Abstract
15-F2T-isoprostanes are byproducts of lipid peroxidation and were determined to be the best marker of oxidative injury in a rodent model of oxidative stress. A previous study compared methods for measurement of urinary F2-isoprostanes (gas chromatography and negative ion chemical ionization–mass spectrometry, GC-NICI-MS; and ELISA) and found poor agreement in dogs, horses, and cows. Surprisingly, fair agreement between these methods was identified in a small population of cats. We evaluated the agreement between GC-NICI-MS and ELISA of urinary F2-isoprostanes in the urine of 50 mature cats ranging from healthy to systemically ill. All urine samples had detectable levels of F2-isoprostanes by both methods. Significant proportional bias and poor agreement were identified between the 2 methods (ρ = 0.364, p = 0.009) for all cats, and in subgroup analysis based on health status. The concentration of urinary F2-isoprostanes was significantly lower in systemically ill cats compared to healthy cats when measured by ELISA (p = 0.002) but not by GC-NICI-MS (p = 0.068). Our results indicate that GC-NICI-MS and ELISA have poor agreement when measuring urinary F2-isoprostanes in cats.
Keywords: antioxidant, biomarkers, oxidative stress, reactive oxygen species
Introduction
Oxidative damage mediated by reactive oxygen species (ROS) has been implicated in a variety of disease states in animals and people. Through direct and indirect mechanisms, ROS cause injury to cellular structural components such as lipids and proteins, as well as to nuclear structures including DNA.8 Oxidative stress is challenging to document in vivo. Ideally, oxidative stress would be measured directly through quantification of ROS in cells and tissues, but there are significant limitations to the measurement of ROS including poor stability in tissues, short half-lives, and lack of specificity or sensitivity in traditional detection methods.7,9 In people, oxidative stress is primarily assessed through the concentration of endogenous small-molecule antioxidants, estimated by measuring the activity of antioxidant enzymes, and presumed through measuring by-products of oxidative damage.8,9 Studies have utilized these indirect measures to document oxidative stress in animals in different states of disease and health, but none have emerged as an ideal biomarker.10 In people, urinary measurement of isoprostanes is a favored approach to measurement of oxidative stress.16,19
Isoprostanes are by-products of the peroxidation of arachidonic acid. Isoprostanes are initially esterified to phospholipids, and then released as free isoprostanes by phospholipase A2.18 This peroxidation of arachidonic acid occurs independently from cyclooxygenase metabolism, and is catalyzed by the interaction of arachidonic acid with ROS.14 Although a variety of isoprostanes are generated from this interaction, the most extensively studied are the F2-isoprostanes, which are isomeric to prostaglandin-F2α. F2-isoprostanes can be measured in a number of tissues including urine, plasma, cerebrospinal fluid, and bronchoalveolar lavage fluid.12
In people, F2-isoprostanes have been evaluated in health and disease. Given that they are produced from oxidative damage to lipids, increased concentrations of F2-isoprostanes in tissue and urine have been shown to correlate with oxidative stress and with disease severity.16,19 However, unmetabolized F2-isoprostanes have been identified to increase with normal aging and through auto-oxidation in the kidneys.19 Studies using isoprostanes as biomarkers of disease primarily utilize measurement of F2-isoprostanes after hepatic glucuronidation.13,19 One of the major metabolites used in research is 15-F2T-isoprostane (also called 8-iso-PFG2α). 15-F2T-isoprostanes are highly stable compounds in all tissues, but especially in urine.11,13 Urinary measurement of 15-F2T-isoprostanes is favored because of minimally invasive collection and the fact that in vitro generation of isoprostanes is unlikely given that urine is a lipid-poor tissue in most species.12,13
The gold standard for measurement of urinary F2-isoprostanes in people is mass spectrometry (MS).9,11 Gas chromatography and negative ion chemical ionization–mass spectrometry (GC-NICI-MS) was one of the earliest developed methods for MS detection of these compounds.15 However, additional methods have been developed, including liquid chromatography–mass spectrometry (LC-MS) and gas chromatography–electronic impact mass spectrometry (GC-EI-MS).2,4,23 These mass spectrometric methods allow for measurement of samples in a research setting but their availability is limited. Immunoassays have been developed but less is known about the specificity of these assays. In people, measurement of F2-isoprostanes by commercial immunoassays demonstrated poor agreement with the GC-NICI-MS, GC-EI-MS, and gas chromatography–tandem mass spectrometry GC-MS/MS methods.2,5,17,21 In one study, it was speculated that the 2 methods (GC-EI-MS and ELISA) did not measure the same compounds.2 In a rodent model of oxidative stress, multi-laboratory validation of many indirect measures of oxidative stress including urinary F2-isoprostanes, hydroxyeicosatetraenoic acid (HETE), low-density lipoproteins (LDL), malondialdehyde (MDA), serum glutamic pyruvic transaminase (SGPT), and thiobarbituric acid reacting substances (TBARS) was performed; plasma free F2-isoprostanes and urinary 15-F2T-isoprostanes detected by GC-NICI-MS were determined to be the best measure of oxidative injury in vivo.19,20
Despite GC-NICI-MS being recommended for measuring F2-isoprostanes in people and rodents, the majority of studies measuring 15-F2T-isoprostanes in cats have utilized ELISAs, including those documenting oxidative stress in feline hyperthyroidism, chronic kidney disease, and obesity.3,7,25 To date, there is no consensus as to the best method for measuring F2-isoprostanes in cats or other companion animal species, and data comparing methods is scarce. A previous method comparison was performed in domestic species, comparing the measurement of urinary F2-isoprostanes by GC-NICI-MS and 2 commercial ELISA kits in dogs, cats, horses, and cows.22 Poor agreement between the 2 immunoassays and the GC-NICI-MS methods was identified in dogs, horses, and cows, resembling the comparisons performed in people. However, in cats, fair agreement was identified between one of the immunoassays and the GC-NICI-MS methods. With these findings, it was recommended that GC-NICI-MS methods be used for urinary F2-isoprostane measurements in the other domestic species, but that feline urinary isoprostanes may be assessed by ELISA.22 Given the small sample size and findings in other species, this conclusion warranted further investigation.
