Abstract
Twenty-eight lactating dairy cattle in New York State were exposed to botulism toxin; 12 died and 16 recovered but never returned to full productivity. Pieces of a raccoon carcass were found in the total mixed ration on the first day of the outbreak. Clinical signs included anorexia, decreased milk production, decreased tongue tone, profound weakness, and recumbency. Clostridium botulinum type A (BoNT/A) was detected in rumen contents from 2 deceased cows via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). In addition, C. botulinum type C was cultured from the liver of a third cow, and C. botulinum neurotoxin-producing type C gene (bont/C) was detected via real-time PCR. On postmortem examination, 4 cows had findings suggestive of toxic myopathy, but the cause and significance of these lesions is unknown given that botulism is typically not associated with gross or histologic lesions. This outbreak of BoNT/A in cattle in North America was diagnosed via MALDI-TOF MS, a rapid and sensitive modality for detection of botulinum preformed neurotoxin.
Keywords: botulism, cattle, Clostridium botulinum type A, matrix-assisted laser desorption/ionization mass spectrometry, neurotoxins
Clostridium botulinum is a gram-positive, rod-shaped, anaerobic bacterium.11 The bacteria form spores in soil and decaying organic material, such as carcasses, poultry litter, and improperly ensiled forages stored under anaerobic conditions.12 The spores, which can be stable for years in the environment,3 can release potent neurotoxins. There are 7 botulinum neurotoxins that can cause botulism in humans and animals: A–G (BoNT/A–G).12 Once in the host, the neurotoxin binds to motor neurons and blocks the release of acetylcholine from the presynaptic membrane of the neuromuscular junction.3 This results in flaccid paralysis of the affected animal, and if enough toxin is present, death from paralysis of the diaphragm.11,12 The U.S Department of Agriculture has designated botulism neurotoxins as federal select agents, tier one, given their “potential to pose a severe threat to both human and animal health” (https://www.selectagents.gov/SelectAgentsandToxinsList.html).
On November 10, 2018, a tie-stall dairy herd in New York State (NYS) with 62 lactating and 12 nonlactating cows experienced a 50% drop in feed intake among the lactating cattle. By the next day, 1 lactating cow was recumbent. Two other cows were weak and exhibiting abdominal effort during respiration. On November 12, the 2 weak cattle progressed to recumbency, and herd milk production dropped by ~ 20%. Over the next 5 d, 28 cattle showed clinical signs of weakness starting in the hind legs and progressing to recumbency, anorexia, abdominal effort during respiration, dry manure, decreased rumen contractility, and a dramatic decrease in milk production. There was slow retraction of the tongues of affected cattle, and many would repeatedly attempt to rise on the hind legs until they were exhausted, and then remain in sternal recumbency. The cattle appeared alert, with normal ear carriage and normal tail tone. Total herd milk production was 50% lower than average, based on bulk tank weights from the milk hauler on day 5 of the outbreak. By November 17, 12 cattle had died, and the remaining 16 showed profound weakness. In some cases, disease progression was rapid; cattle developed clinical signs and died or were euthanized given prolonged recumbency for 36 h in a tie-stall barn. This outbreak had a 45% morbidity rate and a 19% mortality rate.
The day after the lactating cattle became anorexic, the veterinarian found a piece of raccoon carcass, identified by the attached tail, in the total mixed ration (TMR) in the manger in front of a mid-lactation cow. The farmer stated that the TMR batch containing the carcass had only been available for consumption for the past 24 h, given that one bale of hay was used to make a daily supply of feed for the lactating herd. Based on the severe state of decomposition, the raccoon carcass was presumably mowed in the first cutting of grass hay harvested earlier that year. The hay was stored in single round bales and covered with commercial plastic bags to promote fermentation. No spoilage was detected in the hay when opened. It is possible that other round bales from that field contained pieces of raccoon carcass, and previous feeding of the carcass to the cattle had occurred but was not noticed by the farmer. The farmer noted that there was more jimson weed in the hay fields that growing season compared to previous years. The TMR diet included grass haylage, corn silage, grain, cottonseed, and the ionophore monensin (Rumensin; Elanco) in a commercial pellet. After finding the raccoon carcass, the remaining TMR and first cutting haylage were discarded. Only lactating cows fed the TMR diet developed clinical signs. Nonlactating cows housed in the other end of the tie-stall barn, but on a different ration, remained asymptomatic. Young stock on the premises were also unaffected.
