at 4°C. Serum aliquots of 250 µL were stored at −80°C prior to biomarker analysis.
Four intact male retired racing Greyhound dogs underwent histologically diagnosed, experimentally induced ischemic AKI, as part of another study.8 Prior to entering the study, the dogs were determined to be healthy based on physical examination, complete blood count, a limited serum biochemistry profile (albumin, urea, and creatinine only), and urinalysis as described previously. Blood (4 mL from the jugular vein) was collected from these dogs immediately following induction of anesthesia, prior to initiation of AKI (samples C–F); then again 3 h following ischemia (samples G–J). We expected that the baseline blood would contain a low concentration of the biomarkers studied, and that the post-ischemia sample would contain a high concentration of the biomarkers. Blood was collected into serum (4.0-mL Vacutainer plastic serum tube with clot activator), lithium heparin (4.0-mL Vacutainer plastic lithium heparin tube), ethylenediaminetetraacetic acid (4.0-mL Vacutainer plastic K2 EDTA tube), and sodium citrate (2.7-mL Vacutainer Plus sodium citrate tube) collection tubes. Each tube was filled to the manufacturer’s recommended level. Within 60 min of sample collection, blood was centrifuged at 1,358 × g for 10 min at 4°C. Immediately following centrifugation, aliquots (250 µL each) of serum were stored at −80°C prior to biomarker analysis.
Samples from the 2 client-owned dogs were used for all parts of the assay validation and for investigation of hemoglobin interference. Samples from the 4 Greyhound dogs were used for inter-assay validation and for comparison of serum versus plasma biomarker concentrations.
Assay method
The same operator (J. Davis) performed all assays and all parts of the analytical validation. Concentrations of Clus, CysC, KIM-1, MCP-1, and NGAL in all specimens were measured using a commercial magnetic bead–based multiplex immunoassay (MILLIPLEX MAP canine kidney toxicity expanded magnetic bead panel 1). The assay was performed according to the manufacturer’s recommended procedure with all 7 standards and 2 provided quality controls included in each run. Given that this assay is designed for use with canine urine, a specific serum diluent (Serum matrix; MilliporeSigma) was added to wells containing the standards and controls in place of an assay buffer (a step recommended by the manufacturer when the assay is to be used with serum samples). Following dilution with assay buffer (details present in subsequent analytical validation), samples were incubated with a solution of antibody-immobilized magnetic beads for 2 h at room temperature. The plate was washed using a magnetic plate washer (Bio-Plex Pro II wash station; Bio-Rad), detection antibodies added, and plates incubated at room temperature for 1 h. Streptavidin–phycoerythrin was added to detect antibodies, prior to a further 30-min incubation at room temperature. The plate was washed and beads re-suspended with drive fluid (MAGPIX drive fluid; MilliporeSigma). The plate was analyzed 5 min later using a multiplex reader (Bio-Plex MAGPIX multiplate reader; xPONENT software; Bio-Rad). The median fluorescence intensity data was analyzed using a 5-parameter logistic curve to calculate analyte concentrations in each sample. The multiplex reader was calibrated weekly using manufacturer-recommended reagents (MAGPIX calibration kit; MilliporeSigma), and performance verification (MAGPIX performance verification kit; MilliporeSigma) was performed daily prior to use. Unless otherwise stated, samples were run in triplicate and the mean concentration of the triplicate measurements used for further statistical analysis. For all parts of the study, except intra- and inter-assay precision testing, if the coefficient of variation (CV) was > 15% caused by one of the triplicates, that measurement was discarded and the mean value from the remaining duplicate was used (if the CV was < 15%).
Analytical validation
For all validation analyses, aliquots of serum were thawed for 2 h at room temperature (18–22°C) immediately prior to use. Intra-assay precision was determined by 8 repeat measurements of biomarkers from samples A and B on the same assay plate. Inter-assay precision was determined by analyzing samples A–C and G in triplicate across 4 different assay plates. A dilution factor of 1:4 (sample to assay buffer) was used for all intra- and inter-assay precision testing. This dilution was chosen as the dilution most likely to retrieve biomarker concentrations within the measurable range of the assay for each analyte (Table 2), following a preliminary assay run with canine serum that suggested undiluted samples gave measurement above the measurable range for most analytes. Means, standard deviations (SDs), and CVs were calculated. Based on the validation thresholds claimed in the assay data sheet, acceptable intra-assay precision was defined as CV < 10% and acceptable inter-assay precision as CV < 15%.