We investigated the agreement between 2 methodologies, GC-NICI-MS and a commercial ELISA kit, in a larger, mixed population of adult cats ranging from healthy to systemically ill. We hypothesized that there would be significant bias and poor correlation between the 2 methods for measurement of urinary F2-isoprostanes in cats.
Materials and methods
Cats
Skeletally mature cats were recruited from students, staff, and clinical patients of the Purdue University Veterinary Teaching Hospital (PUVTH; West Lafayette, IN). Previous studies have suggested at least 40 samples for a method comparison and that samples should represent the spectrum of diseases expected in routine application of the methods.6 Cats were classified as being either healthy or systemically ill (sick), with the intent to include cats representing a spectrum of disease states. Cats were classified as healthy based on physical examination, complete blood count (CBC), serum biochemistry profile, and urinalysis. Cats classified as systemically ill were recruited from the active patient population of the PUVTH, so all were documented to be exhibiting clinical signs. Any cat that had been presented for an illness-related complaint and that had a CBC, serum biochemistry profile, and urinalysis was eligible for enrollment. Once study enrollment began, the investigator contacted owners of cats meeting the above criteria to inquire about consent. Client consent was obtained for all cats, and the study protocol was approved by the Institutional Animal Care and Use Committee (Protocol 1610001489). Enrollment ended once adequate samples were met.
Systemic illness was classified based on a scoring system generated for our study to allow for classification of mild, moderate, or severe systemic disease for the purpose of meaningful statistical analysis. For this systemic illness score, one point each was attributed to: fever on presentation (≥ 39.4°C [> 103.0°F]), neutrophilia (> 12.0 × 109/L [> 12.0 × 103/µL]), presence of band neutrophils on blood smear examination, biochemical evidence of organ involvement or dysfunction (creatinine > 142 µmol/L; alanine aminotransferase (ALT) > 138 U/L; alkaline phosphatase (ALP) > 157 U/L), and anemia (hematocrit < 0.30 L/L [< 30%]). Systemic illness scores were as follows: 0–1 = mild systemic illness, 2–3 = moderate systemic illness, 4–5 = severe systemic illness.
Sample collection
In all healthy cats, urine was collected at home, noninvasively by the owners from a clean, disposable litter pan (Kitty Lounge; Argee) containing non-absorbable cat litter (No-Sorb; Catco Vet Products). Cats were monitored closely so that urine could be collected shortly after it was voided (within 30 min). Once collected, urine was placed into two 2.0-mL cryovials, and these were placed in home freezers (standard temperatures –15°C to −17°C). Remaining urine was capped in the collection syringe and stored at 4°C. Owners transferred the urine specimens to the investigator within 12 h of collection. All refrigerated urine specimens collected from healthy cats were evaluated, including a dipstick examination (Siemens Healthcare Diagnostics) and microscopic evaluation of the sediment, by the same clinical pathologist (A. Leisering) to confirm a normal urinalysis. The urine contained in cryovials were transferred to a −80°C freezer to be used for isoprostane measurement.
In all systemically ill cats, urine specimens were collected by cystocentesis as part of the routine examination and biochemistry assessment and thus submitted to the institutional clinical pathology laboratory for evaluation, including a chemical examination (Siemens Healthcare Diagnostics) and microscopic evaluation of the sediment. After analysis, urine samples were stored in the laboratory at −20°C. Urine samples were collected from the clinical pathology laboratory after completion of the urinalysis and always within 12 h after collection, and then transferred to −80°C until isoprostane measurement.
For the measurement of isoprostanes, one aliquot was shipped frozen to the Eicosanoid Core Laboratory at the Vanderbilt University Medical Center (Nashville, TN) for 15-F2T-isoprostane determination by mass spectrometry, and one was used for F2-isoprostane measurement by ELISA (Enzyme immunoassay for urinary 8-isoprostane-PFG2α; Oxford Biomedical Research). All specimens were analyzed within 120 d of collection and storage. Urine creatinine for normalization of the isoprostane concentrations was measured by the Jaffe reaction using a commercial chemistry analyzer (COBAS Integra 800; Hoffman-La Roche). This value was used to normalize the isoprostane concentrations from both methods.
