Abstract
Background
COVID-19 is a pandemic disease and questions rise about the coronavirus 2 (Sars-CoV-2) effect on nervous system. This involvement could help explaining the pathogenesis of this condition and lead to novel therapeutic approaches.
Objective
To assess the occurrence of neurological symptoms in COVID-19 patients during the Italian pandemic outbreak, as reported by physicians.
Materials and methods
In the early days of pandemic emergence we developed an online survey open to all Italian clinicians involved in the diagnosis and management of COVID-19 patients. The survey was structured in three sections, with nine different items concerning the presence of different specific clinical abnormalities. Each item was graded from “absent” to “severe” in a 4-point Likert’s scale.
Main outcomes and measures
Likert’s scale data were analyzed by studying the distribution of responses by using medians and bar charts-relative frequencies. Also, in order to analyze differences in symptoms findings depending on the group of specialty, Likert’s scale data were combined into two nominal categories (“absent”/“low” and “moderate”/”high”/”) and a contingency table chi-square test was used.
Results
126 physicians of 9 different medical specialties, from 10 regions of Italy, filled the online survey. The results show that 87.3% of practitioners reported neurological symptoms. In most cases these were mild and non-specific, but they were severe in a minority of patients. The most common symptoms observed were headache, myalgia and taste and smell abnormalities. Whilst there was no difference between neurologists and non-neurologists, we found that experienced clinicians (defined as clinicians that evaluated more than 30 patients) reported neurological symptoms more frequently than non-expert.
Conclusions
Neurological symptoms have frequently been ported during the Italian COVID-19 pandemic, and thus should be monitored for all affected patients. Whilst some of the disturbances reported may be non-specific and common to other infectious diseases, smell and taste abnormalities might indicate nervous system as entry door for SARS-CoV-2 virus. This interpretation should promote research trials to avoid nervous system involvement.
Introduction
In March 2020 Italy became the second most affected country in the world and death toll overtook those in China. Symptoms of COVID-19 include respiratory illness, fever, dry cough and dyspnea [1, 2]. In severe cases, patients have interstitial pneumonia with acute respiratory distress syndrome (ARDS) and require mechanical ventilation [1, 2]. There is a growing evidence that SARS-CoV-2 can involve organs other than the lung, including the nervous system [3, 4]. The most common neurological symptoms reported are headache, anosmia and ageusia [5–7]. Other common symptoms include stroke, impairment of consciousness, seizure, and encephalopathy [8–10]. Some authors suggested that SARS-CoV-2 neurotropism could contribute to the severity of respiratory failure [11, 12]. Netland et al. reported the involvement of cardiorespiratory centers in the medulla of transgenic mice for the SARS-CoV receptor (human angiotensin-converting enzyme 2) [13]. Because SARS-CoV-2 shows highly homological sequence with SARS‐CoV they can share the same neurotropism [14].
This study aimed to rapidly get an insight into the occurrence of neurological manifestations of the rising epidemic outbreak of COVID-19 disease in Italy by a structured web-based, on-line survey among the Italian physicians involved in the management of acute patients. The data may be useful for other countries that are going to face this dramatic emergency.
Materials and methods
Study design and participants
Medical doctors were invited to fill the on-line survey through the Italian medical web community and different social media (WhatsApp, Facebook, Instagram, Websites). 126 colleagues replied in five weeks. The online survey was administered via Google form and was organized in 3 sections:
Section 1 assessed medical specialty, current geographic region of work and number of cases encountered (<10, 10–30, 30–50, >50 cases)
Section 2 estimated the frequency of general symptoms and laboratory findings in COVID-19, rated on a 4-point Likert scale from 1 “Never” to “Always”
Section 3 estimated the frequency of neurological symptoms in COVID-19 patients. The first question addressed the overall presence of neurological symptoms in patients. The second one provided a set of representative symptoms organized in multiple choice grid: each symptom was rated on a 4-point Likert scale from 1 “Never” to 4 “Always”. A 4-point scale was preferred among 5-point scale to avoid neutral responses. In the last item of the survey participants were asked if neurological symptoms were supposed to be “incidental”, “probably correlated” or “directly caused” by SARS-Cov-2.
