Abstract
An 8-y-old Labrador Retriever was presented to a small animal practice in northern Virginia with a history of recent lethargy. Physical examination findings were unremarkable. Ultrasound revealed several large hepatic masses and multiple smaller masses involving the pancreas. Cytologic findings on fine-needle aspirates of the hepatic masses included inflammation and necrosis with eosinophilic, membranous oval structures consistent with cestode infection. Histopathologic findings for biopsies of these masses included extensive necrosis, inflammation, and PAS-positive hyaline-like membranous material interpreted as metacestode cyst wall. A PCR product was generated from aspirate material using primers specific for Echinococcus multilocularis. Subsequent sequence data were 100% homologous to E. multilocularis NADH dehydrogenase subunit I gene sequences. The dog received daily oral albendazole (10 mg/kg) treatment, but its condition deteriorated, and the dog was euthanized. The dog, born in Mississippi, was brought as a puppy to Virginia with no other travel history. To our knowledge, alveolar echinococcosis has not been reported previously in a dog in the United States; E. multilocularis infection was apparently acquired in the mid-Atlantic region of the United States.
Keywords: alveolar echinococcosis, dogs, Echinococcus multilocularis, polymerase chain reaction, United States
An 8-y-old, 33.6 kg, neutered male Labrador Retriever was presented to a small animal practice in Clear Brook, Virginia on November 6, 2018. The owners reported that the dog did not want to eat treats and had recently become lethargic. Physical examination findings were unremarkable. Analysis of a blood sample showed mild nonregenerative anemia, hematocrit 0.30 L/L (reference interval [RI]: 0.37–0.61 L/L), reticulocyte count 105 × 109/L (RI: 10.0–110 × 109/L), and mildly elevated alkaline phosphatase activity (341 IU/L, RI: 23–212 IU/L). The dog was returned home for observation.
Two days later, the dog was returned with no improvement in clinical signs. Abdominal and thoracic radiographs demonstrated loss of detail in the cranial abdomen and a possible hepatic mass. Ultrasound images indicated the presence of focal hepatic masses, some with cavitations, consistent with neoplasia (Fig. 1). Also noted were enlarged adrenal glands and an enlarged pancreas with cystic changes consistent with pseudocyst formation, pancreatitis, or neoplasia. Ultrasound-guided, fine-needle aspirates (FNA) of hepatic masses were submitted to Antech Diagnostics (Fountain Valley, CA). Cytologic findings included eosinophilic oval cystic structures (50–650 µm), with folded membranes and moderate numbers of semi-refractile irregular structures (~ 10–20 µm) that were consistent with material of cestode parasite origin (Fig. 2).
Figure 1.
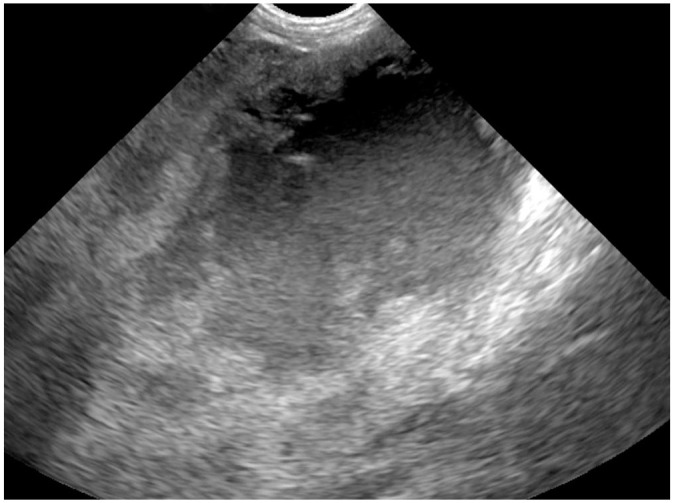
Ultrasound image of a cavitated mass in the liver of an 8-y-old dog with alveolar echinococcosis.
