Abstract
Most of the pigs on a farm in Aichi Prefecture, Japan had chronic diarrhea and severe wasting. The pigs had consumed 8,000 ppm zinc oxide (ZnO) as a feed additive. The pancreas of each of 4 autopsied pigs was less than half the normal size. Acinar cells were considerably decreased. Epithelial duct–like cells were increased and tested positive for cytokeratin AE1/AE3, Ki67, PGP9.5, and Sox9. Pancreatic islet cells were decreased and shrunken. The α and δ cells were relatively decreased, and their distribution was abnormal. Islet cells were positive for PGP9.5. The livers and kidneys had high accumulations of zinc (Zn; 788 µg/g and 613 µg/g, respectively). Copper was deficient in the liver, likely as a result of Zn poisoning. Our immunohistologic examination suggested that the high dose of ZnO could influence the function of islet cells in addition to that of acinar cells. Given that colistin sulfate has been banned as a feed additive in order to reduce antimicrobial use in Japan, the use of ZnO in the livestock industry is expected to increase. Zn supplementation of pig feed must be monitored to prevent Zn poisoning and contamination of soil and water.
Keywords: chronic pancreatitis, Japan, pigs, wasting, zinc oxide, zinc poisoning
Zinc (Zn) is essential for homeostasis in animals. Over 300 enzymatic reactions require Zn as a catalyst or cofactor.15 Zn is an integral part of the antioxidant enzyme copper–zinc superoxide dismutase. In the pancreas, Zn is required for synthesis, storage, and secretion of insulin and glucagon by β and α cells.3,12 Zn binds to metallothionein and is secreted largely via the pancreatic duct.2 Thus, the pancreas plays an important role in Zn homeostasis.
Since the early 2000s in Japan, zinc oxide (ZnO) has been administered at high doses to swine as a feed additive to prevent infectious diseases in weaned piglets,7 and to decrease antimicrobial use. The use of dietary zinc supplements is increasing in the farms of Aichi Prefecture in Japan further to the prohibition of the use of colistin sulfate as a feed additive. Although high doses of Zn (2,500–3,000 ppm, for 1 wk) have demonstrated efficacy against certain microbial pathogens,6,8 the actual dose of Zn varies across countries and farms. Excessively high concentrations of Zn may be toxic to pigs, and can induce pancreatitis.4 It has been reported that high doses of ZnO (4,000–6,000 ppm, 3 wk) induce pancreatic acinar cell apoptosis.1 Nevertheless, we found no reports of Zn poisoning in farm-reared pigs; histologic and immunohistochemical examinations of their organs and tissues are lacking.
We encountered Zn poisoning cases in farm-raised pigs. The swine were given excessive ZnO, which induced chronic pancreatitis and caused substantial financial loss. We studied the pathologic and biochemical effects in this case of long-term, high-dose ZnO administration in swine.
In a farrow-to-finish farm with 330 sows located in Aichi Prefecture in Japan, the pigs had chronic diarrhea (loose white stools) and were wasting. Weaned pigs had accidentally been given 8,000 ppm ZnO in their feed for 2 mo. ZnO (2,000 ppm) was usually added to the feed from weaning until marketing (~5 mo); it was mixed with the feed in tanks on-farm before feeding. Over the 4 mo since the feed mixing error occurred, nearly all of the pigs developed wasting, and each weighed <60 kg just before shipping. The mortality rate did not change at any time before shipping. The feed supplied to this farm, including weaner, grower–finisher, and lactating sow rations, conformed to the Japan pig feed standards (containing <55 ppm Zn).
Four pigs with severe wasting and loose white feces were euthanized ~8 mo after the feed mixing error occurred. Pigs 1 and 2 were 200 d old and weighed 60 kg each; pigs 3 and 4 were 240 d old and weighed 40 kg each. They had been fed 8,000 ppm ZnO for 6–7 mo. Lung, heart, kidney, liver, spleen, pancreas, stomach, intestine (duodenum, jejunum, ileum, cecum, and colon), mesenteric lymph nodes, and central nervous system tissue samples were collected and used in subsequent histologic, microbiologic, and biochemical analyses. Tissue samples were fixed in 10% (v/v) neutral phosphate-buffered formalin, processed routinely, and slides stained with hematoxylin and eosin, and dithizone, for histologic examination. Kidney sections were also stained with rhodamine.