The full survey is available in Supplemental Materials.
According to the Italian regulation concerning surveys for physicians, the study was not required to be approved by ethical committee. The Alberta Research Ethics Community Consensus Initiative (ARECCI) ethic screening tool was also used to assess and address the ethical dimension of our study: the score was 0, corresponding to a “minimal risk” [15]. In particular: 1) survey data were completely anonymous with no personal information collected; 2) the data were not sensitive or confidential in nature and the issues researched were not likely to upset or disturb participants; 3) participants were not recruited from vulnerable or dependent groups and they get no benefit from participation. Finally, because the study was on voluntary basis, the act of completing the survey was considered an indication of the respondent’s consent to participate. The full results of the ARECCI ethic screening tool are available in Supplemental Materials.
Data analysis
The survey was developed on the basis of systematic literature review and through discussions with clinicians. We mainly focused on the estimated frequency of a broad list of neurological symptoms to address further studies. Progression to each next question was not possible before answers to all the preceding questions had been registered. The pilot phase indicated that completing the survey took approximately five minutes.
The Likert’s scale data were evaluated analyzing the distribution of responses by using medians and bar charts-relative frequencies. To test the hypothesis that 1) there were no significant differences in neurological symptoms recognition between neurologists and non-neurologists and 2) there would be no difference in the estimated frequency of neurological symptoms between expert and non-expert physicians (defining “expert” physicians that evaluated more than 30 patients) data were combined into two nominal categories (“absent”/”low” and “moderate”/”high”) a contingency table chi-square test was used.
Results
Replied to the survey 61.1% neurologists, 13.5% specialists in internal medicine and 25.4% physicians from other medical specialties: infectious disease (7.9%), lung disease (2.3%), emergency physician (3.1%) and anesthesiology (0.8%). The majority of participants was therefore not a neurologist. 65.8% surveys were from Lombardy, the area with highest number of patients in Italy. The number of patients evaluated was < 10 for 36.5% of clinicians; between 10 and 30 for 19.0% of them; between 30 and 50 for 19.0% of them and > 50 for 25.4%. Hence, our sample of physicians evaluated a number of patients estimated approximately between 2600 and 4200.
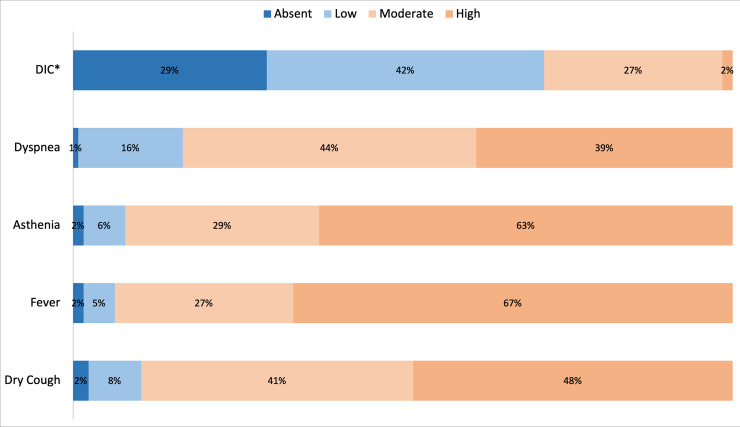
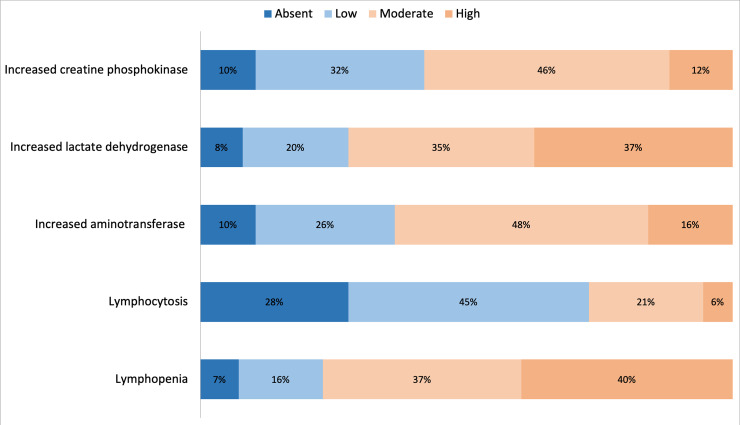
Non-neurological symptoms and laboratory data are consistent with those previously reported thus providing a control variable of the reliability of the survey (Figs 1 and 2) [1, 2].