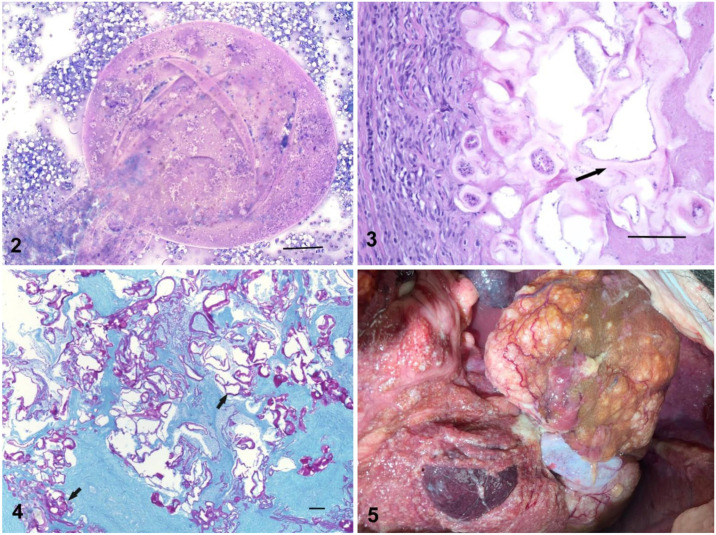
Figures 2–5.
Alveolar echinococcosis in a dog. Figure 2. Example of folded membrane-like structures present in sediment of a fine-needle aspirate of a hepatic mass in the infected dog. Modified Wright Giemsa. Bar = 200 µm. Figure 3. Granulomatous hepatitis, fibrosis, mineralization, and acellular hyaline membrane material (arrow) are present. H&E. Bar = 200 µm. Figure 4. Extensive hyaline membrane (arrows) in periodic acid-Schiff–stained hepatic biopsy tissue. Bar = 200 µm. Figure 5. A multilobular mass was adherent to the liver. The serosal surfaces of abdominal organs were diffusely granular with numerous small white foci.
Based on cytologic results indicating parasite infection, a fecal sample was submitted to Antech Diagnostics for evaluation by centrifugal flotation. Small coccidian oocysts (reported as “consistent with Neospora caninum”) were detected. Because of concern over possible adult tapeworm infection, the patient and 5 canine housemates were each given praziquantel (5 mg/kg orally, daily for 2 d; Droncit; Bayer Animal Health).
Two weeks after initial presentation, the dog was returned to the practice with no change in clinical signs. Blood sample results were unchanged. The dog was sedated as before, and ultrasound-guided FNA of cystic fluid and biopsy samples were collected from liver masses. A portion of a needle biopsy (Tru-Cut; Merit Medical) was fixed in formalin and submitted to Antech Diagnostics. The remainder of the biopsy samples and aspirated fluid were frozen. Histologic examination showed extensive necrosis with little intact hepatic parenchyma. Necrotic debris contained irregular convoluted arrangements of eosinophilic, hyaline membranous material (Fig. 3). Infiltrates of neutrophils, lymphocytes, plasma cells, macrophages, and multinucleate giant cells were present. No protoscolices or calcareous corpuscles were observed in the examined material (Fig. 3). Hyaline membranes stained positively with periodic acid–Schiff (PAS; Fig. 4). Collectively, these findings are typical of infection with the larval stage of Echinococcus multilocularis.3,8,14,15,21
Frozen biopsy material was submitted to the Animal Health Laboratory, University of Guelph (Ontario, Canada) for PCR using primers specific for E. multilocularis.20 A 344-bp product was sequenced and was 100% homologous (344/344 bp) to E. multilocularis NADH dehydrogenase subunit I (NAD1) gene sequences in GenBank (e.g., MG755266, KX384671, KT033489, and KR870967). Additional frozen cyst material was sent to the Zoonotic Parasite Research Unit, University of Saskatchewan (Saskatoon, SK, Canada) for genotyping PCR using primers for the NAD2 mitochondrial DNA locus.13 Over 876 bp, there were single nucleotide variations (SNVs) at 6 positions (42, 157, 234, 246, 252, and 796) consistent with European-type strains in Slovakia, France, and Austria, rather than North American strains (N2) reported in Indiana, Saskatchewan, and Alaska.4 In addition, there was a unique SNV at position 486 that has only been observed in the E5 Slovakian strain.13
In early December 2018, ~ 1 mo after the original presentation, the dog was evaluated for possible surgical excision of the hepatic masses. Following abdominal ultrasound, it was determined that complete excision was impossible and surgery was not attempted. Daily treatment of the dog was started with albendazole (10 mg/kg/d, orally; Valbazen; Zoetis). Analysis of a blood sample 1 wk after initiation of albendazole treatment showed no evidence of bone marrow suppression. On December 14, the dog had a cough, and radiographic evidence of thoracic and abdominal effusions was found. The dog was treated with furosemide (2 mg/kg, orally, q12h). After ~40 d, treatment was changed to a combination product of spironolactone and hydrochlorothiazide (Aldactazide; Pfizer) administered orally, q12h at a spironolactone dose of 0.5–1 mg/kg. Over the following 56 d, the dog’s condition deteriorated, and it was euthanized on February 7, 2019. A limited examination