Based on the history, clinical signs, and number of animals affected simultaneously, a differential diagnoses list included botulism, ionophore toxicity, organophosphate poisoning, mycotoxin toxicity, nitrate poisoning, cyanide poisoning, vitamin E–selenium deficiency (white muscle disease or nutritional myodegeneration), toxic plant ingestion (coffee senna [Cassia occidentalis] seeds, gossypol [Gossypium], jimson weed [Datura stramonium], lupine [Lupinus], or white snakeroot [Ageratina altissima]), and metabolic disorders such as hypocalcemia, hypomagnesemia, and hypokalemia.
Four cattle were euthanized for postmortem examination and diagnostic testing. Cow 1 was autopsied on the farm, and ~ 500 mL of dark-red pericardial effusion was observed. Several mild changes were present in a chemistry panel performed on serum collected antemortem from cow 2: hypernatremia, hyperkalemia, hyperchloremia, elevated anion gap, hypocalcemia, hyperproteinemia, hyperglycemia, mildly elevated aspartate aminotransferase and creatine kinase activities, and hyperbilirubinemia (Table 1). Cow 2 was euthanized and sent with the carcasses of cows 3 and 4, and fresh and formalin-fixed tissues from cow 1, for autopsy and ancillary testing at Cornell University’s Animal Health Diagnostic Center (AHDC; Ithaca, NY). Upon examination, cow 3 also had ~ 500 mL of pericardial effusion, and cow 4 had ecchymotic and petechial hemorrhages affecting the pericardium, aortic adventitia, and tracheal mucosa.
Table 1.
Abnormal results in the serum chemistry panel for cow 2.
| Test name | Unit | Result | Reference interval |
|---|---|---|---|
| Sodium | mmol/L | 158 | 134–144 |
| Potassium | mmol/L | 6.0 | 4.0–5.9 |
| Chloride | mmol/L | 102 | 98–99 |
| Anion gap | mmol/L | 35 | 19–26 |
| Na/K ratio | 26 | ||
| Calcium | mmol/L | 2.1 | 2.2–2.7 |
| Total protein | g/L | 90 | 67–88 |
| Globulin | g/L | 56 | 28–54 |
| Glucose | mmol/L | 4.8 | 3.2–4.4 |
| AST | µkat/L | 4.0 | 0.9–2.3 |
| GGT | µkat/L | 0.1 | 0.3–0.9 |
| Total bilirubin | µmol/L | 6.8 | 0.0–1.7 |
| Indirect bilirubin | µmol/L | 5.1 | 0.0–1.7 |
| Creatine kinase | U/L | 26 | 2–5 |
AST = aspartate aminotransferase; GGT = gamma-glutamyl transpeptidase. Reference intervals sourced from the Clinical Pathology laboratory, Animal Health Diagnostic Center, Cornell University, Ithaca, NY.