Fig 1. Summary of reply distribution to Question 4: “Evaluate the frequency of these symptoms in COVID-19 patients”.
*DIC = disseminated intravascular coagulopathy”.
Fig 2. Reply distribution to Question 5: “Evaluate the frequency of these laboratory findings in COVID-19 patients”.
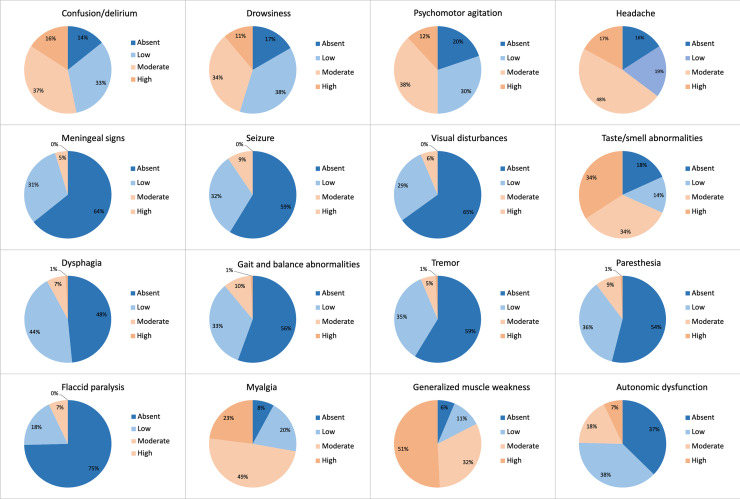
Neurological symptoms are reported in 87.3% of the surveys. They are “rare” for 41.2% of doctors, “occasional” for 21.4%, “frequent” for 26.2% and “always present” for 2.4%. Fig 3 describes the symptoms observed (Fig 3). 73.8% of physicians report some correlation between the COVID-19 and neurological symptoms: 41.2% consider neurological symptoms as “probably correlated”, 21.4% with a causal relationship, and 11.1% “incidental”.
Fig 3. Reply distribution to Question 7: “Evaluate the frequency of these neurological findings in COVID-19 patients”.
Whereas neurologist do not differ from other specialists in detecting neurological symptoms (p = 0.27), “expert” physicians report neurological symptoms with higher frequency than other colleagues (p<0.005). Tables 1 and 2 compare the responses of neurologists and non-neurologist in addressing neurological and non-neurological symptoms and laboratory findings.
Table 1. Non neurological symptoms and laboratory findings in neurologists and non-neurologists.