Measurement of F2-isoprostanes
GC-MS
GC-NICI-MS quantification of 15-F2T-isoprostanes was performed at the Eicosanoid Core Laboratory according to their previously published method.11 Briefly, a stable isotope dilution method was used, in which the F2-isoprostanes were measured against several internal standards for quantification. Isoprostanes were analyzed after conversion to pentafluorobenzyl ester–trimethylsilyl ether derivatives. The precision and accuracy of this test are + 6% and 96%, respectively.11 The lower limit of sensitivity is ~ 20 pg.11 Results are reported as nmol isoprostane/mmol creatinine.
ELISA
ELISA quantification of isoprostanes was performed using a commercial kit (Enzyme immunoassay for urinary 8-isoprostane-PFG2α). Samples were pretreated with glucuronidase to allow for identification of free isoprostanes. Samples were diluted as per the manufacturer’s instructions, and samples and standards were added to the well in which F2-isoprostanes bind to the polyclonal antibody on the plate. Horseradish peroxidase is outcompeted for this binding and creates a colorimetric change for which 3N sulfuric acid (Sigma Aldrich) was added to stop this development. Absorption at 450 nm was measured using a microplate reader (Synergy HT; Biotek) in which the absorbance is inversely proportional to the concentration of isoprostane in the sample. Concentrations were then calculated from the optimal portion of the standard curve. Isoprostane measurement was conducted in duplicate according to instructions in the kit, and the average of the 2 values was used for statistical analysis.
Statistical analysis
The data were analyzed for normality using the Shapiro–Wilk test. Correlation analysis were performed using the Spearman rank correlation test. Agreement between methods was assessed using Passing–Bablok regression analysis, which is most appropriate for this comparison because a true gold standard does not exist, and this type of regression assumes some imprecision in both methods. Bland–Altman plots were also constructed to depict the agreement between methods. Fixed bias was determined if the 95% CI for the intercept did not include 0. Proportional bias was determined if the 95% CI for the slope did not include 1. Measurements were compared with all cats, healthy and sick, as one group, as well as separately. In addition to agreement, the concentrations of urinary F2-isoprostanes were compared between the healthy and sick cats, and between groups of sick cats of different severities, by both methods, using the Mann–Whitney U-test. Spearman-rank correlation coefficients were calculated between the age of cats and the concentrations of urinary F2-isoprostanes. Correlation was graded by the following: 0.0–0.3 = no agreement, 0.3–0.5 = poor agreement, 0.5–0.7 = fair agreement, 0.7–0.9 = strong agreement, 0.9–1.0 = very strong agreement. Values of p ≤ 0.05 were considered significant. Statistics were performed using commercial software (MedCalc Software).
Results
Cats
We enrolled 50 cats in our study, with a mean (SD) age of 8.1 (± 5.2) y. Twenty-five cats were classified as healthy, with a mean (SD) age of 5.2 (± 4.2) y; 18 were castrated males, and 7 were spayed females. Breeds included 15 domestic shorthair cats, 3 domestic medium-hair cats, 2 Abyssinians, 2 mixed-breed cats, and 1 each of Maine Coon, Persian, and domestic longhair cats. Systemically ill cats (n = 25) had a mean (SD) age of 10.9 (± 4.6) y, and were significantly older than the healthy cats (p < 0.001); 15 were spayed females, 9 were castrated males, and 1 was an intact male (Table 1). Breeds included 16 domestic shorthair cats, 4 domestic longhair cats, 2 domestic medium-hair cats, and 1 each of Maine Coon, Siamese, and mixed-breed cats. Diagnoses in the systemically ill cats included chronic kidney disease (n = 5), hyperthyroidism (n = 5), enteritis (multiple causes; n = 4), pancreatitis (n = 2), diabetic ketoacidosis (n = 2), and single, other diagnoses in the remaining 8 cats (Table 1). Systemic illness scores for the sick cats included a diagnosis of mild disease in 12 cats (score 0, n = 5; score 1, n = 7), moderate disease in 12 cats (score 2, n = 10; score 3, n = 2), and severe disease in 1 cat (score 4; Table 1).
Table 1.
Clinical information from 25 systemically ill cats used to study urinary F2-isoprostanes.