Immunohistochemistry (IHC; Table 1) was performed on formalin-fixed, paraffin-embedded (FFPE) pancreas samples from pig 4 using antibodies to trypsin (Dako), cytokeratin AE1/AE3 (Dako), Ki67 (Dako), PGP9.5 (OriGene Technologies), Sox9 (Abcam), insulin (Nichirei Biosciences), glucagon (Nichirei Biosciences), and somatostatin (Dako). IHC was performed only on samples from pig 4 because all of the samples of pancreas from Zn-poisoned pigs had the same lesions.
Table 1.
Conditions for immunohistochemical examination of pig pancreases.
| Antibody | Serum source | Suppression of endogenous peroxidase activity | Blocking | Antigen retrieval | Second antibody | Antibody target |
|---|---|---|---|---|---|---|
| Trypsin | Rabbit | 3% H2O2, RT, 10 min | NGS, RT, 30 min | Proteinase K, 37°C, 20 min | EnVision+ kit (HRP, anti-rabbit), RT, 30 min | Zymogen granule |
| Cytokeratin AE1/AE3 | Mouse | 3% H2O2 in methanol, RT, 10 min | None | Proteinase K, RT, 5 min | EnVision+ kit (HRP, anti-mouse), RT, 30 min | Epithelial membrane |
| Ki67 | Mouse | 3% H2O2 in methanol, RT, 10 min | None | Autoclave, 121°C, 10 min | EnVision+ kit (HRP, anti-mouse), RT, 30 min | Cell proliferation |
| PGP9.5 | Rabbit | 3% H2O2 in methanol, RT, 10 min | NGS, RT, 30 min | Microwave, 170 W, 10 min | Histofine simple stain MAX-PO (rabbit), RT, 30 min | Pancreatic progenitor cell |
| Sox9 | Rabbit | 3% H2O2, RT, 10 min | NGS, RT, 45 min | Microwave, 170 W, 15 min | EnVision+ kit (HRP, anti-rabbit), RT, 30 min | Multipotential progenitor cell |
| Insulin | Mouse | 3% H2O2 in methanol, RT, 15 min | NGS, RT, 10 min | None | Histofine simple stain MAX-PO (mouse), RT, 10 min | β cell |
| Glucagon | Rabbit | 3% H2O2 in methanol, RT, 30 min | NGS, RT, 30 min | None | Histofine simple stain MAX-PO (rabbit), RT, 30 min | α cell |
| Somatostatin | Rabbit | 3% H2O2, RT, 5 min | None | Microwave, 170 W, 15 min | EnVision+ kit (HRP, anti-rabbit), RT, 30 min | δ cell |
HRP = horseradish peroxidase; NGS = 10% (v/v) normal goat serum; RT = room temperature.
No pathogenic bacteria were isolated from any of the organs and tissues on routine culture. To detect pathogenic Mycoplasma, DNA was extracted from the lung (QIAamp MinElute virus spin kit; Qiagen). Mycoplasma hyopneumoniae and M. hyorhinis were detected in the lungs of pigs 3 and 4 by specific PCR.10,14 Tissue Zn, copper, and iron concentrations were determined by flame atomic absorption spectrometry (AA-7000; Shimadzu) after tissue digestion by microwave pretreatment (Multiwave GO; Anton Paar).
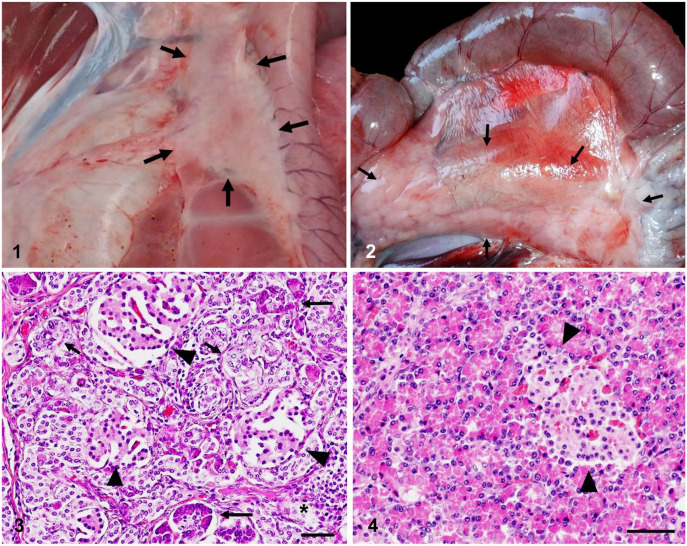
At autopsy, the pancreas had shrunken to 20–50% of normal size (Figs. 1, 2). All 4 pigs had the same gross pancreatic lesions. Livers were slightly swollen. The cranial lobe of each lung was discolored, dark, and purple. The spleens of pigs 1 and 2 were discolored, dark red, and slightly swollen. No gross lesions were evident in the other organs.
