Abstract
Innate immunity plays a central role in the pathogenesis of chronic inflammatory enteropathies (CIE) in dogs, and further evaluation of the innate immune receptor for advanced glycation end-products (RAGE) is warranted. We measured serum concentrations of decoy receptor soluble RAGE (sRAGE) in 102 dogs diagnosed with CIE, and evaluated relationships with clinical disease severity, histologic lesion severity, concentrations of serum C-reactive protein (CRP), and serum and fecal calprotectin, S100A12, and alpha1-proteinase inhibitor (α1PI). Serum sRAGE levels were not associated with clinical disease activity, serum CRP, serum and fecal α1PI, calprotectin, or S100A12 concentrations. Microscopic lesions in the duodenum were more severe in dogs with serum sRAGE concentration ≤ 340 ng/L (p = 0.013). Serum sRAGE levels were weakly and inversely correlated with the severity of lymphoplasmacytic infiltration in the gastric antrum and duodenum, and with crypt dilation and the neutrophilic infiltrate in the duodenum, in univariate analysis (all p < 0.05), but none of the correlations remained statistically significant after correction for multiple comparisons. Our study confirms that CIE in dogs is associated with decreased serum sRAGE concentrations, suggesting a dysregulated sRAGE/RAGE axis.
Keywords: canine, ELISA, inflammatory bowel disease, innate immunity, pattern recognition receptor, RAGE, receptor for advanced glycation end-products
Introduction
Chronic inflammatory enteropathies (CIE) are important diseases in dogs, the diagnosis of which requires the presence of chronic gastrointestinal signs (≥ 2 wk), histopathologic evidence of intestinal mucosal inflammation, and the exclusion of other underlying causes.2,8,34 Canine CIE are classified based on response to dietary changes (food-responsive enteropathy, FRE) and antibiotic trials (antibiotic-responsive enteropathy), or the response to anti-inflammatory or immunosuppressive treatment (immunosuppressant-responsive or -refractory enteropathy, IRE).2,8,10 Several biomarkers of inflammation have been evaluated in dogs with CIE,21 of which C-reactive protein (CRP), calprotectin (S100A8/A9 protein complex), S100A12 (calgranulin C) protein, and alpha1-proteinase inhibitor (α1PI) appear to have clinical utility.14,17,20,23 Similar to inflammatory bowel disease (IBD) in humans, medical treatment of dogs with IRE usually involves corticosteroids and/or other immunosuppressive drugs2,8,10; more targeted treatment strategies would be desirable but are currently lacking.
Dysregulations of the innate immune response, in particular the signaling cascades of pattern recognition receptors (PRRs), have been linked to the development of chronic autoimmune diseases.3,32 The innate immune system plays a key role in the complex pathogenesis of CIE,16,19,27,34 and there is also evidence of PRR dysregulation in dogs with IRE.19 Further research in this area is needed to better characterize the pathogenesis of CIE and to aid in the development of more targeted treatment strategies.19
The receptor of advanced glycation end-products (RAGE) is a PRR that recognizes pathogen-associated molecular patterns and endogenous molecular structures released at sites of inflammation or tissue damage.3,19,32 Full-length RAGE is a type I transmembrane glycoprotein that is constitutively expressed on the surface of certain cells (e.g., alveolar cells); its expression in other cells (e.g., macrophages) is induced by the accumulation of RAGE ligands (i.e., existence of a proinflammatory microenvironment) or the activation of certain transcription factors (e.g., nuclear factor–κB).3,19,25 Sustained RAGE-ligand interaction was suggested to be associated with the perpetuation and amplification of the proinflammatory immune response in autoimmune diseases, such as human IBD.3,26 Shortened isoforms of RAGE, such as the decoy receptor soluble RAGE (sRAGE), which results from alternative splicing or proteolytic cleavage,19,25 can abrogate the RAGE-ligand interaction and thus modulate downstream signaling.3,26 A 2019 experimental study also indicated that the RAGE signaling pathway plays a key role in intestinal inflammation as it promotes oxidative stress and endothelial activation, and that interference with the RAGE pathway presents a promising therapeutic target in patients with IBD.4
The canine RAGE gene and its gene products have been characterized,28 and the expression of RAGE in canine lymphoma and histiocytic sarcoma has been evaluated.29,30 RAGE is also expressed in the intestinal mucosa in dogs (Fig. 1). In a pilot study, serum sRAGE concentrations were decreased in a small group of dogs with CIE compared to healthy controls and normalized only in those dogs that experienced full clinical remission during the induction phase of therapy.15 However, the association between serum sRAGE, disease severity, and other inflammatory biomarkers has not been studied extensively in canine CIE. Thus, further evaluation of the sRAGE/RAGE axis in canine CIE is warranted.
Figure 1.
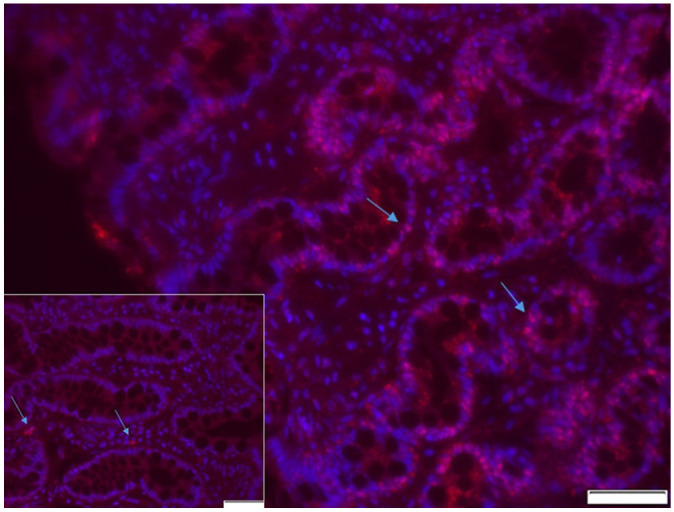
Intestinal mucosal expression of the receptor for advanced glycation end-products (RAGE). Fluorescence microscopy image of a colonic mucosal biopsy obtained from a dog diagnosed with moderate diffuse lymphoplasmacytic and eosinophilic chronic colitis. The image shows multifocal positive immunostaining (pink fluorescence; arrows) for RAGE that is located predominantly in the colonic epithelium. Bar = 50 μm. Inset: representative negative control (primary antibody omitted) confirming absence of nonspecific staining. Arrows indicate artificial autofluorescence caused by erythrocytes. Bar = 50 μm.
