Abstract
Detection of Leptospira interrogans is difficult as a result of intermittent leptospiruria and brief leptospiremia. Hence, diagnosis relies heavily on serologic testing, the reference method of which is the microscopic agglutination test (MAT). In horses, clinical leptospirosis has been associated with abortion, recurrent uveitis, and sporadic cases of hepatic and renal disease. Little information exists on the seroprevalence of antibodies to L. interrogans in equids in the United States; past nationwide studies suggest that the seroprevalence in some areas is as high as 77% (reciprocal titer ≥ 100). We tested sera from 124 apparently healthy horses previously submitted for equine infectious anemia (EIA) serology using MAT for 6 serovars—Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, and Pomona. When using a reciprocal MAT titer cutoff of ≥ 100, 102 of 124 (82%) of the samples were positive for at least one serovar. Seropositivity was significantly associated with increasing age. Query of specimens from clinical cases submitted to the Colorado State University Veterinary Diagnostic Laboratory for MAT since 2010 indicated significantly greater seroprevalence (p = 0.015) of pathogenic serovar Pomona in clinical cases compared to sera submitted from healthy equids for routine EIA testing. Information from our diagnostic laboratory submission forms also suggests a correlation between uveitis or other ophthalmic problems and serovar Pomona.
Keywords: cross-reactions, horses, Leptospira, seroepidemiologic studies, zoonoses
Leptospirosis is a bacterial disease of global public health importance that is caused by a group of spirochete bacteria of the genus Leptospira. The disease causes morbidity and mortality in many different domestic and wild animal species, including equids, canids, and swine.8,10,19 Leptospira spp. also infect people, and animals can serve as a source of transmission to humans. In horses, infection is characterized by nonspecific clinical signs, including fever, listlessness, and anorexia. More progressive disease is characterized by equine recurrent uveitis (ERU), acute renal failure, abortion, placentitis, stillbirth, and hemolytic anemia.6–8,11
Over 300 serovars exist within the genus Leptospira, and those that are host-adapted generally cause asymptomatic infection in their reservoir, or maintenance, host. Serovar Bratislava is considered to be a host-adapted serovar for horses, which is reflected by high seroprevalence in the absence of clinical signs.4,15,18,20 Serovars for which the horse is the incidental host generally cause more significant clinical disease. In horses, the serovars most commonly associated with severe morbidity are Pomona, notably Pomona strain Kennewicki,7 and Grippotyphosa.13
Transmission of leptospires to the incidental host occurs mainly through contact with contaminated urine, reproductive fluids, or an environmental source (water, soil), entering through mucous membranes, wet skin, or open wounds.12,14,17 In incidental hosts, leptospiremia may last 2–6 d post-infection (dpi), with intermittent leptospiruria occasionally lasting up to 4 wk.21 Experimental infection of horses with serovar Pomona strain Kennewicki demonstrated that antibodies to L. interrogans appear 5–6 dpi, peak on 14 dpi, and decline below detectable levels by 40–60 dpi.21
Given that different serovars are adapted to different mammalian reservoir hosts, identification of infecting or circulating serovars is epidemiologically significant. Although PCR is a more rapid and sensitive assay than bacterial culture and isolation, issues exist in nucleic acid detection because of intermittent shedding and leptospiremia.2,3,9 The gold standard serologic assay utilized for serovar determination is the microscopic agglutination test (MAT).5 This test identifies both IgM and IgG antibodies to specific serovars of L. interrogans in patient sera.5 The American College of Veterinary Internal Medicine small animal consensus statement states that MAT is the current test of choice used in the diagnosis of leptospirosis and, although results are subjective and often difficult to interpret, acute and convalescent titers or high titers in the face of clinical illness may be supportive of a leptospirosis diagnosis.19 A similar consensus statement does not exist for equids.
Further information on the seroprevalence of antibodies against Leptospira spp. in horses in the United States would provide data on frequency of infection and cumulative exposure. A 2012 study analyzing diagnostic laboratory samples from 29 states and 1 Canadian province yielded a 45% seroprevalence when using a reciprocal MAT titer of ≥ 200 as a cutoff.4 Similarly, a 2015 nationwide study found 75% seroprevalence when using a reciprocal MAT titer of ≥ 100 as a cutoff (Study Report Restricted Grant-FTLEPTO13 (v1.0) TI-01366, Zoetis)
In October 2015, Zoetis released a vaccine against serovar Pomona, the first vaccine in the United States labeled for use in equids (Lepto EQ Innovator, https://www.zoetisus.com/products/horses/lepto-eq-innovator/index.aspx). Although little is known about antibody response in vaccinated equids, canids vaccinated with a multivalent vaccine against leptospirosis had reciprocal MAT titers of ≥ 800 at various times following vaccination.16 Our objective was to compare the prevalence of anti-Leptospira antibodies in a presumed healthy population of Colorado equids prior to the use of the Zoetis vaccine to seroprevalence in clinical cases submitted to the Colorado State University, Veterinary Diagnostic Laboratory (CSU-VDL; Fort Collins, CO).