of the abdominal organs was performed by the primary care veterinarian. The abdomen contained ~500 mL of red-to-brown, opaque liquid. An ~ 15-cm diameter, multilobular, tan, firm mass invaded the caudal aspect of the left liver lobe. The mass was well-vascularized and covered by small raised white foci. A similar, 10-cm diameter, multilobular mass was adhered to, and invaded, the right lateral liver lobe. An additional mass was associated with the pancreas. The serosal surfaces of all abdominal organs, the mesentery, and omentum were diffusely granular and covered by similar small white foci (Fig. 5).
E. multilocularis is a tapeworm in the family Taeniidae, which contains several common small animal tapeworms in the genus Taenia. However, adult Echinococcus tapeworms are much smaller (< 10 mm in length). Also, the larval stage (metacestode) in the intermediate host is morphologically different from Taenia metacestodes.6,19 Three species of zoonotic Echinococcus (E. multilocularis, E. canadensis, and E. granulosus sensu stricto) are found in the United States and Canada, with metacestodes capable of causing severe disease in humans.4
Adult E. multilocularis develop in the small intestine of wild canid definitive hosts following consumption of an infected rodent intermediate host. Dogs, and less frequently, cats may become infected with the adult tapeworm.19 Intermediate hosts vary by geographic region and include voles, deer mice, and lemmings.4 Following ingestion of eggs by an appropriate intermediate host, the larval tapeworm typically grows invasively in the liver, forming multi-cystic structures bound by a germinal membrane that buds exogenously and generates tapeworm protoscolices. When ingested by a definitive host, protoscolices develop into adult tapeworms.19
Uncommonly, dogs or humans that ingest parasite eggs become aberrant intermediate hosts for E. multilocularis. Dogs may be infected through coprophagy of wild or domestic canid feces that contain eggs. The resultant disease, alveolar echinococcosis (AE), is usually associated with damage to abdominal organs.3 The parasite is a recognized public health problem in parts of the world where infection rates in wild canids are high.5
AE in dogs is typically associated with lesions in the liver and other abdominal organs.1,3 Abdominal distension, lethargy, anorexia, and vomiting are common.3 Lesions are usually first detected through radiographic or ultrasound images.16 Liver masses, often with cavitations and evidence of mineralization, are frequent findings3 and were observed in our case. However, gross morphology is not different from hepatic neoplasia or other inflammatory or proliferative hepatic diseases.8
Microscopic examination of FNA or biopsy material is helpful in identifying AE. Calcareous corpuscles, which are small (2–30 µm), round-to-oval mineral concretions, and protoscolices may be seen, but neither is specific to Echinococcus spp.14,19 Typical of E. multilocularis in hematoxylin and eosin–stained tissue sections is replacement of normal tissue with multiple coalescing cyst-like structures surrounded by hyaline membranes that are PAS positive (Fig. 4).3,8,15,21 Other Echinococcus spp. also produce PAS-staining membranes, but the appearance of a multichambered structure growing invasively in normal tissue is most characteristic of E. multilocularis. The metacestode of Taenia crassiceps, another tapeworm of wild canids, also occurs rarely in dogs and other animals and may appear grossly as a multicystic structure,1 but the metacestode stage of this parasite is the cysticercus, composed of a bladder with an invaginated scolex.7 Additionally, the characteristic PAS-staining laminated layer membranes of Echinococcus spp. are absent.2,19
Final confirmation of E. multilocularis infection in cases of AE is made by either species-specific PCR analysis of fluid aspirate or biopsy material or by genus-specific PCR and genotyping.2,3,8,15 These tests are available through a limited number of academic and reference laboratories.