We hypothesized that serum sRAGE concentrations in dogs with CIE correlate with 1) the severity of clinical signs, 2) the severity of microscopic lesions, and 3) with the concentrations of serum and fecal biomarkers of inflammation or protein loss. To test these hypotheses, we evaluated serum sRAGE concentrations in a large group of dogs with CIE and correlated serum sRAGE concentration with 1) a clinical disease activity score, 2) a histologic disease score, and 3) the concentrations of serum CRP and serum and fecal calprotectin, S100A12, and α1PI.
Materials and methods
Ethics approval
Our study was approved by the Clinical Research Review Committee (CRRC approval TAMU 2009-06, approved 01-15-2009) and the Institutional Animal Care and Use Committee at Texas A&M University (IACUC approval TAMU 2012-083, approved 05-22-2012). A letter of informed consent was signed by the owner to enroll dogs in the study.
Study population
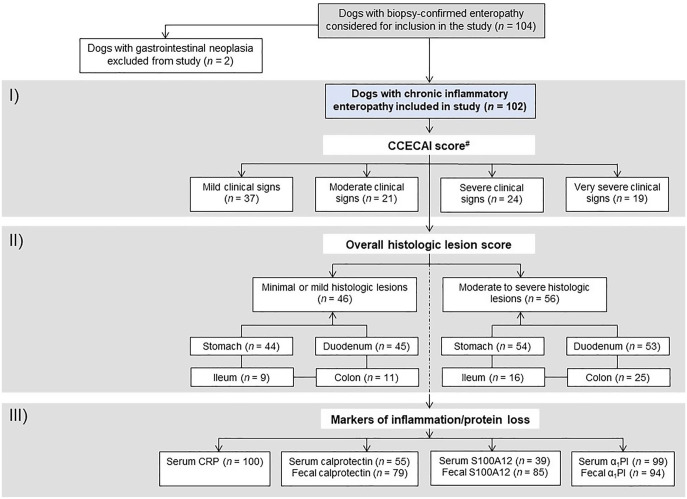
We included 102 dogs diagnosed with CIE in our study (Fig. 2). These dogs were enrolled over a 61-mo period (Sept 2009–Oct 2014) and were recruited at the Veterinary Teaching Hospital at Texas A&M University (College Station, TX; n = 6) or at one of several small animal referral hospitals across the United States (n = 96). Inclusion criteria were the presence of gastrointestinal clinical signs for at least 2–3 wk, exclusion of other identifiable causes (i.e., atypical hypoadrenocorticism, exocrine pancreatic insufficiency), and intestinal mucosal infiltration with inflammatory cells confirmed by histopathology.34 There were no restrictions on canine breed or age. The exclusion criteria were the diagnosis of other causes of the gastrointestinal clinical signs (e.g., alimentary lymphoma), the lack of gastrointestinal tissue biopsies, or insufficient sample material for serum sRAGE analysis. Some of the data from these dogs have been published previously.20
Figure 2.
Study flowchart. Of the 104 dogs considered for inclusion in the study, 102 dogs were included. Data from all 102 dogs were included in the first part of the study (I: correlation with clinical disease severity) and in the second part of the study (II: correlation with the severity of histologic lesions); data from a subset of these dogs were included in the third part of the study (III: correlation with biomarkers of gastrointestinal inflammation or protein loss). #available from 101 dogs.
Routine diagnostic investigation of dogs included a physical examination; hematology (performed at the institution recruiting the case or at the Texas Veterinary Medical Diagnostic Laboratory), serum biochemistry profile (LiquiColor, Sirrus clinical chemistry analyzer; Stanbio Laboratory) with a bile acid stimulation test or an ACTH stimulation test if indicated, and urinalysis (with a urine culture and/or a urine protein-to-creatinine ratio if indicated); fecal endoparasite screen; abdominal diagnostic imaging (ultrasonography and/or radiographs); a gastrointestinal profile including serum canine trypsin-like immunoreactivity (cTLI), specific canine pancreatic lipase (Spec cPL), cobalamin (n = 101), folate (n = 101), and gastrin concentration. Hypoalbuminemia was defined as mild (serum albumin concentration 15–20 g/L), moderate (12–14.9 g/L), or severe (< 12 g/L), and dogs with a serum cobalamin concentration < 300 ng/L were classified as hypocobalaminemic.
At the time of evaluation, each patient (except for one dog) was assigned a canine chronic enteropathy clinical activity index (CCECAI) score,1 which considers the general attitude and activity of the dog, appetite, frequency of vomiting, stool consistency and frequency of defecation, weight loss, serum albumin concentration, peripheral edema or ascites, and pruritus. Individual CCECAI criteria can range from 0–3 (0 = normal, 1 = slightly abnormal, 2 = moderately abnormal, 3 = severely abnormal), and the cumulative CCECAI score is interpreted as clinically insignificant disease (score 0–3), mild disease (score of 4–5), moderate disease (score of 6–8), severe disease (score of 9–11), or very severe disease (score of ≥ 12).1
Endoscopy of the gastrointestinal tract (n = 94) or laparotomy (n = 8) with collection of tissues biopsies was performed on each dog. Several biopsies were taken from different locations in the gastrointestinal tract (stomach—range: 1–24, median: 12; duodenum—range: 1–29, median: 13; ileum—range: 1–22, median: 4; colon—range: 1–44, median: 14). The tissue samples were subjected to routine histologic evaluation9,34 through the Texas A&M University Gastrointestinal Laboratory Histopathology service and were evaluated by 1 of 9 board-certified anatomic pathologists with special expertise in small animal gastrointestinal pathology. Tissue biopsies were histologically evaluated using the structural and inflammatory criteria of the World Small Animal Veterinary Association Gastrointestinal Standardization grading system.9,34 A score of 0 was assigned to normal tissues, 1 for mild histologic lesions, 2 for moderate histologic lesions, and 3 for severe histologic changes. Individual and cumulative lesion scores (calculated as the sum of individual lesion scores of the stomach, duodenum, ileum, and colon) were considered for data and statistical analyses.
Follow-up samples and/or information were available for 20 dogs and for a period of 1–10 mo. Patients were diagnosed with FRE based on the clinical response to an elimination diet with a protein hydrolysate or a novel protein and carbohydrate source (2 of these 5 dogs had received antibiotic treatment without a clinical response), whereas dogs that required anti-inflammatory or immunosuppressive treatment were classified as IRE (8 of these 15 dogs also received metronidazole or tylosin as part of their treatment plan).