Although there were no observed gross lesions, histologic examination of the skeletal and cardiac muscle demonstrated acute mild-to-moderate multifocal monophasic degeneration of individual muscle fibers in the hindquarters of 4 of 4 cattle and hypereosinophilic cardiomyocytes in 2 of 4, raising concern for a toxic myopathy, particularly ionophore toxicity.19 Commercial pellets (Rumensin) from the current supply on the farm were tested by the manufacturer (Elanco Animal Health Feed Assay Service) for monensin levels. Test results (65.2% g/ton as fed, wet weight) indicate 102% of label claim (63.9 g/ton), which is within acceptable limits (85–115%). TMR was not tested for monensin quantification, but the farmer and attending veterinarian confirmed that no mixing errors occurred. At the time of clinical signs or death from ionophore toxicity, the rumen contents may no longer contain representative as-fed ionophore levels, as a result of digestion and absorption.6 This makes interpretation difficult when monensin is purposefully added to the diet; therefore rumen contents from deceased cattle were not tested for ionophore quantification in this outbreak. Feed was not tested for other ionophores, such as lasalocid or salinomycin, given that they were not part of the mixed ration. Monensin and other ionophores remain a potential cause of toxicity but their presence in the feed are unlikely based on the TMR diet formulation and clinical signs.
Ancillary testing included aqueous humor nitrate concentrations on all 4 cattle, liver selenium concentrations on all 3 cows tested (cows 1–3), and serum cholinesterase activity on cow 2. These tests were all within normal limits, ruling out nitrate poisoning, nutritional myopathy, and organophosphate poisoning. The TMR was tested for mycotoxins (Cumberland Valley Analytical Services, Waynesboro, PA), which were found to be within normal limits (Burbank KE, pers. comm., Nov 2018).
Samples of rumen contents from 3 autopsied cows were tested for C. botulinum at the Wadsworth Center Biodefense Laboratory, New York State Department of Health (WCBL-NYSDOH; Albany, NY). Toxin testing was performed using the Bruker matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS; Biotyper; Bruker Daltonics).15 BoNT/A was detected in rumen contents from 2 of 3 cows (Figs. 1–3) and was inconclusive in rumen contents from 1 of 3 cows. BoNT/B, /E, and /F were not detected (0 of 3 cows), and we did not test for BoNT/C and /D because there was insufficient sample volume. Samples were also tested by a multiplex real-time PCR (rtPCR) assay developed at the WCBL-NYSDOH (Egan C, pers. comm., Sept 17, 2019) and culture. Anaerobic culture was negative for all 3 samples, and C. botulinum neurotoxin (bont)-producing genes (bont/A–F as