Abstract
Background and Aims
Leaflet shapes of tomato plants (Solanum lycopersicum) have been reduced to simple geometric shapes in previous functional–structural plant models (FSPMs) in order to facilitate measurements and reduce the time required to reconstruct the plant virtually. The level of error that such simplifications introduce remains unaddressed. This study therefore aims to quantify the modelling error associated with simplifying leaflet shapes.
Methods
Realistic shapes were implemented in a static tomato FSPM based on leaflet scans, and simulation results were compared to simple geometric shapes used in previous tomato FSPMs in terms of light absorption and gross photosynthesis, for both a single plant and a glasshouse scenario.
Key Results
The effect of simplifying leaflet shapes in FSPMs leads to small but significant differences in light absorption, alterations of canopy light conditions and differences in photosynthesis. The magnitude of these differences depends on both the type of leaflet shape simplification used and the canopy shape and density. Incorporation of realistic shapes requires a small increase in initial measurement and modelling work to establish a shape database and comes at the cost of a slight increase in computation time.
Conclusions
Our findings indicate that the error associated with leaflet shape simplification is small, but often unpredictable, and is affected by plant structure but also lamp placement, which is often a primary optimization goal of these static models. Assessment of the cost–benefit of realistic shape inclusion shows relatively little drawbacks for a decrease in model uncertainty.
Keywords: Assimilation light, FSPM, GroIMP, gross photosynthesis, high-pressure sodium (HPS) lamp, light-emitting diode (LED), light modelling, plant modelling, realistic leaflet shape, Solanum lycopersicum, tomato
Introduction
Tomato (Solanum lycopersicum) is a crop which requires a relatively high light intensity and temperature in order to grow. Temperatures below 12 °C can lead to physiological injury (Costa and Heuvelink, 2005), which limits the plant from growing outdoors in more northern climates. Glasshouses have been used for many years to combat this temperature problem. Year-round production in glasshouses is, however, still hindered due to the dark months in winter. The use of assimilation light, generally high-pressure sodium (HPS) lamps (Moerkens et al., 2016), solved this problem and found its way to commercial glasshouses as early as the 1980s (McAvoy and Janes, 1984; Dorais et al., 1991; Marcelis et al., 2002). These innovative technologies are widely applied in Belgium and the Netherlands, and resulted in them being the most efficient tomato=producing countries (in kg m−2). Both countries achieved an average yield of around 50 kg m−2 in 2017, which is 22 % more than the runner-up (UK) (FAO, 2017). Another important type of lamps are light-emitting diodes (LEDs) for which research started in the late 1980s (Morrow, 2008), only a few years after HPS lamp research. However, the first LED prototypes for use as supplemental lighting in glasshouses were only developed around 2006 (Morrow, 2008). In recent years, growers have become increasingly interested in these lamps (Moerkens et al., 2016). Their lower heat radiation makes them also suitable for lighting inside the canopy (Trouwborst et al., 2010; Tewolde et al., 2016). These new technologies raise some interesting research questions regarding optimal lamp and plant placement within the glasshouse. Experiments can provide answers to some of these questions, but are constrained in time and space because tomato plants are grown for an entire year with the use of assimilation light.
As an alternative, a so-called functional–structural plant model (FSPM) of tomato can be built. Such an FSPM combines both structure and functionality (e.g. light absorption, photosynthesis, transpiration) of the plant (Godin and Sinoquet, 2005). When combined with a realistic glasshouse structure and virtual lamps, simulations can be performed to test a wide array of different scenarios in a short time span. FSPMs for tomato have been used for this purpose in the past, but have done so with simplified leaflet structures (Sarlikioti et al., 2011a, b; Chen et al., 2014; de Visser et al., 2014). The rationale behind these simplifications is that the composite leaf structure of tomato consists of many leaflets with a complicated, irregular and diverse outline. This makes a low-parameter description of leaflet shapes difficult, as straightforward methods for accurately modelling leaflet shape require entire, simple leaves [e.g. 3-D curve fitting (Fournier and Pradal, 2012); spline interpolation, polynomial fitting and hermite interpolation (Henke et al., 2014); parametric equation fitting (Coussement et al., 2018b)], making them inapplicable for tomato. An alternative, highly accurate method for modelling any leaf contour is through elliptic Fourier analysis (Iwata et al., 2002; Neto et al., 2006), although this requires up to 100 parameters or more, which makes them inapplicable for reconstruction in an FSPM. As a result, the only viable alternative for realistic leaf shape inclusion is the random selection of leaf contours from a database. In FSPMs, the leaflet structure has therefore always been simplified to either rectangles (de Visser et al., 2014), hexagons that approximate ellipses (Sarlikioti et al., 2011b), ellipses (Sarlikioti et al., 2011a) or rhombuses (Chen et al., 2014). Recently, it was shown that the inclusion of a complex leaf shape, rather than a simplified structure, had a significant influence on growth parameters in a dynamic cucumber FSPM (Schmidt and Kahlen, 2018). In a dynamic model, small differences in light interception and photosynthesis can have a propagating effect on the future growth conditions in the model. In a static model, these deviations are absolute, and quantification is warranted so that informed modelling decision with regards to simplifications can be taken.