Sample collection and analysis
Serum samples from dogs included in our study were also used for measurement of CRP (n = 100), calprotectin (n = 55), S100A12 (n = 39), and α1PI (n = 99). In addition, calprotectin (n = 79), S100A12 (n = 39), and α1PI concentrations (n = 94) were measured in fecal samples from 3 consecutive days. Not all markers could be analyzed in samples from all dogs given the amount of sampling material available.
Serum sRAGE concentrations were measured in archived samples (stored at −80°C for 1–72 mo) by ELISA as described previously.15 Briefly, 96-well plates were coated with 150 ng of polyclonal anti-canine RAGE antibody (sheep anti-recombinant canine RAGE; R&D Systems). Following a wash step, nonspecific binding sites were blocked with 30% (v/v) newborn calf serum and 1% (v/v) Triton X-100 (Thermo Fisher) in phosphate-buffered saline (PBS; assay buffer). Plates were then incubated with test samples (diluted 1 in 2 [50% each] in assay buffer, each sample tested in duplicate), calibrator solutions with different canine RAGE concentrations (5,000, 2,000, 1,000, 500, 200, 100, and 20 ng/L in assay buffer), and assay blanks (assay buffer). After another wash step, plates were incubated with a biotinylated polyclonal anti-canine RAGE antibody (60 ng/well; R&D Systems) and were washed again. NeutrAvidin–horseradish peroxidase (NA-HRP; Thermo Fisher Scientific) in PBS with 1% (w/v) bovine serum albumin (20 ng/well) was added to each plate, and after a wash step each well received a stabilized 3,3’,5,5’-tetramethylbenzidine substrate. After 10 min, the reaction was stopped by adding 4 M acetic acid, and the absorbance in each well was measured at 450 nm by use of an automated plate reader. A 5-parameter logistic curve fit was used to determine canine serum sRAGE concentrations in test samples. The limit of detection (LOD) of the assay has previously been reported at 52 ng/L.15
Serum CRP concentrations were measured using a commercial ELISA (Phase CRP; Tri-Delta Diagnostic). Calprotectin and S100A12 concentrations in serum and fecal samples were tested using previously established and analytically validated species-specific sandwich ELISAs11,18; serum and fecal α1PI were measured using an in-house radioimmunoassay.12,13
Statistical analysis
Normality of the data and equality of variances were tested by using a Shapiro–Wilk test and a Brown–Forsythe test, respectively. Summary statistics are presented as median and interquartile ranges (IQR) or ranges. Serum sRAGE concentrations were compared among the different groups of dogs using nonparametric group comparisons (Wilcoxon rank-sum test or Kruskal–Wallis test with Dunn post-hoc comparisons). A potential relationship of serum sRAGE levels with clinical disease activity (CCECAI scoring system), histologic lesion severity (4-point semi-quantitative grading system), and serum and fecal inflammatory marker concentrations was tested using a likelihood ratio test for association or calculation of a Spearman rank-sum correlation coefficient ρ. Sensitivity and specificity were calculated as the true-positive and true-negative rate, respectively (dichotomous data), or by construction of a receiver operating characteristic curve with a Youden index for determination of the optimum cutoff value (continuous data). Statistical significance was set at p ≤ 0.05, and a Holm sequential Bonferroni correction was applied for multiple comparisons.22 A commercial statistical software package (JMP v.13; SAS) was used for all statistical analyses.
Results
Study population
A total of 102 dogs met the inclusion criteria (Fig. 2, Table 1). Dogs were 4–9 y old, and there was an approximately even sex distribution. Most dogs were purebred (82%). Clinical disease activity (CCECAI) scores and histologic lesions scores varied from mild to severe or very severe. Approximately one third of the dogs were hypoalbuminemic (29%), and 33% of the dogs were hypocobalaminemic.
Table 1.
Characteristics of the 102 dogs included in our study of biomarkers of inflammation in cases of canine chronic inflammatory enteropathy.
| Group characteristic | Value |
|---|---|
| Total number | 102 |
| Age median (IQR) | 6.8 (4–9) |
| Sex: male (castrated)/female (spayed) | 50 (44)/52 (50) |
| Body weight (kg), median (IQR) | 14.6 (6.5–24.8) |
| Dog breed, n | |
| Purebred | 84 (82%) |
| German Shepherd | 14 (14%) |
| Yorkshire Terrier | 7 (7%) |
| Mixed-breed | 18 (18%) |
| Disease duration (mo), median (IQR) | 3 (1–8) |
| Prior glucocorticosteroid treatment | 11 (11%) |
| Biopsy type, n | |
| Endoscopic | 94 (92%) |
| Surgical | 8 (8%) |
| No. of biopsies per site, median (IQR) | |
| Stomach (n = 89) | 12 (10–16) |
| Endoscopic | 13 (10–17) |
| Surgical | 2 (1–4) |
| Duodenum (n = 90) | (8–17) |
| Endoscopic | 13 (11–17) |
| Surgical | 1 (1) |
| Ileum (n = 22) | 4 (1–11) |
| Endoscopic | 8 (4–14) |
| Surgical | 1 (1–2) |
| Colon (n = 28) | 14 (10–17) |
| Endoscopic | 15 (10–17) |
| Surgical | 2 (1–2) |
| Histologic lesion score, median (IQR) | |
| Stomach | 1 (0–2) |
| Duodenum | 1 (1–2) |
| Ileum | 2 (0–2) |
| Colon | 1 (1–2) |
| Overall lesion score | 2 (1–2) |
| CCECAI score, median (IQR)* | 7 (5–11) |
| Clinical disease severity, n | |
| Mild† | 37 (37%) |
| Moderate‡ | 21 (21%) |
| Severe§ | 24 (24%) |
| Very severe¦ | 19 (19%) |
| Serum sRAGE concentration (ng/L), median (IQR) | 287 (154–472) |
| > 340 ng/L, n | 37 (36%) |
| ≤ 340 ng/L, n | 65 (64%) |
| 52–340 ng/L, n | 53 (52%) |
| ≤ 52 ng/L, n | 12 (12%) |
| Serum albumin concentration (g/L), median (IQR) | 26 (19–30) |
| Hypoalbuminemia, n | 29 (29%) |
| Mild# | 14 (14%) |
| Moderate¶ | 9 (9%) |
| Severe** | 6 (6%) |
| Serum cobalamin concentration (ng/L), median (IQR)* | 319 (212–678) |
| Hypocobalaminemia,†† n | 33 (33%) |
| Serum folate (µg/L), median (IQR)* | 12.4 (9.4–16.0) |
| Hypofolatemia, n | 16 (16%) |
| Hyperfolatemia, n | 5 (5%) |
| Serum CRP (mg/L), median (IQR) (n = 100) | 8.6 (1.2–26.6) |
| Fecal calprotectin (µg/g), median (IQR) (n = 79) | 1.5 (0.5–17.0) |
| Serum calprotectin (mg/L), median (IQR) (n = 55) | 6.3 (4.1–8.9) |
| Fecal S100A12 (ng/g), median (IQR) (n = 85) | 131 (21.3–864) |
| Serum S100A12 (µg/L), median (IQR) (n = 39) | 211 (148–339) |
| Fecal α1PI (µg/g), median (IQR) (n = 94) | 7.4 (3.8–23.8) |
| Serum α1PI (mg/L), median (IQR) (n = 99) | 1,290 (1,005–1,493) |
CCECAI = canine chronic enteropathy clinical disease activity index; CRP = C-reactive protein; IQR = interquartile range.