| Non neurological symtoms | absent | low | moderate | high | absent (%) | low (%) | moderate (%) | high (%) | |
| Dry Cough | neurologist | 2 | 7 | 35 | 33 | 2.6% | 9.1% | 45.5% | 42.9% |
| non neurologist | 1 | 3 | 17 | 28 | 2.0% | 6.1% | 34.7% | 57.1% | |
| Fever | neurologist | 2 | 5 | 28 | 42 | 2.6% | 6.5% | 36.4% | 54.5% |
| non neurologist | 0 | 1 | 6 | 42 | 0.0% | 2.0% | 12.2% | 85.7% | |
| Asthenia | neurologist | 2 | 5 | 26 | 44 | 2.6% | 6.5% | 33.8% | 57.1% |
| non neurologist | 0 | 3 | 11 | 35 | 0.0% | 6.1% | 22.4% | 71.4% | |
| Dyspnea | neurologist | 1 | 12 | 43 | 21 | 1.3% | 15.6% | 55.8% | 27.3% |
| non neurologist | 0 | 8 | 13 | 28 | 0.0% | 16.3% | 26.5% | 57.1% | |
| DIC* | neurologist | 19 | 37 | 20 | 1 | 24.7% | 48.1% | 26.0% | 1.3% |
| non neurologist | 18 | 16 | 14 | 1 | 36.7% | 32.7% | 28.6% | 2.0% | |
| Laboratry Findings | absent | low | moderate | high | absent (%) | low (%) | moderate (%) | high (%) | |
| Lymphopenia | neurologist | 5 | 15 | 28 | 29 | 6.5% | 19.5% | 36.4% | 37.7% |
| non neurologist | 4 | 5 | 19 | 21 | 8.2% | 10.2% | 38.8% | 42.9% | |
| Lymphocytosis | neurologist | 17 | 37 | 17 | 6 | 22.1% | 48.1% | 22.1% | 7.8% |
| non neurologist | 18 | 20 | 10 | 1 | 36.7% | 40.8% | 20.4% | 2.0% | |
| Increased aminotransferase | neurologist | 8 | 19 | 37 | 13 | 10.4% | 24.7% | 48.1% | 16.9% |
| non neurologist | 5 | 14 | 23 | 7 | 10.2% | 28.6% | 46.9% | 14.3% | |
| Increased lactate dehydrogenase | neurologist | 5 | 16 | 30 | 26 | 6.5% | 20.8% | 39.0% | 33.8% |
| non neurologist | 5 | 9 | 14 | 21 | 10.2% | 18.4% | 28.6% | 42.9% | |
| Increased CPK | neurologist | 7 | 27 | 34 | 9 | 9.1% | 35.1% | 44.2% | 11.7% |
| non neurologist | 6 | 13 | 24 | 6 | 12.2% | 26.5% | 49.0% | 12.2% |
Table 2. Neurological symptoms in neurologists and non-neurologists.
| Neurological symtoms | absent | low | moderate | high | absent (%) | low (%) | moderate (%) | high (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Confusion/delirium | neurologist | 11 | 20 | 31 | 15 | 14.3% | 26.0% | 40.3% | 19.5% |
| non neurologist | 7 | 21 | 16 | 5 | 14.3% | 42.9% | 32.7% | 10.2% | |
| Drowsiness | neurologist | 13 | 26 | 31 | 7 | 16.9% | 33.8% | 40.3% | 9.1% |
| non neurologist | 8 | 22 | 12 | 7 | 16.3% | 44.9% | 24.5% | 14.3% | |
| Psychomotor agitation | neurologist | 13 | 24 | 30 | 10 | 16.9% | 31.2% | 39.0% | 13.0% |
| non neurologist | 12 | 14 | 18 | 5 | 24.5% | 28.6% | 36.7% | 10.2% | |
| Headache | neurologist | 10 | 17 | 32 | 18 | 13.0% | 22.1% | 41.6% | 23.4% |
| non neurologist | 6 | 11 | 22 | 10 | 12.2% | 22.4% | 44.9% | 20.4% | |
| Meningeal signs | neurologist | 45 | 29 | 3 | 0 | 58.4% | 37.7% | 3.9% | 0.0% |
| non neurologist | 36 | 10 | 6 | 0 | 73.5% | 20.4% | 12.2% | 0.0% | |
| Seizure | neurologist | 36 | 31 | 10 | 0 | 46.8% | 40.3% | 13.0% | 0.0% |
| non neurologist | 38 | 9 | 2 | 0 | 77.6% | 18.4% | 4.1% | 0.0% | |
| Visual symtoms | neurologist | 45 | 24 | 8 | 0 | 58.4% | 31.2% | 10.4% | 0.0% |
| non neurologist | 37 | 12 | 0 | 0 | 75.5% | 24.5% | 0.0% | 0.0% | |
| Taste/smell alterations | neurologist | 12 | 7 | 27 | 31 | 15.6% | 9.1% | 35.1% | 40.3% |
| non neurologist | 11 | 10 | 16 | 12 | 22.4% | 20.4% | 32.7% | 24.5% | |
| Dysphagia | neurologist | 34 | 39 | 3 | 1 | 44.2% | 50.6% | 3.9% | 1.3% |