Table 2.
Manufacturer-stated measurable ranges for renal biomarkers in a commercial multiplex assay marketed for use with canine urine (MILLIPLEX MAP canine kidney toxicity expanded magnetic bead panel 1; MilliporeSigma).
| Biomarker | Measurable range |
|---|---|
| Clus | 1.37–1,000 ng/mL |
| CysC | 0.01–10 ng/mL |
| KIM-1 | 0.96–700 pg/mL |
| MCP-1 | 0.07–50 ng/mL |
| NGAL | 0.03–20 ng/mL |
See Table 1 notes for explanations of biomarker abbreviations.
Because an accepted gold standard measurement technique is not available for any of the proteins measured by this assay, accuracy was determined indirectly using linearity under dilution (LUD) and a spike-recovery (S-R) study. The LUD was assessed by measuring biomarker concentration in triplicates of serum sample A serially diluted using the assay buffer provided in the assay kit, to 100%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, and 10% of serum. The S-R study was performed by measuring biomarker concentration in triplicates of a serum sample A spiked with 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% serum from sample B. Although contrary to the recommended procedure for assay validation, a serum sample from a healthy dog was used for spiking because use of a pathologic sample containing higher concentrations of biomarker resulted in values outside the measurable range of the assay (Table 2).13 The S-R study for CysC and Clus was repeated using serum sample A diluted 1:19 with assay buffer prior to spiking, and spiked with standard 5 from the assay kit (Table 3). Using serum from a healthy dog, the concentration of these analytes with only a 10% spike was still above the measurable range of the assay. For both the LUD and S-R study, percentage recovery was calculated as measured concentration divided by expected concentration (× 100%) for each dilution or spiked sample. Correlation of percentage recovery with expected values for the LUD and S-R were compared using least squares regression test (Prism8 software; GraphPad Software).
Table 3.
Renal biomarker concentrations measured in 10 canine serum samples and 1 assay standard using a commercial multiplex assay. Dilution factor 1:4 with assay buffer.
| Sample* | CysC (ng/mL) | KIM-1 (pg/mL) | NGAL (ng/mL) |
|---|---|---|---|
| A | 26.5 | 11.0 | 1.0 |
| B | 24.2 | 22.4 | 2.9 |
| C | 59.8 | 33.9 | 8.7 |
| D | 48.7 | 88.8 | 14.0 |
| E | 44.0 | 59.9 | 12.7 |
| F | 44.1 | 72.6 | 9.6 |
| G | 27.9 | 21.7 | 8.6 |
| H | 47.3 | 48.7 | 16.7 |
| I | 37.4 | 39.4 | 17.8 |
| J | 21.1 | 44.2 | 11.1 |
| Standard 5 | 1.08 | 79.4 | 2.2 |
See Table 1 notes for explanations of biomarker abbreviations.
A, B = client-owned healthy dogs; C–F = healthy retired Greyhound dogs prior to experimentally induced acute kidney injury (AKI); G–J = dogs C–F 3 h after ischemic AKI.
Observed total error (TEobs) for each biomarker was calculated as: TEobs = 2CV (mean of intra-assay CV & inter-assay CV) + absolute bias%.16 Absolute bias was calculated as the mean of the relative error (100 – recovery %) of each dilution measured in the LUD and S-R series. When published biological variation data were available for a biomarker, allowable total error (TEa) was calculated as: TEa = 1.65 × imprecision goal (%) × bias goal (%).16 Imprecision goal was calculated as: 0.5 × published within subject biological variation.27 Bias goal was calculated as: 0.25 × [(published within subject variation)2 + (published between subject variation)2]1/2.27
The limit of blank (LOB) was defined as the highest measurement result observed for a blank sample. It was calculated for the immunoassay based on data from 10 replicate determinations of the blank (2 replicates per plate for 5 plates run over a 12-mo duration) as the mean + 1.65 SD.12 The limit of quantification (LOQ) was defined as the lowest amount of analyte that could be quantitatively determined with acceptable precision and trueness.12 It was determined as the lowest concentration during serial dilution (LUD series) with recovery within ± 20% and CV between replicates of < 10%.