Available from 101 dogs.
CCECAI score of 4–5.
CCECAI score of 6–8.
CCECAI score of 9–11.
CCECAI score of ≥ 12.
Defined as a serum albumin concentration 15–20 g/L.
Defined as a serum albumin concentration 12–14.9 g/L.
Defined as a serum albumin concentration < 12 g/L.
Defined as a serum cobalamin concentration < 300 ng/L.
Serum sRAGE concentrations
Serum sRAGE concentrations were 52–3,260 ng/L (median: 287 ng/L) in all CIE dogs, with 65 dogs (64%) having a serum sRAGE concentration ≤ 340 ng/L (cutoff value that has previously been shown to provide the best separation of CIE dogs from healthy control dogs15; Table 1). Twelve dogs (12%) had a serum sRAGE concentration below the LOD of 52 ng/L. Prior corticosteroid treatment (p = 0.150, Wilcoxon rank-sum test) or disease duration (p = 0.387, Spearman correlation analysis) did not affect serum sRAGE concentrations. Age was also not correlated with serum sRAGE concentrations (ρ = 0.16, p = 0.108), and serum sRAGE concentrations did not differ between purebred (median: 287 ng/L, IQR: 153–432 ng/L) and mixed-breed (median: 275 ng/L, IQR: 178–637 ng/L; p = 0.772) dogs.
Correlation of serum sRAGE concentrations with clinical and histologic disease severity
Serum sRAGE concentrations were not associated with the severity of clinical signs (CCECAI scores; ρ = −0.07, p = 0.460). A higher maximum overall histologic lesion score was associated with a serum sRAGE concentration ≤ 340 ng/L (p = 0.028; sensitivity: 73%, specificity: 48%), and microscopic lesions in the duodenum were more severe in dogs with serum sRAGE levels ≤ 340 ng/L (p = 0.013; Fig. 3). A cumulative microscopic lesion score in the duodenum of ≥ 4 was detected by a serum sRAGE concentration of ≤ 340 ng/L with a sensitivity of 57% and a specificity of 68%. Serum sRAGE was significantly and inversely correlated with the severity of lymphoplasmacytic infiltration in the gastric antrum (ρ = −0.22, p = 0.038) and duodenum (ρ = −0.25, p = 0.017), and with crypt dilation (ρ = −0.23, p = 0.027) and neutrophilic infiltrate in the duodenum (ρ = −0.21, p = 0.044), in univariate analysis, but none of the results remained statistically significant after correction for multiple comparisons (Table 2). No other correlations of histologic criteria with serum sRAGE concentrations were identified.
Figure 3.
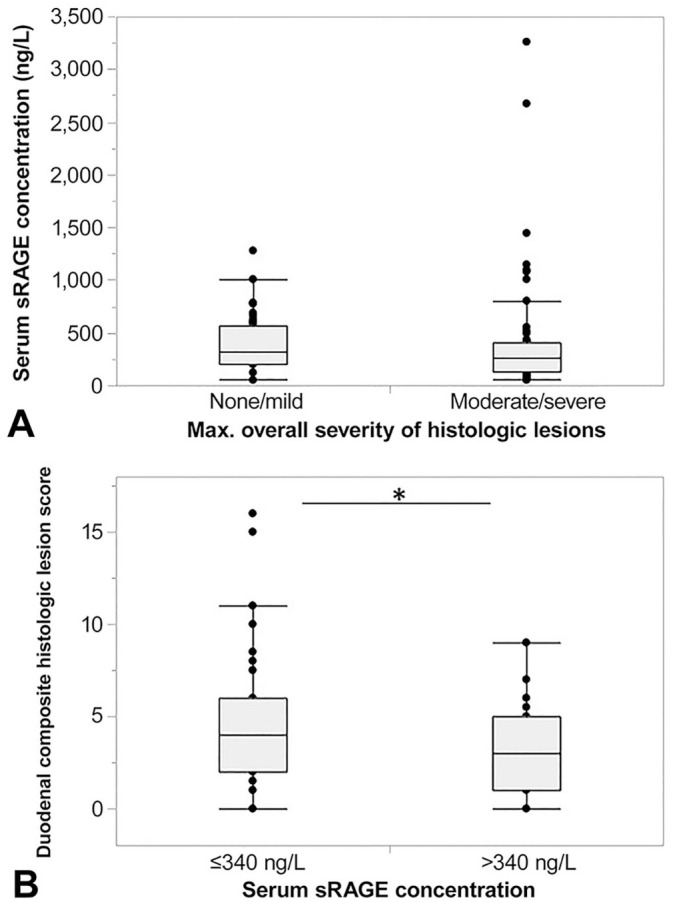
Association of gastrointestinal histologic lesions with serum soluble RAGE (sRAGE) concentrations in dogs with chronic inflammatory enteropathy (CIE; n = 102). A. Patients with moderate-to-severe histologic lesions (score 2–3) had numerically lower serum sRAGE concentrations (median: 257 ng/L, interquartile range [IQR]: 126–413 ng/L; n = 56) compared to dogs with no more than mild histologic lesions (score: 0–1; median: 326 ng/L, IQR: 207–573 ng/L; n = 46), but no significant difference in serum sRAGE concentration was found between the groups of dogs (p = 0.157). B. Cumulative histologic lesion scores in the duodenum in dogs with CIE and a serum sRAGE concentration ≤ 340 ng/L were significantly higher (median: 4, IQR: 2–6; n = 61) compared to dogs with a serum sRAGE concentration > 340 ng/L (median: 3, IQR: 1–5; n = 34; p = 0.013). Asterisk (*) indicates significant difference at p < 0.05.