| Cat | Temp (37.2–39.4°C) |
Hct (0.37–0.55 L/L) |
SegNeut (3.0–12.0 × 109/L) |
BandNeut (0.0 × 109/L) |
Urea (2.5–11.4 mmol/L) |
Creatinine (44–142 µmol/L) |
ALT (3–69 U/L) |
ALP (20–157 U/L) |
Illness score | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.8 | 0.32 | 16.8 | 0 | 3.2 | 88 | 43 | < 20 | 2 | Pancreatitis |
| 2 | 38.3 | 0.37 | 12.6 | 0 | 4.6 | 97 | 1,220 | 101 | 2 | LP hepatitis |
| 3 | 38.6 | 0.32 | 11.7 | 0 | 6.8 | 106 | 38 | 45 | 0 | IBD |
| 4 | 39.2 | 0.31 | 12.5 | 0 | 10 | 88 | 311 | 97 | 2 | Hyperthyroidism |
| 5 | 38.8 | 0.33 | 5.4 | 0 | 10.7 | 142 | 73 | 31 | 1 | CKD Stage 2 |
| 6 | 37.7 | 0.39 | 18.6 | 0.2 | 8.6 | 124 | 40 | 26 | 2 | LP rhinitis |
| 7 | 40.4 | 0.37 | 19.4 | 0.6 | 6.8 | 88 | 38 | < 20 | 3 | Pyothorax |
| 8 | 38.0 | 0.36 | 4.7 | 0 | 7.1 | 62 | 93 | 42 | 0 | Hyperthyroidism |
| 9 | 38.9 | 0.27 | 2.8 | 0 | 5 | 115 | 62 | 57 | 1 | Bacterial pneumonia |
| 10 | 38.5 | 0.35 | 6.1 | 0 | 15.3 | 186 | 76 | 89 | 2 | DKA |
| 11 | 39.3 | 0.39 | 8.4 | 0 | 17.5 | 124 | 119 | 100 | 1 | Urethral obstruction |
| 12 | 38.4 | 0.31 | 6.8 | 0 | 17.5 | 221 | 42 | 32 | 1 | CKD stage 2 |
| 13 | 37.8 | 0.39 | 7.4 | 0 | 16.8 | 159 | 127 | 98 | 1 | Hyperthyroidism |
| 14 | 37.6 | 0.48 | 11.1 | 0 | 10 | 106 | 47 | 67 | 0 | Chronic vomiting |
| 15 | 38.9 | 0.31 | 5.8 | 0 | 9.6 | 115 | 47 | 41 | 0 | Ascites |
| 16 | 38.3 | 0.48 | 12.4 | 0 | 16.1 | 124 | 138 | 54 | 2 | Renal lymphoma |
| 17 | 39.5 | 0.08 | 7.9 | 0.4 | 11.4 | 80 | 62 | < 20 | 3 | IMHA |
| 18 | 40.4 | 0.36 | 20.6 | 0.5 | 15.7 | 168 | 28 | 76 | 4 | Duodenal perforation |
| 19 | 37.7 | 0.43 | 11.8 | 0 | 7.5 | 115 | 46 | 80 | 0 | IBD |
| 20 | 37.8 | 0.28 | 17.3 | 0 | 5 | 44 | 24 | 25 | 1 | IBD |
| 21 | 38.8 | 0.37 | 12.1 | 0 | 3.9 | 53 | 210 | 121 | 2 | Hyperthyroidism |
| 22 | 38.9 | 0.32 | 12.5 | 0 | 13.2 | 203 | 88 | 97 | 2 | CKD stage 2 |
| 23 | 38.2 | 0.27 | 6.1 | 0 | 11.8 | 194 | 86 | 53 | 2 | Pancreatic carcinoma |
| 24 | 37.8 | 0.29 | 6.3 | 0 | 17.1 | 2 | 71 | 31 | 2 | CKD stage 2 |
| 25 | 37.7 | 0.35 | 6.1 | 0 | 15.4 | 2 | 76 | 40 | 1 | CKD stage 2 |
Numbers in parentheses are reference intervals. ALP = alkaline phosphatase; ALT = alanine aminotransferase; BandNeut = band neutrophils; CKD = chronic kidney disease; DKA = diabetic ketoacidosis; Hct = hematocrit; IBD = inflammatory bowel disease; IMHA = immune-mediated hemolytic anemia; LP = lymphoplasmacytic; RI = reference interval; SegNeut = segmented neutrophil. Values in bold met the criteria to contribute to the systemic illness score utilized in our study. One point each was attributed to: fever on presentation (≥ 39.4°C or 103.0°F), neutrophilia (> 12.0 × 109/L), presence of band neutrophils on blood smear analysis, biochemical evidence of organ involvement and/or dysfunction (creatinine > 142 µmol/L; ALT > 138 U/L; ALP > 157 U/L), and anemia (hematocrit < 0.30 L/L). Systemic illness scores were as follows: 0–1 = mild systemic illness, 2–3 = moderate systemic illness, 4–5 = severe systemic illness.
Measurement of F2-isoprostanes by GC-NICI-MS and ELISA
All urine samples contained quantifiable levels of F2-isoprostanes by both methods, and data were not found to be normally distributed. Passing–Bablok regression showed poor agreement based on the assessment of bias between the 2 methods when comparing all cats. The methods demonstrated a nonlinear relationship (Fig. 1) and proportional bias, but fixed bias was not identified (Table 2). Poor agreement was identified when the subgroup analysis compared the healthy and the systemically ill cats separately. The comparison of all cats in a Bland–Altman plot demonstrates the nonlinear relationship, and proportional bias wherein the difference between the 2 methods gets wider as the concentration of urinary F2-isoprostanes increases (Fig. 2). Spearman rank correlation coefficient showed significant, weak-positive correlation in the comparison of all cats (ρ = 0.36, p = 0.009) and supported poor agreement. In subgroup analysis, correlation coefficients were similar but did not reach significance (Table 2).