Evaluation of hemoglobin interference
Potential for hemoglobin interference in serum samples was assessed according to previously published protocols.34 A canine serum sample was spiked with 0.5 g/L, 2 g/L, 4 g/L, and 8 g/L of hemoglobin, using commercial bovine lyophilized hemoglobin powder (hemoglobin from bovine blood lyophilized powder; MilliporeSigma) reconstituted with distilled water prior to use. Additionally, the same serum sample was spiked with an equivalent volume of distilled water for each concentration. Analyte concentration for each hemoglobin-spiked sample was compared to the sample spiked only with distilled water. The percentage change in biomarker concentration compared with the control sample was calculated and plotted against the concentration of hemoglobin to produce interferograms. Measured concentrations of each biomarker in each different matrix were compared statistically using a Friedman test (Prism8 software).
Serum versus plasma measurement
Comparisons of biomarker measurement were made between serum and 3 types of plasma aliquots (lithium heparin, EDTA, sodium citrate) from 8 samples (C–J). All specimens were included in a single assay plate, using serum diluent in blank and standard wells as described above. The percentage change in biomarker concentration for each plasma sample compared to serum was calculated, and a mean percentage difference > 15% was considered unacceptable. Repeated measures ANOVA tests (Friedman test) with post hoc Dunnett tests were used to assess for difference in biomarker concentration for each plasma sample type compared to serum (Prism8 software).
Results
Even when samples were diluted as described, all serum Clus concentrations were above the measurable range of the assay, and all serum MCP-1 concentrations were below the measurable range of the assay; hence data for these 2 biomarkers are not reported (Table 3).
Intra-assay CVs were acceptable for NGAL (8.8%), and unacceptable for CysC (11.1%) and KIM-1 (14.9%). Inter-assay CVs were acceptable for NGAL (mean across replicates: 13.2%), and unacceptable for CysC (49.8%) and KIM-1 (27.6%).
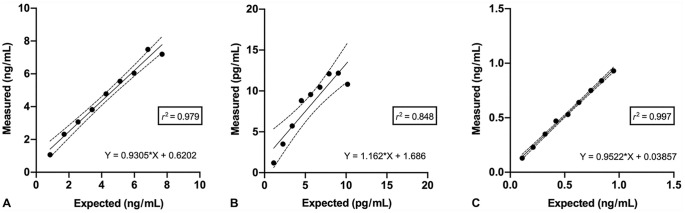
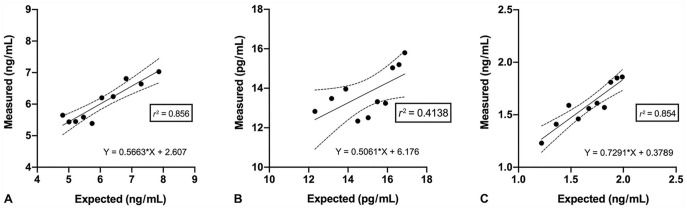
Regression analysis indicated good linearity for CysC and NGAL across all dilutions, and moderate linearity for KIM-1 (Fig. 1). Recovery for the dilution series was acceptable for CysC and NGAL, but unacceptable for KIM-1 (Table 4). Recovery was acceptable for all 3 analytes across the S-R study (Table 4). Results of regression analysis of the S-R series were acceptable for CysC and NGAL, but poor for KIM-1 (Fig. 2).
Figure 1.
Linearity under dilution of renal biomarker concentration in a serially diluted canine serum sample, measured using a commercial multiplex immunoassay. Each data point is the mean of a triplicate measurement. The solid line is the linear correlation between expected and observed values, and dotted lines indicate the 95% CI. Observed values were statistically correlated with the expected value according to a linear model, as demonstrated by the “lack of fit” test. A. Cystatin C. B. Kidney injury molecule 1. C. Neutrophil gelatinase–associated lipocalin.