Adult E. multilocularis infection in dogs is not readily detected. Tapeworm segments are typically too small to be detected in feces. Centrifugal flotation testing with sugar solution will recover eggs present in a fecal sample, but the eggs are indistinguishable from those of other taeniid tapeworms.2,11,15 Further, detection by flotation examination substantially underestimates the infection prevalence.2,11 Fecal testing through PCR is a more reliable means of detecting intestinal Echinococcus infections2,20 and is available commercially in North America. The commercial PCR test can also be used to detect DNA of metacestodes in biopsy material and fluid aspirates.
Albendazole is the drug of choice for canine AE but is variably effective.3 Daily treatment is usually required for the remainder of the dog’s life. By the time most cases of canine AE are diagnosed, complete resection of the parasite lesion is usually not possible. Surgical debulking may be performed to manage clinical signs, but surgery plus daily albendazole treatment is not considered to increase survival times compared to albendazole treatment only.3 Praziquantel is not effective for the treatment of AE but is a label-approved effective treatment for adult Echinococcus spp. When AE is suspected, the patient and other dogs and cats in the household should be treated immediately with praziquantel (5 mg/kg daily for 2 d) because of its efficacy against adult tapeworms. The incubation period of AE in dogs is unknown, but cases in young dogs suggest that clinical disease can develop within a few months of infection.3,16 Consequently, a dog with an adult E. multilocularis infection can be exposed to eggs from its intestinal adults and develop AE while the adults are still present. Furthermore, suspicion of AE is usually based on the assumption that the parasite is circulating in local wildlife, creating an ongoing risk of exposure to infected intermediate hosts and hence adult E. multilocularis infection, which poses a risk of infection and AE to owners as well as dogs.
E. multilocularis is found worldwide in northern temperate areas, including regions in Europe, Asia, and North America.4 For several decades, the parasite has been considered endemic in Alaska, arctic Canada, western and south-central Canada, and north-central United States, with infections reported as far east as Ohio and Michigan.4,5 No reports of canine AE occurred in North America until 2009 when an infected dog in British Columbia with no travel history was described.15 Since then, additional cases have been detected in Canada, mostly in Alberta, Saskatchewan, and Ontario, a province that previously had not been considered in the parasite’s endemic range.8,17,18 Surveys have detected E. multilocularis in up to one third of coyotes in Alberta11 and Ontario.9 Only 2 haplotypes of E. multilocularis had been recognized in North America, one in Alaska and the other in the north-central United States.12 However, the genotype of the first case of canine AE in British Columbia was associated with European infections, not previously recognized in North America.10 Since then, cases of canine AE in Ontario have been associated with both European and North American genotypes,9 and mostly European genotypes have been reported in coyotes in western Canada.7
The dog described in our report was born in Mississippi but was purchased as a young puppy and brought to Virginia, with no subsequent travel history. Given that Mississippi is distant from any described endemic area, infection almost certainly occurred in Virginia. To our knowledge, E multilocular is has not been reported previously as being acquired in the eastern United States, nor has canine AE been reported in the United States. Because the clinical incubation period for AE in dogs is unknown, it is not possible to pinpoint when infection occurred. However, the dog had extensive exposure to the environment where it could have consumed feces or other material contaminated with E. multilocularis eggs. Because of the similarity of the parasite’s genotype to European strains, it seems unlikely that the infection was the result of expansion of the parasite’s range from endemic areas of Ohio and Michigan.4,17 An alternative possibility is range expansion of European-associated strains present in AE cases in southern Canada.