For the control group, banked samples previously submitted to the CSU-VDL for equine infectious anemia (EIA) serology testing were selected at random, after having been stored for ≤ 4 mo at 4°C. Demographic information was obtained from submitted EIA forms for each animal, including age, breed, sex, and location. Animals were stratified by age group (≤ 4 y, 5–7 y, 8–10 y, 11–14 y, and ≥ 15 y). Inclusion criteria consisted of blood samples collected and submitted between 1 June 2015 and 30 September 2015 from horses residing in Colorado at the time of sample collection. To ensure broad geographic representation, exclusion criteria specified that no more than 2 horses per property, and no more than 3 horses per zip code, were to be tested. The leptospirosis MAT was performed using antigen from 6 serovars obtained from the National Veterinary Services Laboratories (Ames, IA; Table 1). Patient sera were diluted at 1:100, 1:200, and 1:400 and added to serovar-specific antigen using 96-well test plates. If a serum sample was positive at 1:400, a second assay was performed with 2-fold dilutions to 1:6,400. Direct fluorescence microscopy was used to determine the highest dilution with 50% agglutination, and the reciprocal was taken as the antibody titer.
Table 1.
Serovars and associated strains utilized for the Leptospira microscopic agglutination test (reagents from National Veterinary Services Laboratories, Ames, IA).
| Species/Serovar | Strain |
|---|---|
| Leptospira interrogans | |
| Bratislava | Jez Bratislava (ARL-050) |
| Canicola | Hond Utrecht IV (CAL-010) |
| Grippotyphosa | Andaman (GRL-020) |
| Hardjo | Hardjoprajtino (SJL-060) |
| Icterohaemorrhagiae Copenhageni (Ictero reference) |
M-20 (ICL-020) |
| Pomona | Pomona (POA-010) |
For the clinical group, the CSU-VDL laboratory information management system was queried to identify all samples tested for L. interrogans nucleic acid via PCR or antibodies via MAT from 1 July 2010 to 26 January 2017. The Fisher exact test was used to compare seroprevalence between animals of different age groups, from different regions, and from different populations (clinical submissions vs. EIA screening) using R statistical software (https://www.r-project.org/).
The results of our study indicate that a large proportion of horses have been exposed to L. interrogans, and may indicate a high degree of ongoing exposure in Colorado, given that 102 of 124 (82%) had a reciprocal MAT titer of ≥ 100 and 71 of 124 (57%) had a reciprocal MAT titer of ≥ 200 for 1 or more of the tested serovars (Fig. 1). These results are consistent with previously published nationwide studies indicating 75% seroprevalence using a ≥ 100 cutoff and 45% seroprevalence using a ≥ 200 cutoff (Study Report Restricted Grant-FTLEPTO13 (v1.0) TI-01366, Zoetis).4 Despite serologic data indicating widespread exposure in Colorado equids, no samples submitted for L. interrogans PCR were positive during this sample time (0 of 24, 0%), indicating a discrepancy between serologic (antibody) and molecular (antigen) prevalence data.
Figure 1.
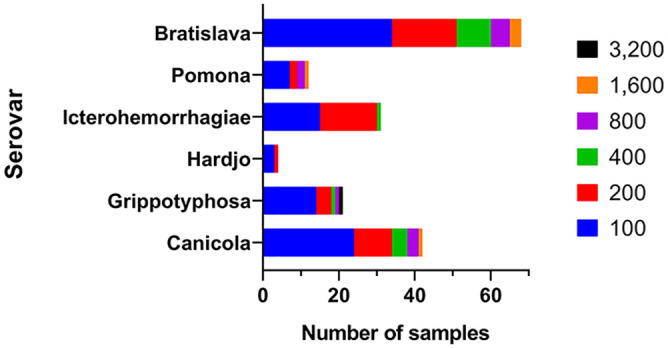
Control group sera (n = 124) Leptospira interrogans microscopic agglutination test reciprocal titers, by serovar (1 June 2015–30 September 2015); 63 of 124 (51%) had a titer to more than 1 serovar.
In samples from the control group, the serovar that yielded the highest seropositivity was Bratislava; 83 of 124 (67%) had a reciprocal MAT titer of ≥ 100 and 42 of 124 (34%) had a reciprocal MAT titer of ≥ 200 (Fig. 1). Given that horses are considered a maintenance host for this serovar, these data indicate that a high proportion of horses have been exposed to serovar Bratislava, perhaps in the absence of clinical illness. These data are consistent with previous findings.20 The Bratislava MAT had been performed on only 3 samples in our clinical cohort (Fig. 2).