Table 4.
Recovery values obtained from a linear dilution and spike-recovery series of canine serum for a commercial multiplex assay.
| Dilution (% canine serum) | CysC recovery % | KIM-1 recovery % | NGAL recovery % | Spike (% spiked solution) | CysC recovery % | KIM-1 recovery % | NGAL recovery % |
|---|---|---|---|---|---|---|---|
| 90 | 94 | 106 | 98 | 10 | 89 | 104 | 101 |
| 80 | 110 | 135 | 100 | 20 | 91 | 102 | 104 |
| 70 | 101 | 153 | 102 | 30 | 100 | 101 | 107 |
| 60 | 108 | 154 | 102 | 40 | 97 | 85 | 93 |
| 50 | 112 | 169 | 101 | 50 | 102 | 83 | 93 |
| 40 | 112 | 195 | 112 | 60 | 94 | 86 | 92 |
| 30 | 120 | 169 | 111 | 70 | 102 | 83 | 86 |
| 20 | 136 | 155 | 110 | 80 | 104 | 92 | 96 |
| 10 | 125 | 106 | 124 | 90 | 109 | 92 | 96 |
Recovery % = measured concentration/expected concentration × 100%. See Table 1 notes for explanations of abbreviations.
Figure 2.
Spike-recovery study of renal biomarker concentration in a canine serum sample spiked with another canine serum sample containing higher biomarker concentrations (or standard 5 for cystatin C), measured using a commercial multiplex immunoassay. Each data point is the mean of a triplicate measurement. The solid line is the linear correlation between expected and observed values, and dotted lines indicate the 95% CI. Observed values were statistically correlated with the expected value according to a linear model, as demonstrated by the “lack of fit” test performed as an indirect assessment of accuracy of a commercial multiple assay for measurement of renal biomarkers in canine serum. A. Cystatin C. B. Kidney injury molecule 1. C. Neutrophil gelatinase–associated lipocalin.
The TEa was calculated for CysC only, because of lack of published biological variation data for the other biomarkers studied. The TEobs for CysC measurement with this assay was in excess of the calculated TEa (Table 5).
Table 5.
Total error observed (TEobs) and calculated total error allowable (TEa) for renal biomarkers measured in canine serum using a commercial multiplex assay.
| Analyte | Total imprecision error (%) | Total bias (%) | TEobs (5) | TEa (5)* |
|---|---|---|---|---|
| CysC | 29.1 | 9.8 | 67.9 | 20.3 |
| KIM-1 | 21.3 | 29.2 | 71.7 | |
| NGAL | 12.2 | 6.6 | 30.9 |
See Table 1 notes for explanations of analyte abbreviations. Bias goal = 0.25 × [(published within subject variation)2 + (published between subject variation)2]1/2; imprecision goal = 0.5 × published within subject biological variation; total bias = mean of the relative error (100 – recovery %) of each dilution measured in the linearity under dilution and spike-recovery series; TEa = 1.65 × imprecision goal (%) × bias goal (%)16; TEobs = [2 × total imprecision error] + total bias16; total imprecision error = mean of intra-assay CV and inter-assay CV. Biological variation values used to calculate TEa for cysC: 14.9% within-subject variation, 12.3% between-subject variation.27
TEa not calculated for KIM-1 or NGAL because of lack of published biological variation data.
The LOB was 1.2 pg/mL, 2.56 ng/mL, and 0.23 ng/mL, and LOQ was 1.5 pg/mL, 0.04 ng/mL, and 0.03 ng/mL for KIM-1, CysC, and NGAL, respectively.
There was no significant interference with measurement of either CysC (p = 0.068), KIM-1 (p = 0.389), or NGAL (p = 0.767) when serum samples contained up to 8 g/L hemoglobin (Fig. 3).
Figure 3.