Table 2.
Correlation between serum soluble RAGE (receptor for advanced glycation end-products) and histologic lesions in dogs with chronic inflammatory enteropathy (n = 102) included in the study.
| Parameter correlated with serum sRAGE concentrations | Spearman ρ | p value | pcorr value |
|---|---|---|---|
| Histologic lesions (composite score) | −0.15 | 0.136 | 0.136 |
| Morphologic criteria (composite score) | −0.13 | 0.198 | 0.396 |
| Inflammatory criteria (composite score) | −0.09 | 0.357 | 0.714 |
| Stomach | |||
| Morphologic criteria | 0.01 | 0.973 | NS |
| Fundus | |||
| Surface epithelial injury | 0.04 | 0.721 | NS |
| Gastric pit epithelial injury | −0.13 | 0.249 | 0.747 |
| Mucosal fibrosis | −0.04 | 0.722 | NS |
| Antrum | |||
| Surface epithelial injury | −0.03 | 0.770 | NS |
| Gastric pit epithelial injury | 0.15 | 0.162 | 0.486 |
| Mucosal fibrosis | −0.01 | 0.903 | NS |
| Inflammatory criteria | −0.12 | 0.269 | 0.269 |
| Fundus | |||
| Intraepithelial lymphocytes | −0.07 | 0.509 | NS |
| Lamina propria LPC | −0.13 | 0.228 | NS |
| Lamina propria eosinophils | 0.14 | 0.204 | NS |
| Lamina propria neutrophils | NA | NA | NA |
| Lamina propria MΦ | 0.07 | 0.486 | NS |
| Lymphoid follicular hyperplasia | −0.01 | 0.978 | NS |
| Antrum | |||
| Intraepithelial lymphocytes | −0.10 | 0.359 | NS |
| Lamina propria LPC | −0.22 | 0.038 | 0.228 |
| Lamina propria eosinophils | −0.03 | 0.789 | NS |
| Lamina propria neutrophils | 0.14 | 0.209 | NS |
| Lamina propria MΦ | −0.03 | 0.812 | NS |
| Lymphoid follicular hyperplasia | −0.10 | 0.369 | NS |
| Duodenum (composite score) | −0.19 | 0.070 | 0.210 |
| Morphologic criteria (sum) | −0.14 | 0.189 | 0.378 |
| Villus stunting | −0.10 | 0.354 | NS |
| Epithelial injury | 0.03 | 0.759 | NS |
| Crypt distension | −0.23 | 0.027 | 0.135 |
| Lacteal dilation | −0.11 | 0.310 | NS |
| Mucosal fibrosis | 0.07 | 0.512 | NS |
| Inflammatory criteria (sum) | −0.14 | 0.189 | 0.378 |
| Intraepithelial lymphocytes | −0.05 | 0.602 | NS |
| Lamina propria LPC | −0.25 | 0.017 | 0.085 |
| Lamina propria eosinophils | 0.01 | 0.902 | NS |
| Lamina propria neutrophils | −0.21 | 0.044 | 0.176 |
| Lamina propria MΦ | 0.12 | 0.256 | 0.768 |
| Ileum (composite score) | 0.11 | 0.629 | NS |
| Morphologic criteria (sum) | 0.09 | 0.670 | NS |
| Villus stunting | 0.13 | 0.545 | NS |
| Epithelial injury | 0.20 | 0.346 | NS |
| Crypt distension | 0.24 | 0.254 | NS |
| Lacteal dilation | −0.25 | 0.238 | NS |
| Mucosal fibrosis | NA | NA | NA |
| Inflammatory criteria (sum) | 0.10 | 0.645 | NS |
| Intraepithelial lymphocytes | 0.04 | 0.843 | NS |
| Lamina propria LPC | 0.17 | 0.417 | NS |
| Lamina propria eosinophils | 0.06 | 0.781 | NS |
| Lamina propria neutrophils | −0.06 | 0.786 | NS |
| Lamina propria MΦ | 0.20 | 0.341 | NS |
| Colon (composite score) | −0.07 | 0.692 | NS |
| Morphologic criteria (sum) | −0.20 | 0.262 | 0.524 |
| Epithelial injury | −0.11 | 0.550 | NS |
| Crypt distension | −0.23 | 0.177 | 0.708 |
| Change in goblet cells | 0.04 | 0.833 | NS |
| Mucosal fibrosis | −0.11 | 0.542 | NS |
| Inflammatory criteria (sum) | 0.08 | 0.649 | NS |
| Intraepithelial lymphocytes | −0.14 | 0.418 | NS |
| Lamina propria LPC | 0.08 | 0.668 | NS |
| Lamina propria eosinophils | 0.15 | 0.389 | NS |
| Lamina propria neutrophils | −0.06 | 0.731 | NS |
| Lamina propria MΦ | 0.02 | 0.910 | NS |
LPC = lymphocytes/plasma cells; MΦ = macrophages; NA = not applicable; NS = not significant; pcorr = Holm–Bonferroni corrected p value (n = 2, 3, 4, 5, or 6). Values in bold indicate significance at p < 0.05.
Correlation of serum sRAGE concentrations with other inflammatory biomarkers
Serum sRAGE concentrations were not associated with serum CRP concentrations (n = 100; ρ = 0.04, p = 0.708), nor with the concentration of serum (n = 55; ρ = −0.08, p = 0.551), or fecal calprotectin (n = 79; ρ = 0.12, p = 0.277), or fecal S100A12 (n = 85; ρ = 0.17, p = 0.132). Serum sRAGE and S100A12 concentrations were also not significantly correlated (n = 39; ρ = −0.17, p = 0.095; Fig. 4).
Figure 4.
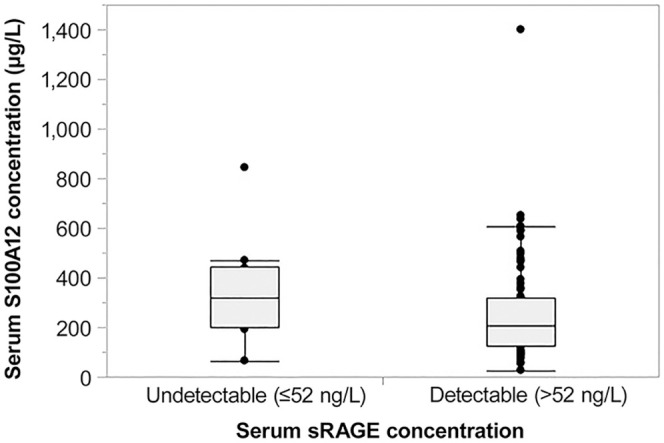
Association of serum sRAGE and S100A12 concentrations in dogs with chronic inflammatory enteropathy (CIE; n = 93). Dogs with an undetectable serum sRAGE concentration (≤ 52 ng/L; n = 10) had numerically higher serum S100A12 concentrations (median: 316 μg/L, interquartile range [IQR]: 197–447 μg/L) compared to dogs with detectable serum sRAGE concentrations (median: 207 μg/L, IQR: 127–320 μg/L; n = 83) but there was no significant difference between the groups (p = 0.081).