well as Clostridium baratii bont/F) were negative by rtPCR for all toxin genes for all samples.7 Several days later, liver and rumen contents from cow 5, another botulism suspect, were submitted directly to the WCBL-NYSDOH by the attending veterinarian. C. botulinum type C was anaerobically cultured from the liver, and bont/C gene was detected by rtPCR from both the liver sample directly and the culture isolate. The BoNT/C toxin was not detected directly from the liver sample by MALDI-TOF MS but was detected from the organism isolated from the liver.
Figure 1.
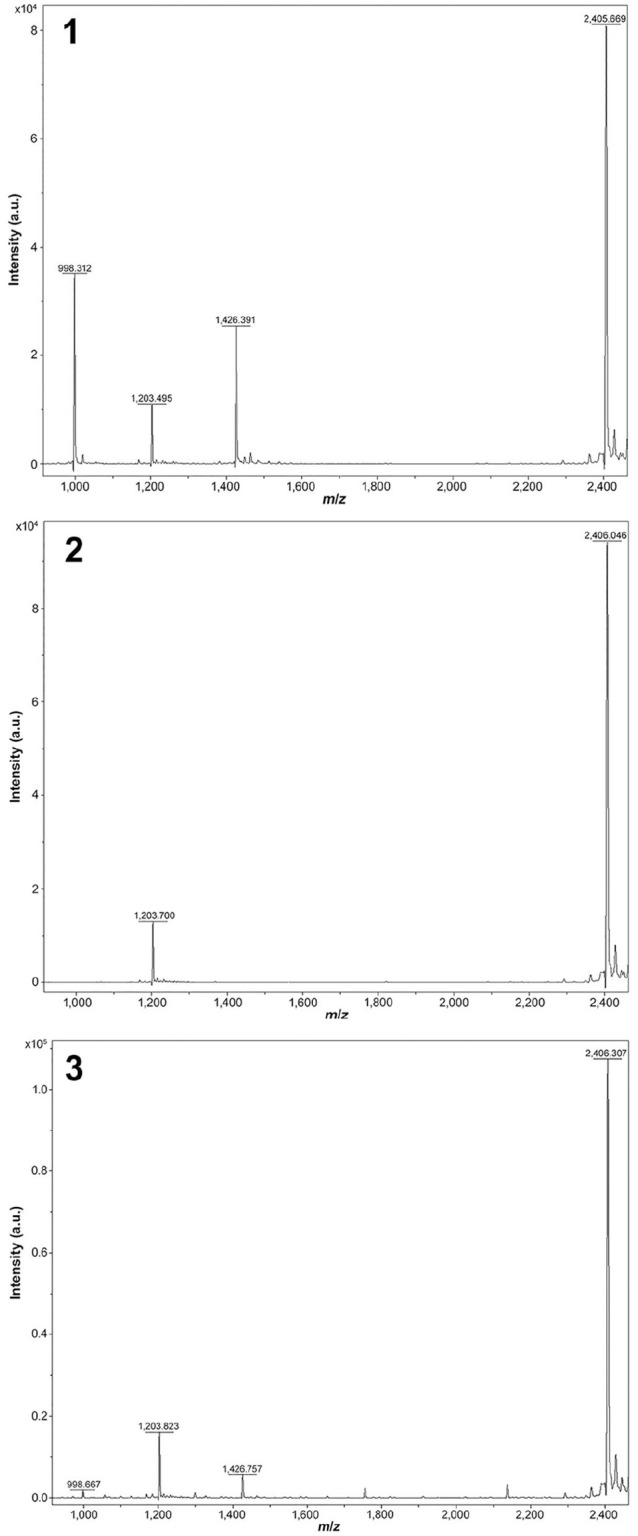
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) Clostridium botulinum type A (BoNT/A) positive control. The intact peptide substrate peak is 2,406 m/z (mass-to-charge). BoNT/A cleavage of the peptide creates 2 fragments (daughter peptides), present at 998 and 1,426 m/z. Figure 2. MALDI-TOF MS BoNT/A negative control. The intact peptide substrate peak is 2,406 m/z, and no daughter peptides are present. Figure 3. MALDI-TOF MS detection of BoNT/A in the rumen contents from cow 3. As in Figure 1, daughter peptides at 998 and 1,426 m/z indicate cleavage by BoNT/A. The intact peptide substrate peak is 2,406 m/z.
The herd diagnosis was botulism caused by BoNT/A. The detection of preformed toxin A by MALDI-TOF MS in the rumen contents of cows 2 and 3 is considered diagnostic for exposure. The positive culture of C. botulinum and the rtPCR detection of bont/C and preformed toxin C by MALDI-TOF MS from the culture isolate from the liver of cow 5 may have contributed to the clinical signs of botulism, but it likewise may represent an overgrowth of bacterial contamination with C. botulinum in the carcass postmortem. It is also possible that BoNT/C was present in the liver, but the concentration of the toxin was below the limit of detection (LOD). The 16 remaining affected cows were monitored and given supportive care as needed. Cattle remained weak for ~ 3 wk and never regained healthy body condition or milk productivity. Within 10 mo of the outbreak, 11 of 16 recovered cattle were culled because of poor body condition and decreased milk production.
The gold standard for diagnosing botulism is detection of the toxin through the mouse bioassay (MBA),1,11 which is offered at only 2 veterinary laboratories in the United States. Although considered the most sensitive test, the LOD of the MBA may exceed the relatively low concentrations of preformed neurotoxin sufficient to intoxicate highly susceptible animals, such as horses, and less so cattle, both known to be more sensitive to the toxin than mice.11,13 Cattle are ~ 13 times more sensitive to BoNT/C than mice,13 and this can lead to false-negative laboratory MBA results in cases of bovine botulism.