Figures 1–4.
Chronic pancreatitis in zinc oxide (ZnO)-poisoned pigs. Figure 1. Severe pancreatic atrophy (2 × 4 cm; arrows) in a ZnO-poisoned pig 3. Figure 2. Pancreas (3 × 11 cm; arrows) of a control 140-d-old pig. Figure 3. Histology of the pancreas of an affected pig. Marked increase in epithelial duct-like cells (short arrows) in the pancreas of pig 4. Epithelial duct-like cells are located next to exocrine acinar cells (long arrows). Islet cells (arrowheads) were shrunken and decreased. A few lymphocytes have infiltrated the interstitium (asterisk). H&E. Bar = 50 μm. Figure 4. Pancreas of a control 200-d-old pig. Islets are marked by arrowheads. H&E. Bar = 50 μm.
Histologically, the pancreatic parenchyma of affected pigs was replaced by fat cells. The exocrine acinar cells were dramatically decreased and adjacent to increased numbers of irregular cuboidal epithelial duct–like cells (Figs. 3, 4). These cells were separated by collagen fibers, fibroblasts, and a few lymphocytes (Fig. 3). A portion of the duct lumen was filled with necrotic epithelial cell debris. IHC revealed that the decreased numbers of acinar cells contained zymogen granules that were positive for trypsin (Table 2). The epithelial duct–like cells were positive for cytokeratin AE1/AE3, Ki67, PGP9.5 (Fig. 5), and Sox9 (Table 2).
Table 2.
Results of the immunohistochemical examination of the pancreas of pig 4.
| Pancreatic cell | Antibody to: | ||||
|---|---|---|---|---|---|
| Trypsin | Cytokeratin AE1/AE3 | Ki67 | PGP9.5 | Sox9 | |
| Acinar cells | +++ | – | – | – | – |
| Epithelial duct–like cells* | – | +++ | +++ | + | +++ |
– = negative; + = <50% of cells were positive for the antibody; ++ = 50% of the cells were positive for the antibody; +++ = most cells were positive for the antibody.
Epithelial duct–like cells were irregularly increased cuboidal epithelial cells in pig pancreas (Fig. 3).
Figures 5–7.

Figure 5. PGP9.5 immunostaining of the pancreas of an affected pig. Positive reaction against PGP9.5 antigen in epithelial duct–like cells (arrows) and islet cells (arrowheads) in pig 4. No positive reaction was detected in acinar cells. Bar = 25 μm. Figure 6. Insulin immunostaining of the pancreas of an affected pig. Positive reaction against insulin antigen in islet cells both within and outside the islet (arrows) in pig 4. Almost all islet cells were positive for insulin antigen. Bar = 50 μm. Figure 7. IHC for insulin in the pancreas of a control 200-d-old pig. Positive reaction was detected only in islet cells. Bar = 25 μm.
The numbers of the pancreatic islet cells were decreased, and they were shrunken (Fig. 3). Most of them were β cells that expressed insulin (Figs. 6, 7). A few insulin-positive cells were detected outside the islets. IHC with glucagon and somatostatin antibodies revealed shrunken α and δ cells. The α cells were located centrally in islets; δ cells were scattered in islets and were also detected outside islets. Islet cells were positive for PGP9.5. Dithizone staining disclosed no Zn granules in any organs, except in the duodenal lumen.
Lymphocytes aggregated around the bronchi in the lungs of 4 pigs, consistent with enzootic pneumonia. Abundant hemosiderin was detected in the red pulp of the spleen of pigs 2 and 4. Rhodamine staining disclosed no copper granules in the kidneys.