Correlation of serum sRAGE concentrations with other prognostic markers
Dogs with more moderate or severe hypoalbuminemia (i.e., a serum albumin concentration < 15 g/L) had numerically lower serum sRAGE concentrations (median: 242 ng/L, IQR: 76–340 ng/L) compared to dogs with normoalbuminemia or mild hypoalbuminemia (i.e., a serum albumin concentration ≥ 15 g/L; median: 297 ng/L, IQR: 161–519 ng/L; Fig. 5), but this difference was not statistically significant (p = 0.095). There was also no significant difference in serum sRAGE concentrations between dogs with hypocobalaminemia (i.e., a serum cobalamin concentration < 300 ng/L; median: 266 ng/L, IQR: 201–422 ng/L) and dogs with normocobalaminemia (i.e., serum cobalamin concentrations ≥ 300 ng/L; median: 296 ng/L, IQR: 140–499 ng/L; p = 0.891). Also, there was no correlation between serum sRAGE and serum (n = 99; ρ = −0.05, p = 0.640) or fecal α1PI concentrations (n = 94; ρ = −0.15, p = 0.154).
Figure 5.
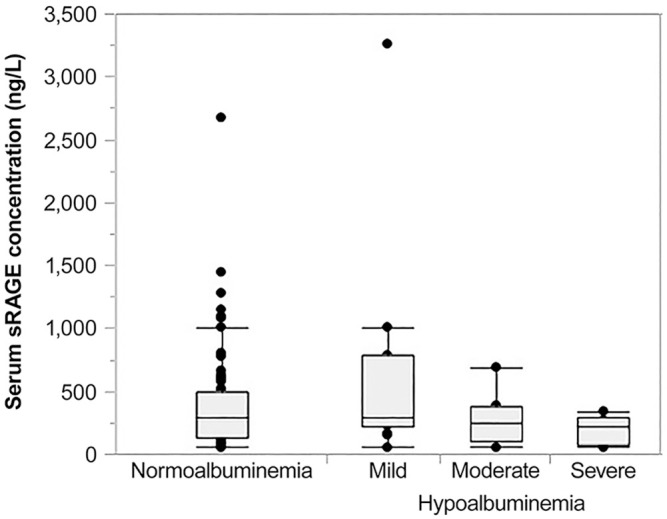
Association of hypoalbuminemia and serum sRAGE concentrations in dogs with chronic inflammatory enteropathy (CIE). Dogs with more severe hypoalbuminemia (n = 15) had numerically lower serum sRAGE concentrations (moderate hypoalbuminemia—median: 252 ng/L, interquartile range [IQR]: 103–381 ng/L; severe hypoalbuminemia—median: 215 ng/L, IQR: 70–286 ng/L) than dogs with normoalbuminemia (median: 297 ng/L, IQR: 137–499 ng/L) or mild hypoalbuminemia (median: 285 ng/L, IQR: 212–782 ng/L; n = 86), but there were no significant differences among those groups of dogs (p = 0.303).
Association of serum sRAGE concentrations with response to treatment
Serum sRAGE concentrations were lower in dogs diagnosed with IRE (n = 15; median: 248 ng/L, IQR: 52–554 ng/L) compared to dogs with FRE (n = 5; median: 415 ng/L, IQR: 217–565 ng/L), but the difference was not statistically significant (p = 0.189; Table 3).
Table 3.
Characteristics of dogs with food-responsive enteropathy (FRE) or immunosuppressant-responsive/-refractory enteropathy (IRE) with available case outcomes (n = 20).
| Group characteristic | FRE | IRE |
|---|---|---|
| Total number | 5 | 15 |
| Age (y), median (IQR) | 8 (1–10) | 8 (7–10) |
| Sex: male/female | 3/2 | 8/7 |
| Body weight (kg), median (IQR) | 19.4 (9.1–37.6) | 12.4 (4.4–25.0) |
| Follow-up time (mo), median (IQR) | 15 (6–18) | 9 (4–12) |
| CCECAI score, median (IQR) | 6 (3–8) | 9 (5–10) |
| Clinical disease severity, n | ||
| Mild* | 2 (40%) | 4 (27%) |
| Moderate† | 2 (40%) | 2 (13%) |
| Severe‡ | 1 (20%) | 7 (47%) |
| Very severe§ | 0 | 2 (13%) |
| Serum sRAGE (ng/L), median (IQR) | 415 (217–565) | 248 (52–554) |
| > 340 ng/L, n | 3 (60%) | 4 (27%) |
| ≤ 340 ng/L, n | 2 (40%) | 11 (73%) |
| 52–340 ng/L, n | 2 (40%) | 7 (47%) |
| ≤ 52 ng/L, n | 0 (0%) | 4 (27%) |
| Serum albumin (g/L), median (IQR) | 31 (30–35) | 22 (17–29) |
| Hypoalbuminemia, n | 0 | 6 (40%) |
| Mild¦ | − | 4 (27%) |
| Moderate# | − | 0 |
| Severe¶ | − | 2 (13%) |
| Serum cobalamin (ng/L), median (IQR) | 367 (293–872) | 226 (178–331) |
| Hypocobalaminemia, n** | 0 | 8 (53%) |
| Serum folate (µg/L), median (IQR) | 15.1 (12.7–21.2) | 15.8 (9.4–19.7) |
| Hypofolatemia, n | 0 | 3 (20%) |
| Hyperfolatemia, n | 0 | 1 (7%) |
| Serum CRP (mg/L), median (IQR) | 1.4 (0.6–2.8) | 14.3 (8.5–37.8)†† |
| Fecal calprotectin (µg/g), median (IQR) | 0.9 (0.5–1.6) | 1.6 (0.8–17.7) |
| Serum calprotectin (mg/L), median (IQR) | 6.1 (2.6–9.6)‡‡ | 9.3 (4.8–11.1)§§ |
| Fecal S100A12 (ng/g), median (IQR) | 72 (35–193) | 205 (59–1,087) |
| Serum S100A12 (µg/L), median (IQR) | 93 (40–233) | 232 (152–393)¦¦ |
| Fecal α1PI (µg/g), median (IQR) | 8.6 (4.7–20.9) | 11.0 (4.0–23.5) |
| Serum α1PI (mg/L), median (IQR) | 1,478 (1,178–1,781) | 1,173 (855–1,571)## |
CCECAI = canine chronic enteropathy clinical disease activity index; CRP = C-reactive protein; IQR = interquartile range.