BoNTs are metalloproteinases, and in the neuromuscular junction, BoNTs cleave key vesicular transport proteins of the N-ethylmaleimide–sensitive fusion attachment protein receptor (SNARE) complex, thus disrupting fusion with the synaptic membrane and release of acetylcholine from the synaptic vesicle. Innovative activity-based detection assays leverage the specific endoproteinase activities of the BoNTs and detect BoNT type-specific cleavage products of SNARE proteins or peptide substrates.1 In 2005, the Centers for Disease Control and Prevention (CDC) validated the Endopep-MS assay for BoNT/A–F, utilizing mass spectrometry detection of BoNT-specific cleavage products of 4 target peptide substrates.1 In 2017, the WCBL-NYSDOH validated their Bruker MALDI-TOF MS Biotyper for toxins A, B, E, and F, the botulinum toxins pertinent to human medicine (Egan C, pers. comm., Sept 17, 2019 ).15 The MALDI-TOF MS Biotyper assay is as sensitive as, or superior to, MBA with a toxin LOD of 0.3–25 mLD50 (50% mouse lethal dose), depending on the toxin type (A, B, E, and F); specifically, the BoNT/A LOD was 18 mLD50.15 The target peptide has subsequently been optimized for detection of BoNT/C and /D20 and the NYSDOH incorporated this method into their algorithm for testing animal and environmental samples. The mLD50 for BoNT/D in cattle is 22.5,11 which indicates that this technique is sufficiently sensitive to detect preformed botulinum neurotoxins in quantities clinically relevant to cause disease and death in cattle. Results can be obtained as quickly as 4 h, accelerating the detection of botulism in comparison to the MBA, which takes 1–4 d. PCR sensitively detects C. botulinum neurotoxin (bont) genes and eliminates the use of live mice, but is not discriminatory for toxin production. Some bacteria can have multiple genes and only produce a single toxin.15 The utilization of the MALDI-TOF MS in our outbreak resulted in quicker detection of BoNT/A and highlights the importance of collaboration between human and animal health diagnostic laboratories. Further validation of this technology for diagnosing botulism in animals may replace the MBA in the future.
BoNT/B–D have historically been found as the causative neurotoxins in bovine botulism.4,11,12 BoNT/C and /D are associated with pica and phosphorus deficiency and have been reported in cattle in South Africa, South America, and Australia.11 Outbreaks of BoNT/C and /D in confined dairy cattle are typically linked to feed contaminated by dead carcasses or poultry litter, and have been reported in North America and Europe.3,5,14 BoNT/B has caused botulism in improperly ensiled forages such as rye silage,4 plastic packed hay,22 and round bale barley haylage fed to cattle.10 The ensiling process creates an anaerobic environment, and if the pH does not drop below 4.5, C. botulinum spores from soil can propagate.3,4,10 There are documented geographic differences in the type of botulism toxin prevalent in soil, with type A found in the western United States and type B found in the eastern United States.17 Humans are affected by BoNT/A, /B, /E, and /F,1,15 and cases of human BoNT/A are not uncommon on the east coast.15 Human botulism is primarily linked to ingestion of preformed toxin in food. Human illness may also be caused by introduction of spores through open wounds in the skin, inadvertent intoxication through therapeutic or cosmetic use, or colonization of spores in the intestinal tract of infants.18 Horses are commonly affected by BoNT/B, but /A and /C have also been reported.9 A review of type A botulism in horses in the United States from 1998 to 2008 described 11 cases confirmed by MBA; 1 case occurred in Florida; the rest of the cases occurred west of the Mississippi River.9 Bovine botulism caused by BoNT/A has been reported in a zebu herd in Brazil; BoNT/A was detected by MBA from bones and tendons found in the rumen of 2 cattle, demonstrating an association with animal carcasses.16