The livers and kidneys of affected pigs contained high concentrations of Zn; cardiac Zn levels were low (Table 3). The renal copper level was extremely high. Nevertheless, hepatic and cardiac copper levels were relatively low. Iron accumulation was observed in all organs tested.
Table 3.
Concentration (µg/g [wet weight]) of accumulated zinc, copper, and iron in the organs of 4 zinc-poisoned pigs.
| Element/organ | 1 | 2 | 3 | 4 | Mean | Standard error |
|---|---|---|---|---|---|---|
| Zinc | ||||||
| Liver | 691 | 456 | 1,180 | 819 | 788 | 132 |
| Kidney | 477 | 304.1 | 793 | 880 | 613 | 117 |
| Spleen | 24.5 | 15.9 | 24.6 | 21.0 | 21.5 | 1.8 |
| Heart | 18.7 | 14.4 | 14.1 | 16.9 | 16.0 | 0.9 |
| Lung | 18.8 | 11.6 | 19.2 | 21.7 | 17.8 | 1.9 |
| Duodenum | — | — | 266 | 201 | 234 | 16.2 |
| Copper | ||||||
| Liver | 4.1 | 1.7 | 9.6 | 6.9 | 5.6 | 1.5 |
| Kidney | 164 | 116 | 184 | 199 | 166 | 15.5 |
| Spleen | ND | ND | ND | ND | — | — |
| Heart | 2.6 | 1.5 | 2.5 | 2.6 | 2.3 | 0.2 |
| Lung | ND | ND | ND | ND | — | — |
| Duodenum | — | — | 0.4 | 0.1 | 0.2 | 0.1 |
| Iron | ||||||
| Liver | 508 | 277 | 308 | 324 | 354 | 45.2 |
| Kidney | 81.4 | 55.6 | 76.3 | 91.6 | 76.2 | 6.6 |
| Spleen | 2,900 | 1,390 | 638 | 607 | 1,380 | 465 |
| Heart | 68.9 | 64.5 | 65.9 | 68.6 | 66.9 | 0.9 |
| Lung | 106 | 78.9 | 133 | 114 | 108 | 9.7 |
| Duodenum | — | — | 59.4 | 68.0 | 63.7 | 2.2 |
— = no data; ND = not detected.
These farm-reared pigs had excessive diarrhea and wasting. In error, they had been given excessive ZnO as a feed additive. Gross and histopathology and biochemical analyses indicated that these signs were the result of Zn poisoning caused by ingestion of excessive ZnO. The severe wasting was halted by reducing the ZnO dosage to the normal 2,000 ppm concentration ~7 mo after the onset of clinical signs. Considerable time, effort, and expense were required to determine the cause of this outbreak by the veterinarian at this farm. The financial loss incurred was ~$182,000 by the time Zn poisoning was ameliorated. In a previous study, Zn toxicity was reported in 4-d-old piglets maintained on total parenteral nutrition containing 50 µg/mL Zn for 3 wk.4 However, to our knowledge, no prior case of ZnO poisoning has been reported in farm-reared pigs.
In our cases, Zn overdose was characterized by clinical signs such as loose white feces and severe wasting. In an earlier study of ZnO toxicity in pigs, no significant differences were observed between the treated and control animals.17 Similarly, there was no significant difference in body weight between treated and control pigs.1,4 However, the exposure periods were shorter and the ZnO concentrations were lower than in our study.
The pigs given excess ZnO showed a marked decrease in acinar cells and an increase in epithelial duct–like cells, along with fibrosis and infiltration of lymphocytes in the pancreas. These histopathologic changes resembled those reported in the past.4 Previous studies disclosed a reduction in the number of zymogen granules in the pancreatic acinar cells of pigs.4 In our case, the zymogen granules appeared normal, and their content was confirmed using trypsin antibody. However, the acinar cells were considerably decreased. The epithelial duct–like cells were positive for cytokeratin AE1/AE3, Ki67, Sox9, and PGP9.5. Thus, these cells were actively proliferating, and the epithelial stem cells observed may have been involved in tissue regeneration. Given that the epithelial duct–like cells were adjacent to acinar cells, the former may have been derived from intercalated duct or centroacinar cells. These histologic changes can be considered to result from ongoing pancreatitis, necrosis of acinar cells, replacement by fibrocytes and lymphocytes, and regeneration by proliferation of the epithelial duct–like cells.