CCECAI score of 4–5.
CCECAI score of 6–8.
CCECAI score of 9–11.
CCECAI score of ≥ 12.
Defined as a serum albumin concentration 15–20 g/L.
Defined as a serum albumin concentration 12–14.9 g/L.
Defined as a serum albumin concentration < 12 g/L.
Defined as a serum cobalamin concentration < 300 ng/L.
Available from 14 dogs diagnosed with IRE.
Available from 2 dogs with FRE.
Available from 5 dogs with IRE.
Available from 11 dogs with IRE.
Available from 12 dogs with IRE.
Discussion
The weak correlation between the severity of histologic lesions in the proximal gastrointestinal tract (i.e., duodenal lymphoplasmacytic and neutrophilic infiltrate, duodenal crypt dilation, and lymphoplasmacytic infiltration in the gastric antrum)—which did not remain significant after correction for multiple comparisons—together with the decrease in serum sRAGE concentrations might suggest a dysregulation of the sRAGE/RAGE axis in canine CIE, suggesting that sRAGE and also intestinal RAGE expression is of interest to further study the pathology of CIE. The serum sRAGE decoy receptor deficiency might signal a perturbation in membrane-bound RAGE signaling (i.e., an increased activation of the receptor causing proinflammatory intracellular signaling), either as a cause or consequence of the disease. This finding is also interesting, considering the potential worse prognosis of CIE dogs with more severe mucosal lesions in the duodenum1 and our previous finding that serum sRAGE concentrations subsequently increased only in those dogs with a complete response to treatment.15 These findings differ slightly from the results of our previous pilot study in which no correlation was seen between histologic lesions and serum sRAGE concentrations,15 but a limitation of that study was the small sample size. Also, endoscopic lesion scores were not obtained in our current study and could not be evaluated for a possible association with serum sRAGE concentrations. Our results agree with the inverse relationship between serum sRAGE concentrations and the severity of histologic lesions in patients with Crohn disease,7 although the primary disease location in human Crohn disease differs from the distribution of lesions in dogs with CIE.5 Intestinal expression of RAGE at the protein level was also shown to correlate with inflammatory lesions in human patients with IBD.31 Although histologic lesion severity was not evaluated, another study found that plasma sRAGE concentrations correlated with the severity of endoscopic lesions in patients with IBD.25 Whether genetic defects in the RAGE gene also contribute to a dysregulation in the sRAGE/RAGE axis33 in canine CIE is unknown and requires further investigation.
In contrast to the severity of histologic lesions, the severity of clinical signs was not correlated with serum sRAGE concentrations in our study. This finding is consistent with the lack of correlation between RAGE positivity and clinical disease activity index in human IBD patients, but serum sRAGE concentrations were not evaluated in that study.6 However, our findings are in contrast with another study that showed an inverse correlation between clinical disease activity and serum sRAGE concentrations in Crohn disease and ulcerative colitis patients.7 Similar to our results, a study6 also found no correlation with prior treatment or disease duration in people.
Serum sRAGE concentrations were not correlated with any of the serum or fecal biomarkers of inflammation or protein loss that we evaluated, and only a trend of an association was seen for serum S100A12 concentrations. This could be explained by spatial and/or temporal differences in the expression of these molecules and also by the recognition of various 3-dimensional molecular structures by RAGE, which includes exogenous ligands and also endogenous molecules such as the S100/calgranulin proteins.3 Consistent with the results of our study, a correlation of serum sRAGE with fecal calprotectin or serum S100A12 concentrations was also not found in patients with Crohn disease, whereas an inverse correlation existed between serum sRAGE and fecal calprotectin concentration in patients with ulcerative colitis.7 Lack of a relationship between serum sRAGE and CRP concentrations in canine CIE also agrees with the results in patients with ulcerative colitis7 but differs from findings in patients with Crohn disease.7,25 Thus, further evaluation of the ligand-RAGE pathways and the sRAGE/RAGE axis is warranted.
We acknowledge that our study had some limitations. First, the possibility of a concurrent disease process (e.g., chronic or subclinical pancreatitis) being present and affecting serum sRAGE concentrations in some dogs cannot be excluded entirely. Also, fecal viral screening tests are not included in the standard diagnostic work-up of patients with a suspicion of CIE.2,8,10,24 Thus, the possibility of a (concurrent) infectious (e.g., chronic viral or occult parasitic) gastrointestinal disease cannot be entirely excluded in the dogs in our study. Although we included many dogs with CIE, the smaller size of the subgroups of dogs with FRE and dogs with SRE or IRE presents another limitation. Thus, long-term outcome and prognosis could not be evaluated for most dogs in our study. Third, a limited number of dogs were included in the analysis of some markers in serum and fecal samples. Thus, the possibility of a type II error for finding no significant differences or associations cannot be excluded. Further, histopathology of gastrointestinal tissue biopsies was evaluated by 9 different board-certified pathologists (albeit with special expertise in canine gastrointestinal pathology), which might be associated with a high interobserver variation despite the use of standardized criteria.24 Last, sRAGE concentrations in serum were analyzed after sample storage for up to 6 y. The stability of sRAGE in canine serum is unknown and could also affect the results of our study.
Our study confirms our previous finding that serum sRAGE concentrations are significantly decreased in dogs with CIE. Our current study further suggests that serum sRAGE might be associated with the severity of histologic lesions. It remains to be determined whether the decrease detected in the systemic concentrations of this decoy receptor is a cause (decreased systemic production contributing to the pathogenesis of canine CIE) or consequence (consumption in the context of inflammation) of CIE in these patients. With the hypothesis that the expression of full-length transmembrane RAGE in the intestinal epithelium correlates with the serum sRAGE level, further research is now needed to investigate the expression of the RAGE receptor along the gastrointestinal tract in dogs with CIE and in healthy controls.
Acknowledgments
We thank Drs. Martina Protschka and Gottfried Alber for their assistance with acquiring the fluorescence image for RAGE immunostaining. Part of the data was presented as an abstract at the 2018 Annual Congress of the European College of Veterinary Internal Medicine, Sept 2018, Rotterdam, The Netherlands.