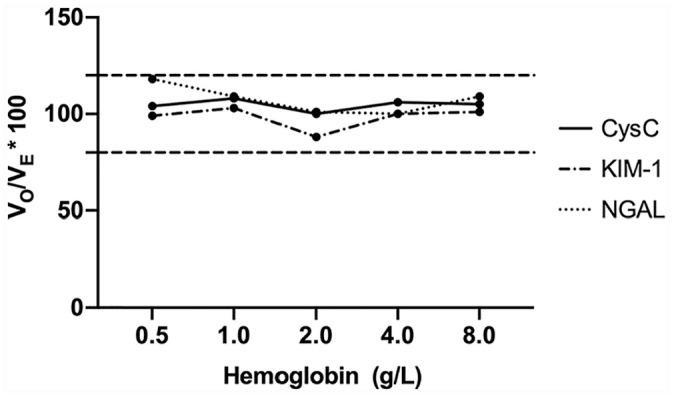
Interferogram for hemoglobin interference during measurement of renal biomarkers in canine serum using a commercial multiplex immunoassay. VO/VE = observed concentration/measured concentration. CysC = cystatin C; KIM-1 = kidney injury molecule 1; NGAL = neutrophil gelatinase–associated lipocalin. Horizontal dashed lines indicate acceptable recovery range of 80–120%.
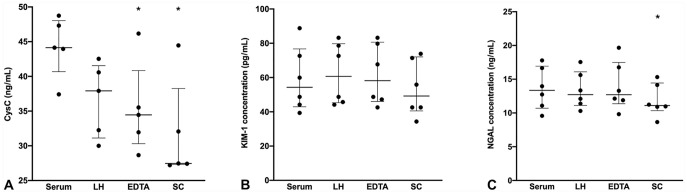
The mean percentage difference when serum biomarker concentration was compared to that in lithium heparin, EDTA, or sodium citrate plasma was < 15% for both KIM-1 (11.9%, 7.4%, 9.1%, respectively) and NGAL (9.5%, 8.5%, and 12.2%, respectively). The mean percentage difference for CysC concentration between serum and all 3 plasma types was unacceptably high: 17.0% for lithium heparin, 20.5% for EDTA, and 28.7% for sodium citrate. There was a statistically significant difference for CysC concentration between serum and EDTA plasma (p = 0.018), and between serum and sodium citrate plasma (p = 0.015); plasma samples consistently measured lower biomarker concentrations. There was a significant difference for NGAL concentration between serum and sodium citrate plasma (p = 0.045), with a lower measured biomarker concentration in the sodium citrate plasma sample (Fig. 4).
Figure 4.
Effect of sample type on renal biomarker measurement in canine blood using a commercial multiplex assay. Median and interquartile range. EDTA = EDTA plasma; LH = lithium heparin plasma; SC = sodium citrate plasma. * p ≤ 0.05 when compared to serum. A. Cystatin C (CysC). B. Kidney injury molecule 1 (KIM-1). C. Neutrophil gelatinase–associated lipocalin (NGAL).
Discussion
The first part of our study was to perform independent validation of a multiplex assay (MILLIPLEX MAP canine toxicity panel 1) using a MAGPIX analyzer, for use with canine serum. Magnetic bead–based multiplex assays such as this offer advantages over single-analyte ELISAs including reduced sample volume, reduced expense, shorter overall assay running time, and ability to customize specific panels of biomarkers to be analyzed. However, a significant limitation anticipated with this technology is increased potential for antibody cross-reactivity as a result of the use of a complex mixture of multiple individual antibodies to allow measurement of multiple analytes simultaneously. Hence, antibody quality is a particularly important factor in determining assay performance.6,38,39 Unfortunately, many commercial immunoassays for measurement of AKI biomarkers in dogs are produced against recombinant antibodies rather than native protein. This results in excellent assay precision and accuracy when assessed by manufacturers using the recombinant proteins, but poorer performance when assessed against native protein in clinical samples.13 Based on the findings of our validation study, we cannot recommend use of this assay for measurement of KIM-1 in canine serum. Precision and accuracy were unacceptable for this analyte. Poor quality antibody or cross-reactivity might be to blame. Although accuracy was acceptable for CysC measurement, precision (both within- and between-run) was not and led to a high TEobs. Imprecision may result from technical laboratory errors (e.g., bead aggregation, loss of beads during plate washing, incomplete plate washing, analyzer related errors); however, if this were the cause of imprecision in our study, we would expect similar poor performance across all analytes on the plate. The finding that the TEobs for CysC in our study is well above the TEa calculated suggests that this assay is not a suitable technique for the measurement of CysC in canine serum.