Pancreases of the affected pigs had fewer but shrunken islet cells. IHC revealed reduced and abnormally distributed α and δ cells. Moreover, β and δ cells were detected outside the pancreatic islets. The islet cells were positive for PGP9.5 but negative for Ki67. Therefore, the β and δ cells may have been regenerated from duct stem cells in or out of the islet and ceased proliferation. Earlier studies on Zn poisoning reported no change in the size, shape, or distribution of islet cells after short-term (3 wk) Zn exposure in piglets.1,4 However, no prior study has reported the effects of long-term, high-dose ZnO (8,000 ppm; >4 mo). Pancreatic islet β cells use Zn for insulin synthesis, storage, and secretion.3,12 After partial pancreatectomy, a diet rich in Zn may enhance early cell proliferation and endocrine and exocrine function in the pancreatic remnant.9 Sustained administration of high Zn concentrations may either damage the islet cells or induce them to regenerate. Post-intoxication islet cell distribution may change during healing and remodeling, and could influence the function of islets in pigs, but this mechanism requires further investigation.
Zn accumulated especially in the liver (mean: 788 μg/g) and kidney (mean: 613 μg/g) of affected pigs. The hepatic Zn levels were higher than those previously reported in intoxicated swine (514 μg/g; 491 μg/g).4 Very high Zn concentrations accumulated in the liver and kidney in our study, but no Zn granules were detected by dithizone staining in FFPE liver, kidney, or pancreas. Zn injection has induced metallothionein biosynthesis in the pancreas17 and kidneys19 of pigs and the livers of rats.5 We did not investigate the expression of metallothionein in our case; however, high levels of induced metallothionein might have bound Zn in the tissues and blocked staining of the Zn.
The level of copper in the liver was relatively low, likely as a result of these pigs having Zn-induced copper deficiency caused by competitive absorption-inhibition. In contrast, the renal copper level was high. This phenomenon was reported in a previous study.13 High dietary Zn (2,100 ppm; 2 wk) induced metallothionein and copper transport 1 gene (Ctr1) expression and repressed antioxidant 1 copper chaperone gene (Atox1) expression associated with copper metabolism.19 In our case, lesions such as renal tubular and hepatic cell necrosis, which occur in response to copper intoxication, were not detected. In addition, we also did not detect copper granules in the kidney. We did not evaluate the expression of the Ctr1 and Atxo1 genes. Nevertheless, similar to changes reported above, the accumulated copper in the kidney without lesions could be the result of binding to increased metallothionein.
Iron was abundant in all tissues. However, excess dietary Zn causes copper deficiency and reduces iron absorption18 and storage in the liver and spleen.16 Zn-poisoned pigs become copper-deficient, which in turn, inhibits Cu-Zn superoxide dismutase in the erythrocytes, resulting in these cells becoming deformed and undergoing hemolysis.11 In our study, the observed high hepatic and splenic iron levels may have been the result of hemolysis. Hemosiderosis was detected only in pigs 2 and 4. These findings suggest that defective Zn–copper–iron balance may lead to iron accumulation, but this mechanism warrants further research. Zn supplementation must be monitored carefully to prevent livestock poisoning and pollution of soil and water.
Acknowledgments
We thank Dr. M. Ikezawa (National Institute of Animal Health, National Agriculture and Food Research Organization [NARO]) for advice on histologic examinations and discussion. We also thank Editage (www.editage.jp) for English language editing.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tomoyuki Shibahara  https://orcid.org/0000-0001-9335-5596
https://orcid.org/0000-0001-9335-5596
Contributor Information
Tetsuya Komatsu, Aichi Prefectural Chuo Livestock Hygiene Service Center, Okazaki, Aichi, Japan.
Kennosuke Sugie, Aichi Prefectural Chuo Livestock Hygiene Service Center, Okazaki, Aichi, Japan.
Naoko Inukai, Aichi Prefectural Chuo Livestock Hygiene Service Center, Okazaki, Aichi, Japan.