Footnotes
Declaration of conflicting interests: Drs. Suchodolski and Steiner are affiliated with the Gastrointestinal Laboratory at the Texas A&M University College of Veterinary Medicine and Biomedical Sciences, where serum CRP and fecal α1PI testing are offered on a fee-for-service basis. The authors declared no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support from a formal grant for the research, authorship, and/or publication of this article. Materials and supplies for the study were generously provided by the Gastrointestinal Laboratory at Texas A&M University; publication costs were paid by the Leipzig University Department for Small Animals.
ORCID iDs: Angela Isabel Cabrera-García  https://orcid.org/0000-0001-7971-816X
https://orcid.org/0000-0001-7971-816X
Romy M. Heilmann  https://orcid.org/0000-0003-3485-5157
https://orcid.org/0000-0003-3485-5157
References
- 1. Allenspach K, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 2. Allenspach K, et al. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec 2016;178:178–368. [DOI] [PubMed] [Google Scholar]
- 3. Bierhaus A, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 2005;83:876–886. [DOI] [PubMed] [Google Scholar]
- 4. Body-Malapel M, et al. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for inflammatory bowel disease. Mucosal Immunol 2019;12:468–478. [DOI] [PubMed] [Google Scholar]
- 5. Cerquetella M, et al. Inflammatory bowel disease in the dog: differences and similarities with humans. World J Gastroenterol 2010;16:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciccocioppo R, et al. Role of the advanced glycation end products receptor in Crohn’s disease inflammation. World J Gastroenterol 2013;19:8269–8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciccocioppo R, et al. The circulating level of soluble receptor for advanced glycation end products displays different patterns in ulcerative colitis and Crohn’s disease: a cross-sectional study. Dig Dis Sci 2015;60:2327–2337. [DOI] [PubMed] [Google Scholar]
- 8. Dandrieux JR. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract 2016;57:589–599. [DOI] [PubMed] [Google Scholar]
- 9. Day MJ, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 10. Erdmann C, Heilmann RM. Diagnostic and therapeutic approach to chronic inflammatory enteropathies in dogs. Tierarztl Prax Ausg Kleintiere Heimtiere 2017;45:317–327. [DOI] [PubMed] [Google Scholar]
- 11. Heilmann RM, et al. Development and analytical validation of an enzyme-linked immunosorbent assay for the quantification of canine calprotectin in serum and feces from dogs [abstract]. J Vet Intern Med 2011;25:693. [Google Scholar]
- 12. Heilmann RM, et al. Development and analytical validation of a radioimmunoassay for the measurement of alpha1-proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. J Vet Diagn Invest 2011;23:476–485. [DOI] [PubMed] [Google Scholar]
- 13. Heilmann RM, et al. Serum alpha1-proteinase inhibitor concentrations in healthy dogs—method validation and determination of reference interval and intra-individual variation. Vet Clin Pathol 2013;42:190–195. [DOI] [PubMed] [Google Scholar]
- 14. Heilmann RM, et al. Association between fecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet Immunol Immunopathol 2014;158:156–166. [DOI] [PubMed] [Google Scholar]
- 15. Heilmann RM, et al. Systemic levels of the anti-inflammatory decoy receptor soluble RAGE (receptor for advanced glycation end products) are decreased in dogs with inflammatory bowel disease. Vet Immunol Immunopathol 2014;161:184–192. [DOI] [PubMed] [Google Scholar]
- 16. Heilmann RM, Suchodolski JS. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet Immunol Immunopathol 2015;168:131–134. [DOI] [PubMed] [Google Scholar]
- 17. Heilmann RM, et al. Fecal S100A12 concentration predicts a lack of response to treatment in dogs affected with chronic enteropathy. Vet J 2016;215:96–100. [DOI] [PubMed] [Google Scholar]
- 18. Heilmann RM, et al. Validation of an enzyme-linked immunosorbent assay (ELISA) for the measurement of canine S100A12. Vet Clin Pathol 2016;45:135–147. [DOI] [PubMed] [Google Scholar]
- 19. Heilmann RM, Allenspach K. Pattern-recognition receptors: signaling pathways and dysregulation in canine chronic enteropathies—brief review. J Vet Diagn Invest 2017;29:781–787. [DOI] [PubMed] [Google Scholar]
- 20. Heilmann RM, et al. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J Vet Intern Med 2018;32:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heilmann RM, Steiner JM. Clinical utility of current biomarkers in canine chronic inflammatory enteropathies. J Vet Intern Med 2018;32:1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:64–70. [Google Scholar]
- 23. Jergens AE, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized-controlled trial. J Vet Intern Med 2010;24:269–277. [DOI] [PubMed] [Google Scholar]
- 24. Jergens AE, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol 2014;51:946–950. [DOI] [PubMed] [Google Scholar]
- 25. Meijer B, et al. Total soluble and endogenous secretory receptor for advanced glycation end products (RAGE) in IBD. J Crohns Colitis 2014;8:513–520. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt AM, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitz S, et al. Effects of ex-vivo and in-vivo treatment with probiotics on the inflammasome in dogs with chronic enteropathy. PLoS One 2015;10:e0120779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterenczak KA, et al. Cloning, characterisation, and comparative quantitative expression analyses of receptor for advanced glycation end products (RAGE) transcript forms. Gene 2009;434:35–42. [DOI] [PubMed] [Google Scholar]
- 29. Sterenczak KA, et al. High-mobility group B1 (HMGB1) and receptor for advanced glycation end-products (RAGE) expression in canine lymphoma. Anticancer Res 2010;30: 5043–5048. [PubMed] [Google Scholar]
- 30. Sterenczak KA, et al. Quantitative PCR and immunohistochemical analyses of HMGB1 and RAGE expression in canine disseminated histiocytic sarcoma (malignant histiocytosis). Anticancer Res 2011;31:1541–1548. [PubMed] [Google Scholar]
- 31. Stintzing S, et al. Role of cannabinoid receptors and RAGE in inflammatory bowel disease. Histol Histopathol 2001;26:735–745. [DOI] [PubMed] [Google Scholar]
- 32. Walsh D, et al. Pattern recognition receptors—molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev 2013;24:91–104. [DOI] [PubMed] [Google Scholar]
- 33. Wang ZT, et al. RAGE gene three polymorphisms with Crohn’s disease susceptibility in Chinese Han population. World J Gastroenterol 2014;20:2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Washabau RJ, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]