When assessing the precision of laboratory tests, it is recommended to use samples, or pooled samples, that span the measurable range of the assay.19 During a multiplex assay, all analytes are measured from the same diluted sample; hence one dilution must be chosen, with the hope that the concentration of each analyte will fall within the measurable range. A limitation of our study is that only samples from healthy dogs were used to assess intra-assay (within-run) precision. These samples were chosen given concern that samples from diseased dogs contained concentrations of Clus above the measurable range of the assay (authors’ unpublished data). It is expected that precision will worsen toward the lower extreme of the measurable range. Once diluted, the samples studied contained concentrations of CysC and NGAL in the middle of the measurable range; KIM-1 concentration of both of these samples was toward the lower end of the measurable range. Low concentrations of KIM-1 in the samples may have resulted in an overestimation of imprecision for KIM-1, and underestimation for CysC and NGAL.
We were unable to measure Clus or MCP-1 reliably with this multiplex assay as a result of concentrations of these proteins being above and below the measurable range of the assay, respectively, when serum was appropriately diluted to allow measurement of the other 3 analytes by the assay. It has been noted previously that Clus is present in canine serum in concentrations 1,000-fold higher than those in urine, at concentrations of up to 100 µg/mL.41 The most concentrated Clus standard in the current assay is 1 µg/mL, suggesting that canine serum would require at least a 1:99 dilution to achieve values within the measurable range. Studies investigating serum MCP-1 in healthy dogs (measured by a commercial ELISA), report MCP-1 concentrations of 4–260 pg/mL.10,18 The lowest standard for MCP-1 in the current assay is 70 pg/mL; thus, even in undiluted canine serum, it is likely that MCP-1 concentration will fall below the measurable range. We conclude that it is inappropriate to measure these 5 analytes in canine serum using the same multiplex assay with the current standards range because it is likely that 3 different dilutions of each sample would have to be analyzed in order to produce measurements within the measurable range of the assay. The need to analyze each sample at several different dilutions negates the cost and time benefit of a multiplex assay.
There is potential for quantification of protein biomarkers in blood samples to be affected by the sample type, whether serum or plasma, and, if plasma, the type of anticoagulant used. The addition of substances released from cells during the clotting process, and a volume displacement effect that occurs after clot formation, means that serum samples may contain higher concentrations of certain proteins and metabolites than plasma.42 Despite a previous suggestion that NGAL concentration in human serum samples may be higher as a result of the release of NGAL from neutrophils during serum preparation, our findings do not support the presence of this effect in canine blood.7 The measured concentration of CysC was significantly lower in all plasma samples compared to serum, and the concentration of NGAL was lower in citrated plasma samples compared to serum. The finding of lower biomarker concentration in plasma samples may, in part, be the result of a dilution effect. The additive in serum, EDTA, and lithium heparin tubes is sprayed into the collection tube (present in a minute volume); the liquid citrate additive produces a 1:9 ratio of additive-to-sample when the tube is correctly filled. Dilution alone cannot account for the almost 30% difference in CysC measurement between serum and citrated plasma; however, dilution in combination with the high level of imprecision for measurement of CysC with this assay could explain the bias observed. Our findings indicate that biomarker concentrations measured in citrated plasma should not be directly compared to those measured in any other sample type. Although NGAL concentration was statistically lower in citrated plasma compared to serum, recovery was still within 80–120%, suggesting that the difference may not be clinically relevant.