Osamu Eguchi, Eguchi Swine Clinic, Fuso, Aichi, Japan.
Toshifumi Oyamada, School of Veterinary Medicine, Kitasato University, Towada, Aomori, Japan.
Hiroshi Sawada, National Institute of Animal Health, National Agriculture and Food Research Organization (NARO), Tsukuba, Ibaraki, Japan.
Noriko Yamanaka, National Institute of Animal Health, National Agriculture and Food Research Organization (NARO), Tsukuba, Ibaraki, Japan.
Tomoyuki Shibahara, National Institute of Animal Health, National Agriculture and Food Research Organization (NARO), Tsukuba, Ibaraki, Japan.
References
- 1. Burrough ER, et al. Zinc overload in weaned pigs: tissue accumulation, pathology, and growth impacts. J Vet Diagn Invest 2019;31:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Lisle RC, et al. Metallothionein is a component of exocrine pancreas secretion: implications for zinc homeostasis. Am J Physiol 1996;271:1103–1110. [DOI] [PubMed] [Google Scholar]
- 3. Dodson G, et al. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol 1998;8:189–194. [DOI] [PubMed] [Google Scholar]
- 4. Gabrielson KL, et al. Zinc toxicity with pancreatic acinar necrosis in piglets receiving total parenteral nutrition. Vet Pathol 1996;33:692–696. [DOI] [PubMed] [Google Scholar]
- 5. Garla R, et al. Induction of metallothionein in rat liver by zinc exposure: a dose and time dependent study. Protein J 2017;36:433–442. [DOI] [PubMed] [Google Scholar]
- 6. Hahn JD, et al. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci 1993;71:3020–3024. [DOI] [PubMed] [Google Scholar]
- 7. Hill GM, et al. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci 2001;79:934–941. [DOI] [PubMed] [Google Scholar]
- 8. Jensen-Waern M, et al. Dietary zinc oxide in weaned pigs—effects on performance, tissue concentrations, morphology, neutrophil functions and faecal microflora. Res Vet Sci 1998;64:225–231. [DOI] [PubMed] [Google Scholar]
- 9. Kato K, et al. Effect of zinc administration on pancreatic regeneration after 80% pancreatectomy. Pancreas 1997;14:158–165. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi H, et al. Mycoplasma hyorhinis infection levels in lungs of piglets with porcine reproductive and respiratory syndrome (PRRS). J Vet Med Sci 1996;58:109–113. [DOI] [PubMed] [Google Scholar]
- 11. Lenartowicz M, et al. Haemolysis and perturbations in the systemic iron metabolism of suckling, copper-deficient mosaic mutant mice—an animal model of Menkes disease. PLoS One 2014;9:e107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maret W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev Nutr Food Sci 2017;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez MM, et al. Pharmacological zinc and phytase supplementation enhance metallothionein mRNA abundance and protein concentration in newly weaned pigs. J Nutr 2004;134:538–544. [DOI] [PubMed] [Google Scholar]
- 14. Mattsson JG, et al. Detection of Mycoplasma hyopneumoniae in nose swabs from pigs by in vitro amplification of the 16S rRNA gene. J Clin Microbiol 1995;33:893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCall KA, et al. Function and mechanism of zinc metalloenzymes. J Nutr 2000;130:1437–1446. [DOI] [PubMed] [Google Scholar]
- 16. O’Neil-Cutting MA, et al. Effect of excess dietary zinc on tissue storage of iron in rats. J Nutr 1981;111:1969–1979. [DOI] [PubMed] [Google Scholar]
- 17. Pieper R, et al. Impact of high dietary zinc on zinc accumulation, enzyme activity and proteomic profiles in the pancreas of piglets. J Trace Elem Med Biol 2015;30:30–36. [DOI] [PubMed] [Google Scholar]
- 18. Storey ML, et al. Iron, zinc and copper interactions: chronic versus acute responses of rats. J Nutr 1987;117:1434–1442. [DOI] [PubMed] [Google Scholar]
- 19. Zetzsche A, et al. Accumulation of copper in the kidney of pigs fed high dietary zinc is due to metallothionein expression with minor effects on genes involved in copper metabolism. J Trace Elem Med Biol 2016;35:1–6. [DOI] [PubMed] [Google Scholar]