Figure 1.
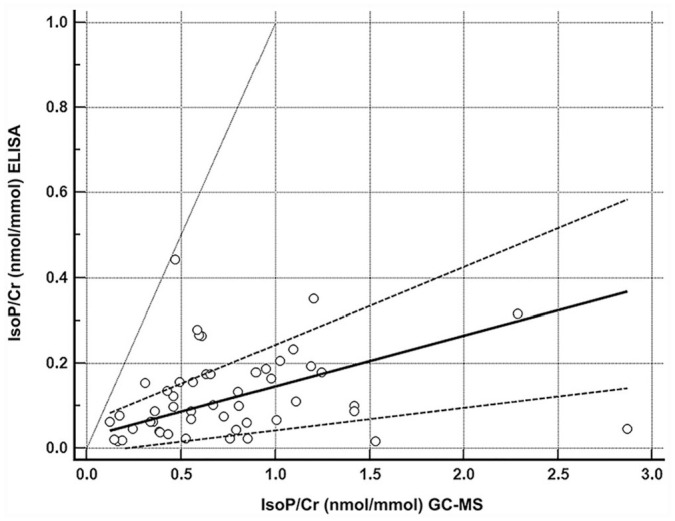
Passing–Bablok regression depicting the comparison of means to differences between the 2 methods (ELISA and gas chromatography–mass spectrometry, GC-MS) of urinary F2-isoprostane measurement normalized to creatinine (IsoP/Cr) in cats. The open circles represent the 50 cats. The solid line is the regression line, with the dashed lines representing the 95% CI of this regression. The gray line indicates the identity line (x = y), which would indicate perfect agreement between the 2 methods.
Table 2.
Passing–Bablok regression and Spearman-rank correlation coefficients for agreement between GC-NICI-MS and ELISA measurements of urinary F2-isoprostanes in 50 sick and healthy cats.
| Comparison | Fixed bias/intercept* | Proportional bias/slope† | Correlation coefficient |
|---|---|---|---|
| GC-NICI-MS versus ELISA | |||
| All cats | 0.09 (–0.04 to 0.2) | 0.12 (0.05–0.18) | 0.36 ( p = 0.009) |
| Healthy cats | 0.22 (–0.04 to 0.46) | 0.09 (0.02–0.18) | 0.37 ( p = 0.07) |
| Systemically ill cats | 0.1 (–0.07 to 0.2) | 0.06 (0–0.23) | 0.23 ( p = 0.27) |
GC-NICI-MS = gas chromatography and negative ion chemical ionization–mass spectrometry.
Fixed bias was not identified in any comparison, as the 95% CI (in parentheses) for all intercepts includes 0.
Proportional bias was present in all comparisons given that the 95% CI (in parentheses) for all slopes did not include 1. Values of p ≤ 0.05 are considered significant.
Figure 2.
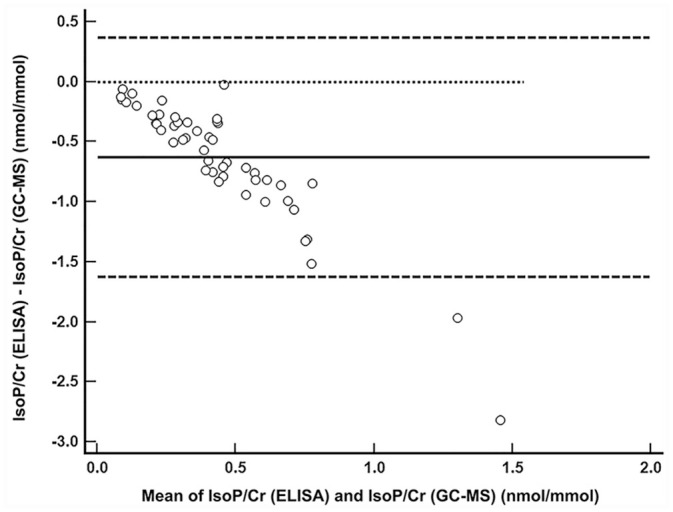
Bland–Altman plot depicting the comparison of means to differences between 2 methods (ELISA and gas chromatography–mass spectrometry, GC-MS) of urinary F2-isoprostane measurement normalized to creatinine (IsoP/Cr) in cats. The open circles represent the 50 cats. The solid line represents the mean difference; the dashed lines represent the 95% CI. The dotted line would indicate perfect agreement between the 2 methods. Proportional bias can be seen: as the mean of the 2 methods increases, the difference between the 2 methods becomes greater, and this difference is nonlinear.
The concentration of urinary F2-isoprostanes was significantly higher when measured by GC-NICI-MS compared to ELISA in all samples (median [range]; 0.62 nmol/mmol [0.12–2.87] vs. 0.099 nmol/mmol [0.016–0.44]; p < 0.001; Fig. 3). The concentrations of urinary F2-isoprostanes measured by ELISA were significantly higher in healthy cats when compared to systemically ill cats (0.16 nmol/mmol [0.04-0.32] vs. 0.062 nmol/mmol [0.016–0.44]; p = 0.002), whereas no significant difference was found when measured by GC-NICI-MS (0.73 nmol/mmol [0.31–2.28] vs. 0.47 nmol/mmol [0.12–2.87]; p = 0.068; Fig. 4).