Figure 2.
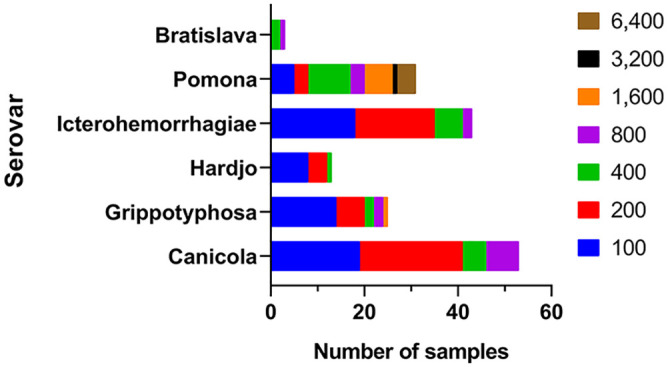
Clinical group sera Leptospira interrogans microscopic agglutination test reciprocal titers, by serovar (01 July 2010–26 January 2017; n = 160 for Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, and Pomona; n = 3 for Bratislava). Many samples (106 of 163, 65%) had a titer ≥ 100 to more than 1 serovar.
Serovars Pomona and Grippotyphosa have been associated with clinical illness in equids, with Pomona strain Kennewicki existing as the most prominent Leptospira equine pathogen in North America.7,13 It is therefore not surprising that the proportion of samples in the clinical group with reciprocal titers ≥ 1,600 to serovar Pomona (11 of 160, 7%) was markedly higher than that detected in sera from the control group (1 of 124, 1%; p = 0.015). No significant difference existed for serovar Grippotyphosa reciprocal titers ≥ 1,600 (p > 0.05). Evaluation of data for samples with a reciprocal titer ≥ 800 to any serovar yielded interesting patterns when comparing serovar to history of illness. Of the 14 animals in the clinical group with reciprocal MAT titers ≥ 800 against serovar Pomona, 13 (93%) had uveitis, ERU, or another ophthalmic ailment listed on the diagnostic laboratory submission form. The equids that had a reciprocal titer of ≥ 800 to serovars Bratislava and Icterohaemorrhagiae also had a high reciprocal titer to serovar Pomona (≥ 1,600), potentially indicating cross-reactivity in the MAT (Table 2).
Table 2.
Equid cases submitted to the Colorado State University, Veterinary Diagnostic Laboratory for Leptospira microscopic agglutination test with reciprocal titers of ≥ 800 stratified by animal history provided on the diagnostic laboratory submission form (n = 24).
| Abortion or pregnancy issue | Uveitis, ERU, or another ophthalmic ailment | Screening (history of Leptospira on property) | None listed | |
|---|---|---|---|---|
| Canicola | 3 | 1 | 1 | 2 |
| Grippotyphosa | 0 | 1 | 0 | 2 |
| Pomona | 0 | 13 | 0 | 1 |
| Bratislava | 0 | 1* | 0 | 0 |
| Icterohaemorrhagiae | 0 | 2* | 0 | 0 |
ERU = equine recurrent uveitis.
Animal(s) also had a titer ≥ 1:1,600 against serovar Pomona.
In examining the seroprevalence to any L. interrogans serovar between age groups using a reciprocal MAT titer cutoff of ≥ 200, there was a significant difference between the ≤ 4 y group and all following groups (p = 0.038, 0.009, 0.006, and 0.003, respectively; Table 3). Although there were no significant differences between any of the other age groups, it appeared that the proportion of seropositive animals increased with age group, also as noted in other studies.1,15
Table 3.
Percentage of equids seropositive at microscopic agglutination test reciprocal titer ≥ 200 stratified by age group (n = 123 because of missing age data on 1 equine infectious anemia submission form).
| Age group (y) | No. of sera tested | Horses seropositive at reciprocal titer ≥ 200 |
|---|---|---|
| ≤ 4 | 14 | 2 (14) |
| 5–7 | 25 | 13 (52) |
| 8–10 | 34 | 20 (49) |
| 11–14 | 23 | 17 (74) |
| 15+ | 27 | 18 (67) |
Numbers in parentheses are percentages.