| non neurologist | 27 | 16 | 6 | 0 | 55.1% | 32.7% | 12.2% | 0.0% | |
| Gait and balance alterations | neurologist | 34 | 32 | 10 | 1 | 44.2% | 41.6% | 13.0% | 1.3% |
| non neurologist | 36 | 10 | 3 | 0 | 73.5% | 20.4% | 6.1% | 0.0% | |
| Tremors | neurologist | 49 | 24 | 4 | 0 | 63.6% | 31.2% | 5.2% | 0.0% |
| non neurologist | 25 | 20 | 3 | 1 | 51.0% | 40.8% | 6.1% | 2.0% | |
| Paresthesia | neurologist | 34 | 34 | 9 | 0 | 44.2% | 44.2% | 11.7% | 0.0% |
| non neurologist | 31 | 11 | 3 | 1 | 12.5% | 27.1% | 50.0% | 12.5% | |
| Flaccid paralysis | neurologist | 49 | 20 | 8 | 0 | 44.2% | 44.2% | 11.7% | 0.0% |
| non neurologist | 45 | 3 | 1 | 0 | 12.5% | 27.1% | 50.0% | 12.5% | |
| Myalgia | neurologist | 8 | 18 | 34 | 17 | 44.2% | 44.2% | 11.7% | 0.0% |
| non neurologist | 2 | 7 | 28 | 12 | 12.5% | 27.1% | 50.0% | 12.5% | |
| Generalized muscle weakness | neurologist | 7 | 10 | 23 | 37 | 44.2% | 44.2% | 11.7% | 0.0% |
| non neurologist | 1 | 4 | 17 | 27 | 12.5% | 27.1% | 50.0% | 12.5% | |
| Autonomic dysfunction | neurologist | 25 | 33 | 11 | 8 | 44.2% | 44.2% | 11.7% | 0.0% |
| non neurologist | 22 | 15 | 11 | 1 | 12.5% | 27.1% | 50.0% | 12.5% |
Discussion
This is the first medical survey on the occurrence of neurological symptoms in patients with COVID-19. In a healthcare emergency, an online web-based survey is capable to produce a large amount of data in a short time for a fairly low cost and therefore provides a wide ‘snapshot of how things are at a specific time’ [16]. The main finding is that the majority (87.3%) of physicians involved in the diagnosis and management of acute COVID-19 patients observed neurological symptoms. The most common reported symptoms are headache, myalgias, altered state of conscience and taste and smell abnormalities. Because it is still not clear whether myalgia is caused by inflammation or by direct muscle damage from the virus itself, we decided to include it within neurological symptoms.
Though not directly comparable, our data provides results qualitatively similar to those described in the retrospective analysis by Mao et al. [10]. The pattern of symptoms is similar: headache, myalgias and smell and test abnormalities are the three out of the four most commonly reported disturbances in both studies. Despite the qualitative similarity, they found an overall frequency of neurological symptoms (36.4%) lower than suggested by our survey. The difference could arise from the retrospective design of the study of Mao et al: because the “tip of iceberg” of COVID-19 is the respiratory impairment, neurological symptoms could have been under-reported and/or obscured by more prominent respiratory symptoms. An alternative explanation could be that the virus responsible for the Italian outbreak is different from the one that caused the Chinese disease but there is no demonstration that viral genetic mutations have a clinical impact [17, 18]. Lastly, because the pathogenesis of coronavirus-induced disease is influenced by host genotype [19], the increased occurrence of neurological symptoms could arise -older average age of the European population and a different prevalence of comorbidities aside- from different genetic features of Asiatic and European populations.