Figure 3.
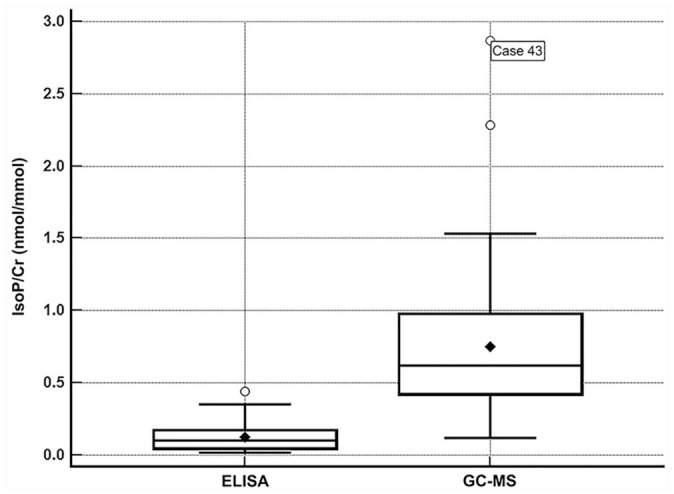
Box-and-whisker plot depicting the concentrations of urinary isoprostanes normalized to creatinine (IsoP/Cr) detected in 50 cats, with a comparison between methods (ELISA and gas chromatography–mass spectrometry, GC-MS). The height of the box represents the interquartile range, and the horizontal line inside the box represents the median; the closed diamond represents the mean. The whiskers represent the range; outliers are represented by open circles. Isoprostanes were significantly higher when measured by the GC-MS method compared to ELISA (p < 0.0001).
Figure 4.
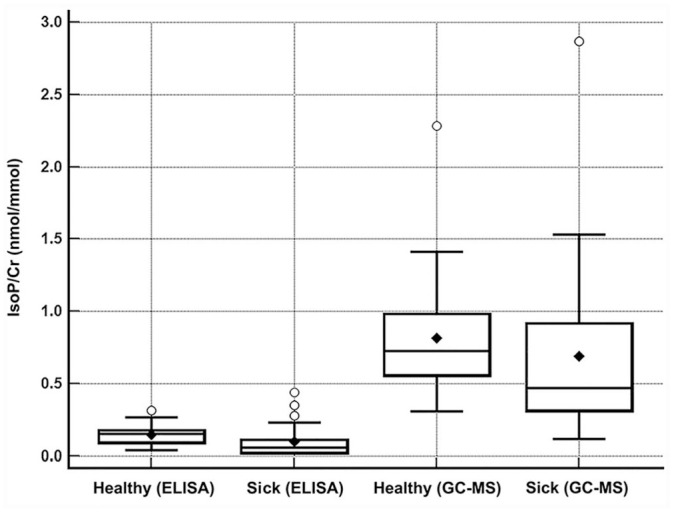
Box-and-whisker plot depicting the concentrations of urinary F2-isoprostanes normalized to creatinine (IsoP/Cr) detected in 50 cats, comparing health status and methods (ELISA and gas chromatography–mass spectrometry, GC/MS). The concentration of F2-isoprostanes in sick cats, measured by ELISA, was significantly lower than in healthy cats (p = 0.0002); there was no difference when measured by GC-MS (p = 0.068).
The concentrations of urinary F2-isoprostanes measured by ELISA (p = 0.48) or GC-NICI-MS (p = 0.30) were not significantly different in the sick cats when comparing the different severities of systemic illness. Cats with chronic kidney disease (n = 5) had significantly lower concentrations of urinary F2-isoprostanes compared to healthy cats when measured by ELISA (0.02 nmol/mmol [0.017–0.0.063] vs. 0.16 nmol/mmol [0.04–0.32]; p = 0.001) and GC-NICI-MS (0.19 nmol/mmol [0.12–1.53] vs. 0.73 nmol/mmol [0.31–2.28]; p = 0.002). No significant difference was found when comparing concentrations of urinary F2-isoprostanes in cats with chronic kidney disease to the remaining sick cats (ELISA, p = 0.23; GC-NICI-MS, p = 0.41). Correlation between age and concentration of urinary F2-isoprostanes was evaluated, and no significant correlation was identified with either method. When measured by ELISA, there was a weak-negative correlation between age and concentration of urinary F2-isoprostanes (ρ = −0.40, p = 0.14). When measured by GC-NICI-MS, there was a weak-positive correlation between age and concentration of urinary F2-isoprostanes (ρ = 0.24, p = 0.24).