Limitations of our study included a small sample size and opportunistic sampling, potentially resulting in sampling bias. In performing the MAT, results are subjective, and it is likely that cross-reactivity occurs between serovars. To ameliorate subjectivity in the analysis of MAT results, a single individual performed all MAT testing for our study. The nature in which our data were obtained did not allow for differentiation of acute and chronic infection, or temporality of exposure. The MAT cannot differentiate between IgM and IgG antibodies, and because of opportunistic sampling, our study was not designed to obtain acute and convalescent samples. Demographic data on clinically healthy horses were gathered through EIA submission forms, one of which was incomplete, resulting in a missing data point in equid age data. Although seldom reported anecdotally in the state of Colorado, off-label use of the canine or bovine leptospirosis vaccines in horses could have contributed to antibody presence in the absence of exposure to L. interrogans. Despite these limitations, our data provide valuable information on background prevalence of anti-Leptospira spp. antibodies in Colorado equids prior to introduction of the equine Leptospira serovar Pomona vaccine, and provide a comparison of serologic profiles between a presumed healthy cohort and clinical cases submitted to the CSU-VDL.
Acknowledgments
We thank Julia Veir and Michael Lappin for information on leptospirosis in Colorado canids, and Kara Maslyn for data organization and maintenance. We also thank Cynthia Hirota for performing MATs.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by the Colorado State University Center for Companion Animal Studies Young Investigator Grant.
ORCID iDs: Anna C. Fagre  https://orcid.org/0000-0002-0969-5078
https://orcid.org/0000-0002-0969-5078
Kristy L. Pabilonia  https://orcid.org/0000-0001-7741-8497
https://orcid.org/0000-0001-7741-8497
References
- 1. Båverud V, et al. Leptospira seroprevalence and associations between seropositivity, clinical disease and host factors in horses. Acta Vet Scand 2009;51:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boonsilp S, et al. Molecular detection and speciation of pathogenic Leptospira spp. in blood from patients with culture-negative leptospirosis. BMC Infect Dis 2011;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown PD, et al. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. J Med Microbiol 1995;43:110–114. [DOI] [PubMed] [Google Scholar]
- 4. Carter CN, et al. Seroepidemiology of equine leptospirosis utilizing diagnostic laboratory specimens from 29 states (US) and one Canadian province. Proc Am Assoc Vet Lab Diagn, Ann Meeting; Oct 2012; San Diego, CA. [Google Scholar]
- 5. Cumberland P, et al. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg 1999;61:731–734. [DOI] [PubMed] [Google Scholar]
- 6. Delph K, et al. Haemolytic anaemia and bilateral uveitis associated with leptospirosis in a 6-year-old Quarter Horse gelding. Equine Vet Educ 2018;30:132–136. [Google Scholar]
- 7. Divers TJ, et al. Leptospirosis: an important infectious disease in North American horses. Equine Vet J 2019;51:287–292. [DOI] [PubMed] [Google Scholar]
- 8. Donahue JM, et al. Diagnosis and prevalence of Leptospira infection in aborted and stillborn horses. J Vet Diagn Invest 1991;3:148–151. [DOI] [PubMed] [Google Scholar]
- 9. Erol E, et al. A diagnostic evaluation of real-time PCR, fluorescent antibody and microscopic agglutination tests in cases of equine leptospiral abortion. Equine Vet J 2015;47:171–174. [DOI] [PubMed] [Google Scholar]
- 10. Fish N, et al. Bacteriological and pathological studies of natural and experimental swine abortion due to Leptospira Pomona. Can Vet J 1963;4:317. [PMC free article] [PubMed] [Google Scholar]
- 11. Gerding JC, et al. Prognosis and impact of equine recurrent uveitis. Equine Vet J 2016;48:290–298. [DOI] [PubMed] [Google Scholar]
- 12. Haake DA, et al. Leptospirosis, water sports, and chemoprophylaxis. Clin Infect Dis 2002;34:e40–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartskeerl R, et al. Classification of Leptospira from the eyes of horses suffering from recurrent uveitis. J Vet Med B Infect Dis Vet Public Health 2004;51:110–115. [DOI] [PubMed] [Google Scholar]
- 14. Jansen A, et al. Leptospirosis in Germany, 1962-2003. Emerg Infect Dis 2005;11:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitson-Piggot A, et al. Leptospirosis in horses in Ontario. Can J Vet Res 1987;51:448. [PMC free article] [PubMed] [Google Scholar]
- 16. Martin LER, et al. Vaccine-associated Leptospira antibodies in client-owned dogs. J Vet Intern Med 2014;28:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mwachui MA, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis 2015;9:e0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park YG, et al. Factors for seropositivity to leptospirosis in horses. Prev Vet Med 1992;13:121–127. [Google Scholar]
- 19. Sykes J, et al. 2010. ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011;25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trimble AC, et al. Seroprevalence, frequency of leptospiuria, and associated risk factors in horses in Kansas, Missouri, and Nebraska from 2016-2017. PLoS One 2018;13:e0206639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan W, et al. Experimental Leptospira interrogans serovar Kennewicki infection of horses. J Vet Intern Med 2010;24:912–917. [DOI] [PubMed] [Google Scholar]