The presence of BoNT/A in rumen contents from 2 NYS dairy cows documents an outbreak of bovine botulism from toxin A in North America, detected by MALDI-TOF MS. The detection of BoNT/A in this herd may be related, in part, to the sensitivity of the MALDI-TOF MS. Toxin A was not detected in the third sample of rumen contents, possibly as a result of low toxin concentration. The source of both BoNT/A toxin and bont/C gene is most likely the raccoon carcass, but the carcass was not available for testing. Improperly fermented haylage cannot be completely ruled out as the cause, but previous reports of bovine forage poisoning have been attributed to BoNT/B.4,10,22
In our outbreak, 2 autopsied cows had pericardial effusion, and all 4 had histologic findings of monophasic, multifocal degeneration variably affecting skeletal muscle and cardiac muscle consistent with toxic myopathy. Given that it targets the neuromuscular synapse, botulism is not associated with gross or histologic lesions,12 although prolonged recumbency secondary to botulism can cause myodegeneration. Muscle toxins include ionophores, toxic plants, and plant-origin toxins that produce nonspecific segmental necrosis. In cattle, the clinical signs of acute monensin toxicity are nonspecific and include anorexia, diarrhea, dullness, weakness, and death.6,19 Monophasic, multifocal degeneration is usually present by 48 h after exposure.19 In this herd, elevated ionophore levels were not detected in the commercial pellets (Rumensin), and other toxins were considered unlikely based on exposure history. Ultimately, the cause of these lesions is unknown.
Marked pericardial effusion consistent with cardiac failure was observed in 2 of 4 autopsied cows; pericardial effusion with fibrin strands is reported as a relatively consistent feature of shaker foal syndrome (toxicoinfectious botulism), as are pulmonary edema and congestion.21 Petechial and ecchymotic hemorrhages have been reported sporadically with botulism toxicity2; however, these findings are also nonspecific agonal changes.
There are no established meat and milk withdrawals for cattle affected by botulism. Botulinum neurotoxin has not been detected in milk from Holstein cattle injected with various doses of BoNT/C.13 Cooking and pasteurization will denature botulinum toxin. Although the risk for milk and meat contamination appears low, in previous reports of bovine botulism, carcasses have been incinerated and milk was not shipped for human consumption.5,8,10 In our outbreak, the farmer was instructed to not drink raw milk and not feed raw milk to calves. Bovine veterinarians, diagnostic laboratories, and federal and state health officials should be aware of C. botulinum type A in dairy cattle, and the sensitive and rapid molecular testing capabilities for C. botulinum toxin detection available through rtPCR and MALDI-TOF MS.
Acknowledgments
We thank Amy Rourke, Melissa D’Amico, Dominick Centurioni, and Maureen Conlon from the Wadsworth Center Biodefense Laboratory, New York State Department of Health, for providing laboratory technical support; Dr. Kelly A. Johnson, Cornell University Outreach & Information Resources Librarian for reference organization and manuscript review; Dr. Jen Nightingale from Countryside Veterinary Clinic for her sample submission; and Dr. Francisco Uzal, California Animal Health and Food Safety Laboratory System for manuscript review.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Elisha A. Frye  https://orcid.org/0000-0002-7784-6771
https://orcid.org/0000-0002-7784-6771
References