Discussion
The 2 methods (GC-NICI-MS and ELISA) for quantification of feline urinary F2-isoprostanes had overall poor agreement in our study, which is in line with what has been shown in other species, including rats, dogs, cows, horses, and people.17,19,22 This finding is different than the fair agreement between these 2 methods that was identified in feline urine in a previous study.22 Our study overcame the major limitation of the original study through using a larger sample size. The Passing–Bablok regression was able to identify the significant proportional bias that is seen in comparison of these methods in other species.17,19,22
Historically, GC-MS has been a key method for our understanding of the biology and pharmacology of eicosanoids and their isoforms and metabolites.15,23 Given that the measurement of F2-isoprostanes does not have a clear clinical indication at this time, mass spectrometry has only been challenged in its performance by a small number of studies already discussed. Although the ELISA is an appealing assay given its availability and ease of use, it has wide variability and poor specificity that make its results unreliable or uninterpretable. In our study, the differences between the F2-isoprostane concentrations detected by the 2 methods was significant, and this difference widened as concentrations increased.
The assessment of bias aids in the strength of our study given that the majority of past research has utilized correlation as a primary means of method comparison. Given a difference in detection methods, simply assessing correlation does not allow comprehensive evaluation of agreement, whereas inclusion of a bias assessment allows a more in-depth analysis of agreement. Methods that, when compared, demonstrate a fixed bias may allow for application of an algorithm to the methods to account for this fixed difference, and thus, allows for some indirect agreement. However, the presence of proportional bias in method comparison means that the difference between method results becomes more disparate as values increase. This makes assessment challenging, especially when evaluating biomarkers with which higher values would be indicative of disease states.
In the previous study that measured F2-isoprostanes by these 2 methods in domestic species, it was recommended that the GC-NICI-MS method be used for F2-isoprostane measurement.22 Although the results of our study are in line with what was found in the other species (dog, horse, cow), it is difficult to recommend either method for cats without a better understanding of the eicosanoid and isoprostane behavior in feline urine compared to people. To our knowledge, ours is the largest study to utilize the GC-NICI-MS method in cats. However, there were not enough samples to establish a reference interval, and little is known about the potential for ex vivo production of isoprostanes or isoprostane-like compounds in feline urine. In people, there is significant ex vivo production of eicosanoids, especially leukotrienes, in serum and plasma.1,23 However, there is negligible ex vivo production of eicosanoids in urine, making it an appealing sample for isoprostane analysis.14 Cats have a higher lipid content in their urine than do humans, thus more research would be necessary to assess the potential for ex vivo production of eicosanoids that could lead to artifactual increases in F2-isoprostane concentrations.14
A limitation of our study is that collection and storage of urine samples was not standardized. We utilized collection and storage recommendations as used in people, given that most urine samples collected in people are voided, and although the urine collection method has not been specifically investigated, isoprostane research in people has included urine collected by multiple methods.4,19 The non-absorbable litter used in our study is chemically inert and unlikely to affect isoprostane concentrations, but this was not directly evaluated. Despite using recommended methods, our healthy and sick cat samples were not collected using a standardized protocol, meaning the potential for ex vivo metabolism is inconsistent between sample types.
The clinical utility of urinary F2-isoprostanes remains unknown in cats. We expected that sick cats would have higher isoprostane concentrations compared to healthy cats, based on the presence of systemic illness. However, this was not true of the cats in our study. The F2-isoprostane concentrations measured by ELISA were significantly higher in the healthy cats. Although the difference was not significant when measured by GC-NICI-MS, the healthy cats still had higher values, generally, compared to the systemically ill cats. This may indicate that urine is not the appropriate sample, that the 15-F2T-isoprostanes are not a useful marker of oxidative stress in cats, or that neither of the 2 methods that we used is the most appropriate for measuring F2-isoprostanes in cats. Cats have higher susceptibility to oxidative stress because their antioxidant defenses are limited, especially in the liver and erythrocytes.24 It is expected that isoprostane metabolites (i.e., 15-F2T-isoprostane) should increase with oxidative stress. One could reason that the assays being tested may not adequately detect these isoprostanes and may detect unmetabolized or other prostaglandin-like compounds. If this were true, the difference between healthy and sick cats would still be a concern because unmetabolized isoprostanes would be expected to increase with age, and the sick cats were significantly older than the healthy cats. Additionally, validation of the GC-NICI-MS assay has not been performed in cats, nor have reference intervals been established. As more is learned about the use of this assay in cats, future studies should target individual diseases to allow for a more thorough investigation of oxidative stress.
We included cats with a wide variety of systemic disease, many of which have been speculated as causes of oxidative stress, including chronic kidney disease and hyperthyroidism.3,25 A previous assessment of cats with chronic kidney disease (CKD) evaluated urinary 15-F2T-isoprostanes measured by ELISA and showed that F2-isoprostanes were marginally increased in cats with stage 1 CKD compared to healthy cats, and significantly decreased with each subsequent stage.25 F2-isoprostane concentrations were inversely correlated with creatinine concentration.25 Similar findings were seen in our study, in which cats with CKD, all in stage 2, had significantly lower urinary 15-F2T-isoprostane concentrations compared to healthy cats when measured by both methods. However, conclusions cannot be drawn from these findings because there were only 5 CKD cats in our study. The previous study speculated that an increase in isoprostanes earlier in CKD may indicate that oxidative stress is highest in that stage.25 Given the inverse correlation with creatinine, and the fact that urinary 15-F2T-isoprostanes represent hepatic-produced metabolites that undergo renal excretion, it possible that nephron loss and changes with glomerular filtration rate in advancing stages of CKD will impact urinary concentrations. Further research is necessary to explore the role of renal disease in urinary F2-isoprostane concentrations.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded by departmental startup funding for A. D. Woolcock.