Our finding that presence of up to 8 g/L hemoglobin does not interfere with the measurement of Clus, KIM-1, or NGAL contradicts reports that hemolysis affects NGAL measurement by a commercial ELISA in human samples.29 To our knowledge, the effect of hemolysis on the measurement of AKI biomarkers in canine serum has not been reported previously. Hemolysis is reported to be the most frequent preanalytical source of laboratory error, so it is imperative to test for hemoglobin interference during validation of assays for use with serum samples.5 Ideally, investigation for interference by lipids and bilirubin in serum samples should also be carried out; however, limited funding precluded this investigation in our study.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: This study was funded by an internal research grant from the School of Veterinary and Life Sciences, Murdoch University, Perth, Australia
ORCID iDs: Jennifer Davis  https://orcid.org/0000-0002-7078-1645
https://orcid.org/0000-0002-7078-1645
Gabriele Rossi  https://orcid.org/0000-0003-4879-9504
https://orcid.org/0000-0003-4879-9504
References
- 1. Ahn HJ, Hyun C. Evaluation of serum neutrophil gelatinase-associated lipocalin (NGAL) activity in dogs with chronic kidney disease. Vet Rec 2013;173:452. [DOI] [PubMed] [Google Scholar]
- 2. Australian Research Council. Australian code for the care and use of animals for scientific purposes. 8th ed. Australian Government publication, 2013. [cited 2020 Jun 10]. https://www.nhmrc.gov.au/about-us/publications/australian-code-care-and-use-animals-scientific-purposes [Google Scholar]
- 3. Basile DP, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 2016;27:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braun J, et al. Plasma cystatin C in the dog: reference values and variations with renal failure. Comp Clin Pathol 2002;11:44–49. [Google Scholar]
- 5. Chawla R, et al. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1-year study at G.B. Pant Hospital. Lab Med 2010;41:89–92. [Google Scholar]
- 6. Christopher-Hennings J, et al. Opportunities for bead-based multiplex assays in veterinary diagnostic laboratories. J Vet Diagn Invest 2013;25:671–691. [DOI] [PubMed] [Google Scholar]
- 7. Clerico A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med 2012;50:1505. [DOI] [PubMed] [Google Scholar]
- 8. Davis J, et al. Urinary neutrophil gelatinase-associated lipocalin concentration changes after acute haemorrhage and colloid-mediated reperfusion in anaesthetized dogs. Vet Anaesth Analg 2016;43:262–270. [DOI] [PubMed] [Google Scholar]
- 9. De Loor J, et al. Urinary biomarkers for acute kidney injury in dogs. J Vet Intern Med 2013;27:998–1010. [DOI] [PubMed] [Google Scholar]
- 10. Duffy AL, et al. Serum concentrations of monocyte chemoattractant protein-1 in healthy and critically ill dogs. Vet Clin Pathol 2010;39:302–305. [DOI] [PubMed] [Google Scholar]
- 11. Ernst R, et al. Comparative performance of IDEXX SDMA test and the DLD SDMA ELISA for the measurement of SDMA in canine and feline serum. PLoS One 2018;13:e0205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flatland B, et al. ASVCP quality assurance guidelines: control of general analytical factors in veterinary laboratories. Vet Clin Pathol 2010;39:264–277. [DOI] [PubMed] [Google Scholar]
- 13. Floras AN, et al. Investigation of a commercial ELISA for the detection of canine procalcitonin. J Vet Intern Med 2014;28:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Martinez JD, et al. Urinary clusterin as a renal marker in dogs. J Vet Diagn Invest 2012;24:301–306. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Martinez JD, et al. Urinary ferritin and cystatin C concentrations at different stages of kidney disease in leishmaniotic dogs. Res Vet Sci 2015;99:204–207. [DOI] [PubMed] [Google Scholar]
- 16. Harr KE, et al. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 2013;42:424–436. [DOI] [PubMed] [Google Scholar]
- 17. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol 2016;45:28–56. [DOI] [PubMed] [Google Scholar]
- 18. Ishioka K, et al. Monocyte chemoattractant protein-1 in dogs affected with neoplasia or inflammation. J Vet Med Sci 2013;75:173–177. [DOI] [PubMed] [Google Scholar]
- 19. Jensen AL, Kjelgaard-Hansen M. Diagnostic test validation. In: Weiss DJ, Wardrop KJ, eds. Schalm’s Veterinary Hematology. 6th ed. Ames, IA: Wiley-Blackwell, 2010:1027–1033. [Google Scholar]