Whilst some neurological symptoms reported in the survey can be non-specific, others, such as smell and taste abnormalities, are consistent with the involvement of the central nervous system or cranial nerves. A possible explanation is that SARS-CoV-2 enters into human neurons interacting with angiotensin-converting enzyme 2 (ACE2) receptor expressed also in the brain [20, 21]. Studies in animals demonstrated that SARS-CoV spreads to the cardiorespiratory centers in the brainstem through the olfactory pathway, ultimately leading to death even without major lung involvement [13]. By analogy, also SARS-CoV-2 can be neurotropic and through a central mechanism could contribute to the severe respiratory impairment of COVID-19 patients [4, 13, 22].
Whatever the mechanism, neurological symptoms should be carefully monitored in COVID-19 patients. An important issue will be to assess whether neurological disturbances influence the prognosis of COVID-19.
This study has some limitations: first neurologists and non-neurologists are not represented in equal proportions; second our case number is too small to represent an epidemiology survey of the country. Despite the limited data, we believe that this work provides a comprehensive snapshot of the clinicians’ experience on neurological symptoms of COVID-19.
Conclusions
87.3% of physicians managing COVID-19 report in their patients the occurrence of neurological symptoms. They can be relevant to understand the pathogenetic mechanism of the disease and develop new treatment strategy. Whilst some of the disturbances reported may be non-specific and common to other infectious diseases, smell and taste abnormalities might indicate nervous system as entry door for SARS-CoV-2 virus. This interpretation should promote research trials to avoid nervous system involvement.
Supporting information
Survey on neurological symptoms in COVID-19 patients.
(PDF)
(PDF)
Original responses in sheets.
(XLSX)
(PDF)
Acknowledgments
The authors would like to thank colleagues for generously complete the survey.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J, Tu W-J, Cheng W, Yu L, Liu Y-K, Hu X, et al. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–755. 10.1093/cid/ciaa243/5814897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan L, Mu M, Ren HG, Yang P, Sun Y, MD7, Wang R, Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020; Epub March 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filatov A, Sharma P, Hindi F, et al. (March 21, 2020) Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 12(3): e7352; 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, Agrawal S, Ish P, Gaind R., Usha G et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90(1). 10.4081/monaldi.2020.1294 [DOI] [PubMed] [Google Scholar]
- 6.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020. 10.1002/lary.28692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. Published online April 18, 2020 10.1111/epi.16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxley TJ, Mocco J, Majidi S, Majidi S, Kellner CP, Shoirah H et al. Large-vessel stroke as a presenting feature of COVID-19 in the young.N Engl J Med. 2020;382(20):e60 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020; 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020; February 27 10.1002/jmv.25728 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohmwald K, Gálvez MS, Ríos M, Kalergis AM. Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci. 2018; October 26; 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008; 82, 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Zhao S, Teng T, Abdalla AE, Zhu W, Xie L et al. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020; February 22;12(2). pii: E244 10.3390/v12020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberta Innovates. ARECCI Ethics Guideline Tool. Retrieved from https://albertainnovates.ca/wp-content/uploads/2017/11/ARECCI-Ethics-Guideline-Tool.pdf. Accessed 23 March 2020.
- 16.Denscombe M. The Good Research Guide: For Small-scale Social Research Projects. Buckingham: Open University Press; 1998. [Google Scholar]
- 17.Villa M. COVID-19 and italy’s case fatality rate: what’s the catch? Italian institute for international political studies. 27 March 2020; www.ispionline.it/it/pubblicazione/coronavirus-la-letalita-italia-tra-apparenza-e-realta-25563. [Google Scholar]
- 18.Wang M, Li M, Ren R, Brave A, van der Werf S, Chen E-Q et al. International Expansion of a Novel SARS-CoV-2 Mutant; preprint; Infectious Diseases. 2020; 10.1101/2020.03.15.20035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gralinski LE, Ferris MT, Aylor DL, Whitmore AC, Green R, Frieman MB et al. Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross. PLoS Genet. 2015; October 9;11(10): e1005504 10.1371/journal.pgen.1005504 eCollection Oct 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS‐ CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol. 2004; 203:740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baig M, Ali SK, Ali S, Huda N. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. Chem Neurosci. 2020; March 13 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey on neurological symptoms in COVID-19 patients.
(PDF)
(PDF)
Original responses in sheets.
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.