ORCID iDs: Andrew D. Woolcock  https://orcid.org/0000-0001-7102-3224
https://orcid.org/0000-0001-7102-3224
Pierre Deshuillers  https://orcid.org/0000-0002-2019-1663
https://orcid.org/0000-0002-2019-1663
References
- 1. Awad JA, et al. Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J Biol Chem 1993;268:4161–4169. [PubMed] [Google Scholar]
- 2. Bessard J, et al. Determination of isoprostaglandin F2alpha type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J Chromatogr B Biomed Sci Appl 2001;754:333–343. [DOI] [PubMed] [Google Scholar]
- 3. Branter E, et al. Antioxidant status in hyperthyroid cats before and after radioiodine treatment. J Vet Intern Med 2012;26:582–588. [DOI] [PubMed] [Google Scholar]
- 4. Goettel M, et al. Analysis of urinary eicosanoids by LC-MS/MS reveals alterations in the metabolic profile after smoking cessation. Chem Res Toxicol 2018;31:176–182. [DOI] [PubMed] [Google Scholar]
- 5. Il’yasova D, et al. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15- F2t-isoprostane. Ann Epidemiol 2004;14:793–797. [DOI] [PubMed] [Google Scholar]
- 6. Jensen AL, Kjelgaard-Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol 2006;35:276–286. [DOI] [PubMed] [Google Scholar]
- 7. Jeusette I, et al. Effects of consuming diets containing various fats or citrus flavanones on plasma lipid and urinary F2-isoprostane concentrations in overweight cats. Am J Vet Res 2010;71:1039–1044. [DOI] [PubMed] [Google Scholar]
- 8. Kadiiska MB, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biol Med 2005;38:698–710. [DOI] [PubMed] [Google Scholar]
- 9. Kadiiska MB, et al. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl(4) poisoning? Free Radical Biol Med 2000;28:838–845. [DOI] [PubMed] [Google Scholar]
- 10. McMichael MA. Oxidative stress, antioxidants, and assessment of oxidative stress in dogs and cats. J Am Vet Med Assoc 2007;231:714–720. [DOI] [PubMed] [Google Scholar]
- 11. Milne GL, et al. Measurement of F2-isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radical Biol Med 2013;59:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milne GL, et al. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers 2005;10(Suppl 1):S10–S23. [DOI] [PubMed] [Google Scholar]
- 13. Milne GL, et al. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Meth Enzymol 2007;433:113–126. [DOI] [PubMed] [Google Scholar]
- 14. Morrow JD, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest 1992;90:2502–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morrow JD, Roberts LJ. Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Meth Enzymol 1994;233:163–174. [DOI] [PubMed] [Google Scholar]
- 16. Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 1997;36:1–21. [DOI] [PubMed] [Google Scholar]
- 17. Proudfoot J, et al. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation—a comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal Biochem 1999;272:209–215. [DOI] [PubMed] [Google Scholar]
- 18. Roberts LJ, Morrow JD. Isoprostanes. Novel markers of endogenous lipid peroxidation and potential mediators of oxidant injury. Ann N Y Acad Sci 1994;744:237–242. [DOI] [PubMed] [Google Scholar]
- 19. Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radical Biol Med 2000;28:505–513. [DOI] [PubMed] [Google Scholar]
- 20. Roberts LJ, Reckelhoff JF. Measurement of F(2)-isoprostanes unveils profound oxidative stress in aged rats. Biochem Biophys Res Commun 2001;287:254–256. [DOI] [PubMed] [Google Scholar]
- 21. Sasaki DM, et al. Enzyme immunoassays for 15- F2T isoprostane-M, an urinary biomarker for oxidant stress. Adv Exp Med Biol 2002;507:537–541. [DOI] [PubMed] [Google Scholar]
- 22. Soffler C, et al. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation: a comparison of enzyme immunoassays with gas chromatography-mass spectrometry in domestic animal species. J Vet Diagn Invest 2010;22:200–209. [DOI] [PubMed] [Google Scholar]
- 23. Tsikas D, Zoerner AA. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J Chromatogr B Analyt Technol Biomed Life Sci 2014;964:79–88. [DOI] [PubMed] [Google Scholar]
- 24. Webb C, et al. Use of flow cytometry and monochlorobimane to quantitate intracellular glutathione concentrations in feline leukocytes. Vet Immunol Immunopathol 2006;112:129–140. [DOI] [PubMed] [Google Scholar]
- 25. Whitehouse W, et al. Urinary F2-isoprostanes in cats with international renal interest society stage 1–4 chronic kidney disease. J Vet Intern Med 2017;31:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]