- 20. Kellum JA, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [cited 2020 Jun 10]. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf [Google Scholar]
- 21. Kuleš J, et al. Glomerular and tubular kidney damage markers in canine babesiosis caused by Babesia canis. Ticks Tick Borne Dis 2018;9:1508–1517. [DOI] [PubMed] [Google Scholar]
- 22. Legatti SAM, et al. Acute kidney injury in cats and dogs: a proportional meta-analysis of case series studies. PLoS One 2018;13:e0190772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maddens BE, et al. Validation of immunoassays for the candidate renal markers C-reactive protein, immunoglobulin G, thromboxane B2 and retinol binding protein in canine urine. Vet Immunol Immunopathol 2010;134:259–264. [DOI] [PubMed] [Google Scholar]
- 24. Malhotra R, Siew ED. Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 2017;12:149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyagawa Y, et al. Evaluation of the measurement of serum cystatin C by an enzyme-linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J Vet Med Sci 2009;71:1169–1176. [DOI] [PubMed] [Google Scholar]
- 26. Nabity MB, et al. Urinary biomarkers of renal disease in dogs with X-linked hereditary nephropathy. J Vet Intern Med 2012;26:282–293. [DOI] [PubMed] [Google Scholar]
- 27. Pagitz M, et al. Evaluation of biological variance of cystatin C in comparison with other endogenous markers of glomerular filtration rate in healthy dogs. J Vet Intern Med 2007;21:936–942. [DOI] [PubMed] [Google Scholar]
- 28. Pasa A, et al. Serum cystatin C concentration as a marker acute renal dysfunction in critically ill dogs. Vet Res Commun 2009;33:529–534. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen KR, et al. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Invest 2010;70:374–382. [DOI] [PubMed] [Google Scholar]
- 30. Perry JA, et al. Increased monocyte chemotactic protein-1 concentration and monocyte count independently associate with a poor prognosis in dogs with lymphoma. Vet Comp Oncol 2011;9:55–64. [DOI] [PubMed] [Google Scholar]
- 31. Pozzoli S, et al. Predicting acute kidney injury: current status and future challenges. J Nephrol 2018;31:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raila J, et al. The distribution of vitamin A and retinol-binding protein in the blood plasma, urine, liver and kidneys of carnivores. Vet Res 2000;31:541–551. [DOI] [PubMed] [Google Scholar]
- 33. Rifai N, et al. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol 2006;24:971–983. [DOI] [PubMed] [Google Scholar]
- 34. Rossi S, et al. Homocysteine measurement by an enzymatic method and potential role of homocysteine as a biomarker in dogs. J Vet Diagn Invest 2008;20:644–649. [DOI] [PubMed] [Google Scholar]
- 35. Shimizu N, et al. Evaluation of urinary and serum level of chemokine (C-C motif) ligand 2 as a potential biomarker in canine urothelial tumours. Vet Comp Oncol 2019;17:11–20. [DOI] [PubMed] [Google Scholar]
- 36. Steinbach S, et al. Plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) in dogs with acute kidney injury or chronic kidney disease. J Vet Intern Med 2014;28:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Susantitaphong P, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tighe P, et al. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods 2013;61:23–29. [DOI] [PubMed] [Google Scholar]
- 39. Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods 2000;243:243–255. [DOI] [PubMed] [Google Scholar]
- 40. Wagoner MP, et al. Evaluation of temporal changes in urine-based metabolomic and kidney injury markers to detect compound induced acute kidney tubular toxicity in beagle dogs. Curr Top Med Chem 2017;17:2767–2780. [DOI] [PubMed] [Google Scholar]
- 41. Yerramilli M. Kidney disease and the nexus of chronic kidney disease and acute kidney injury: the role of novel biomarkers as early and accurate diagnostics. Vet Clin North Am Small Anim Pract 2016;46:961–993. [DOI] [PubMed] [Google Scholar]
- 42. Yu Z, et al. Differences between human plasma and serum metabolite profiles. PLoS One 2011;6:e21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou X, et al. Evaluation of the usefulness of novel biomarkers for drug-induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol 2014;280:30–35. [DOI] [PubMed] [Google Scholar]