- 1. Boyer AE, et al. From the mouse to the mass spectrometer: detection and differentiation of the endoproteinase activities of botulinum neurotoxins A-G by mass spectrometry. Anal Chem 2005;77:3916–3924. [DOI] [PubMed] [Google Scholar]
- 2. Câmara ACL, et al. Epidemiologia, sinais clínicos, achados laboratoriais e patológicos em oito surtos de botulismo em bovinos no Rio Grande do Norte [Epidemiology, clinical signs, laboratory and pathological findings in eight outbreaks of botulism in cattle in Rio Grande do Norte state, Northeastern Brazil]. Acta Sci Vet 2014;42. Portuguese. [Google Scholar]
- 3. Constable PD, et al. Diseases of the nervous system. In: Veterinary Medicine. 11th ed. Saunders, 2017:1155–1370. [Google Scholar]
- 4. Divers TJ, et al. Clostridium botulinum type B toxicosis in a herd of cattle and a group of mules. J Am Vet Med Assoc 1986;188:382–386. [PubMed] [Google Scholar]
- 5. Galey FD, et al. Type C botulism in dairy cattle from feed contaminated with a dead cat. J Vet Diagn Invest 2000;12:204–209. [DOI] [PubMed] [Google Scholar]
- 6. Hall JO. Ionophore use and toxicosis in cattle. Vet Clin North Am Food Anim Pract 2000;16:497–509. [DOI] [PubMed] [Google Scholar]
- 7. Halpin JL, et al. Molecular characterization of Clostridium botulinum harboring the bont/B7 gene. Foodborne Pathog Dis 2019;16:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heider LC, et al. Presumptive diagnosis of Clostridium botulinum type D intoxication in a herd of feedlot cattle. Can Vet J 2001;42:210. [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson AL, et al. Type A botulism in horses in the United States: a review of the past ten years (1998–2008). J Vet Diagn Invest 2010;22:165–173. [DOI] [PubMed] [Google Scholar]
- 10. Kelch WJ, et al. Fatal Clostridium botulinum toxicosis in eleven Holstein cattle fed round bale barley haylage. J Vet Diagn Invest 2000;12:453–455. [DOI] [PubMed] [Google Scholar]
- 11. Maréchal CL, et al. Botulism. In: Uzal FA, et al. , eds. Clostridial Diseases of Animals. 1st ed Wiley, 2016:303–330. [Google Scholar]
- 12. MacKay RJ. Botulism. In: Smith BP. ed. Large Animal Internal Medicine. 6th ed. Mosby, 2020:1101–1104. [Google Scholar]
- 13. Moeller RB, et al. Determination of the median toxic dose of type C botulinum toxin in lactating dairy cows. J Vet Diagn Invest 2003;15:523–526. [DOI] [PubMed] [Google Scholar]
- 14. Neill SD, et al. Type C botulism in cattle being fed ensiled poultry litter. Vet Rec 1989;124:558–560. [DOI] [PubMed] [Google Scholar]
- 15. Perry M, et al. Implementing the Bruker MALDI Biotyper in the public health laboratory for C. botulinum neurotoxin detection. Toxins (Basel) 2017;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schocken-Iturrino RP, et al. First case of type A botulism in zebu (Bos indicus). Vet Rec 1990;126:217–218. [PubMed] [Google Scholar]
- 17. Smith LD. The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Lab Sci 1978;15:74–80. [PubMed] [Google Scholar]
- 18. Stevens DL, et al. Clostridium. In: Jorgensen JH, et al. , eds. Manual of Clinical Microbiology. 11th ed. ASM Press, 2015:940–966. [Google Scholar]
- 19. Van Vleet JF, et al. Clinical, clinicopathologic, and pathologic alterations in acute monensin toxicosis in cattle. Am J Vet Res 1983;44:2133–2144. [PubMed] [Google Scholar]
- 20. Wang D, et al. Enhanced detection of type C botulinum neurotoxin by the Endopep-MS assay through optimization of peptide substrates. Bioorgan Med Chem 2015;23:3667–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkins PA, Palmer JE. Botulism in foals less than 6 months of age: 30 cases (1989–2002). J Vet Intern Med 2003;17:702–707. [DOI] [PubMed] [Google Scholar]
- 22. Wilson RB, et al. Presumptive botulism in cattle associated with plastic-packaged hay. J Vet Diagn Invest 1995;7:167–169. [DOI] [PubMed] [Google Scholar]