Despite the proximity of endemic areas of E. multilocularis, practitioners in the eastern United States have not routinely included this parasite in a differential list for hepatic masses or discussed its zoonotic importance with clients. Research is needed to determine whether infection is endemic in the mid-Atlantic region. However, given evidence of expansion of the parasite’s range in Canada, pet owners should be encouraged to avoid opportunities for pets to consume feces or small rodents. For dogs with access to infected intermediate hosts (and thus, potential for adult tapeworm infection), regular cestocidal treatment can be used to limit further contamination of the environment with eggs that cause AE in both humans and dogs.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anne Zajac  https://orcid.org/0000-0002-7159-7954
https://orcid.org/0000-0002-7159-7954
References
- 1. Alic A, et al. Fatal pulmonary cysticercosis caused by Cysticercus longicollis in a captive ring-tailed lemur (Lemur catta). Vet Parasitol 2017;241:1–4. [DOI] [PubMed] [Google Scholar]
- 2. Conraths F, Deplazes P. Echinococcus multilocularis: epidemiology, surveillance and state-of-the-art diagnostics from a veterinary public health perspective. Vet Parasitol 2015;213: 149–161. [DOI] [PubMed] [Google Scholar]
- 3. Corsini M, et al. Clinical presentation, diagnosis, therapy and outcome of alveolar echinococcosis in dogs. Vet Rec 2015; 177:569. [DOI] [PubMed] [Google Scholar]
- 4. Davidson R, et al. Echinococcus across the north: current knowledge, future challenges. Food Waterborne Parasitol 2016;4:39–53. [Google Scholar]
- 5. Deplazes P, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol 2017;95:317–493. [DOI] [PubMed] [Google Scholar]
- 6. Gardner C, Poynton S. An Atlas of Metazoan Parasites in Animal Tissues. Armed Forces Institute of Pathology, 2006. [Google Scholar]
- 7. Gesy K, Jenkins E. Introduced and native haplotypes of Echinococcus multilocularis in wildlife in Saskatchewan, Canada. J Wildl Dis 2015;51:743–748. [DOI] [PubMed] [Google Scholar]
- 8. Goldsmith D, et al. Pathology in practice. J Am Vet Med Assoc 2018;253:563–565. [DOI] [PubMed] [Google Scholar]
- 9. Kotwa D, et al. Echinococcus multilocularis infection, southern Ontario, Canada. Emerg Infect Dis 2019;25:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkins EJ, et al. Detection of a European strain of Echinococcus multilocularis in North America. Emerg Infect Dis 2012;18:1010–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liccioli S, et al. Sensitivity of double centrifugation sugar fecal flotation for detecting intestinal helminths in coyotes (Canis latrans). J Wild Dis 2012;48:717–723. [DOI] [PubMed] [Google Scholar]
- 12. Massolo A, et al. Echinococcus multilocularis in North America: the great unknown. Parasite 2014;21:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakao M, et al. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int 2009;58:384–389. [DOI] [PubMed] [Google Scholar]
- 14. Oscos-Snowball A, et al. What is your diagnosis? Fluid aspirated from an abdominal mass in a dog. Vet Clin Pathol 2015;44:167–168. [DOI] [PubMed] [Google Scholar]
- 15. Peregrine A, et al. Alveolar hydatid disease (Echinococcus multilocularis) in the liver of a Canadian dog in, British Columbia, a newly endemic region. Can Vet J 2012;53:870–874. [PMC free article] [PubMed] [Google Scholar]
- 16. Scharf G, et al. Radiographic, ultrasonographic, and computed tomographic appearance of alveolar echinococcosis in dogs. Vet Radiol Ultrasound 2004;45:411–418. [DOI] [PubMed] [Google Scholar]
- 17. Schurer JM, et al. Echinococcus in wild canids in Quebec (Canada) and Maine (USA). PLoS Negl Trop Dis 2018;12:e0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skelding A, et al. Hepatic alveolar hydatid disease (Echinococcus multilocularis) in a boxer dog from southern Ontario. Can Vet J 2014;55:551–553. [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson RCA. Biology and systematics of Echinococcus. Adv Parasitol 2017;95:66–109. [DOI] [PubMed] [Google Scholar]
- 20. Traschel D, et al. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007;134:911–920. [DOI] [PubMed] [Google Scholar]
- 21. Weiss A, et al. Canine alveolar echinococcosis: morphology and inflammatory response. J Comp Pathol 2010;143:233–238. [DOI] [PubMed] [Google Scholar]