In this study, a static FSPM for tomato is constructed to evaluate deviations in simulated light conditions and photosynthesis as a result of leaf shape simplifications used in previous research. Tomato plants were grown under a commercial glasshouse set-up with assimilation lighting, where the 3-D plant structure, assimilation light conditions and glasshouse were characterized and reconstructed in a virtual FSPM. Realistic leaflet shapes of tomato plants were reconstructed using scanned images of leaflets that were converted to triangulation points, which can be easily used by 3-D simulation software. Using such an FSPM, we investigated to what extent simplified leaflet shapes alter light absorption and gross photosynthesis in a tomato canopy and how these results can be translated to other crops.
MATERIALS AND METHODS
Experimental design
Tomato plants (Solanum lycopersicum L. ‘Merlice’) were sown in rockwool blocks (Grodan PRO Blok, Grodan, Roermond, The Netherlands) on 31 August 2016. They were grafted on a ‘Maxifort’ rootstock and planted on 19 October 2016 on top of rockwool slabs (Grodan GT Master, Grodan) in a semi-commercial glasshouse (51.08°N, 4.53°E) (Research Station for Vegetable Production, Sint-Katelijne-Waver, Belgium). Two main stems were maintained per plant. An initial interplant distance of 48 cm was used with a row distance of 1.6 m. In both week 46 and week 52, an axillary stem was maintained every 1 : 3 main stems, resulting in a final stem density of 4.34 stems m−2. Half of the plants were lighted using a combination of HPS lamps (1000 W SON-T, Philips, Eindhoven, The Netherlands) (164 µmol m−2 s−1) and LED toplights (Oreon Grow Light 2.1, Lemnis Oreon, Ijsselstein, The Netherlands) (94 µmol m−2 s−1). The other half were lighted with a combination of HPS lamps (177 µmol m−2 s−1) and LED interlights (HORTILED Inter, Hortilux, Monster, The Netherlands) (80 µmol m−2 s−1). Lamps were switched on from 0000 to 1800 h. Four distinct periods were applied during each day: (1) the first part of the artificial day between 0000 h and sunrise when light was only supplied by the lamps, (2) the natural day between sunrise and sunset when light was supplied both by the lamps and by the sun, (3) the second part of the artificial day between sunset and 1800 h, and (4) night-time when lamps were switched off. While these treatments were originally intended to investigate the effects of top and interlighting lamps on tomato plants and their economic feasibility, data from this setup were also used to generate the 3-D structure of glasshouse-grown tomato plants for use in an FSPM. This study focuses solely on the light conditions during periods 1 and 3 because light conditions are very stable during these periods. Incorporation of natural daylight would be possible in the model, but would lead to very variable light conditions, which makes straightforward comparison and quantification of the different leaflet shapes in terms of light interception and photosynthesis difficult. Additionally, light from HPS lamps is often the only light received by commercially grown tomato plants for a large part of the day, i.e. easily up to 9 h per day in wintertime. This ‘artificial day’ is thus a very important part of the day and the focus of this study.
Plant characterization and virtual reconstruction
Accurate approximations of within-canopy light conditions and photosynthesis require four different plant characteristics to be integrated in the model. First, the accurate 3-D topology of the canopy is essential for assessing light interception. A second component, and the main focus of this study, is the accurate inclusion of leaflet shapes. Third, leaf spectral characteristics, which determine whether individual light rays are absorbed, reflected or transmitted when interacting with a leaf, are required. Last, the relationship between absorbed radiation and photosynthesis needs to be included on the individual leaflet level. Characterization and virtual reconstruction in the tomato FSPM of these four model components is outlined below. The static model is constructed in the GroIMP (version 1.5) modelling platform (Kniemeyer, 2008).
Two plants in each treatment (i.e. four plants in total) were destructively measured on 3 and 4 April 2017. The length and thickness of all internodes were measured using a calliper. For trusses, the total length was measured, as well as peduncle thickness, and height and width of all fruits. The length and width of all composite leaves was measured using a ruler. The leaf angle relative to the main stem was measured using a protractor. The thickness of the petiole was measured using a calliper, as well as the thickness of the rachis near the middle and the tip of the leaf. The angle of the petiolules relative to the rachis was measured using a protractor. No structural differences were found between the two light treatments and thus all data were pooled. Allometric relationships between measured values of all plant organs and their position in the plant were established (Supplementary Material S1) and used to model the static structure of a fully-grown tomato plant. Each plant consisted of 11 sympodial units, where one sympodial unit consisted of four phytomers. The first and second phytomer consisted of an internode with a leaf. The third phytomer was just an internode as its leaf was consistently cut at a young age, as is done in practice. The fourth phytomer had a truss on its internode. An exception was made for the first two phytomers where all leaves were cut, also according to practice in glasshouse tomato cultivation. This gave rise to plants with a total of 18 leaves with leaf number 1 being the oldest leaf at the bottom of the canopy and leaf number 18 being the youngest leaf at the top of the canopy.
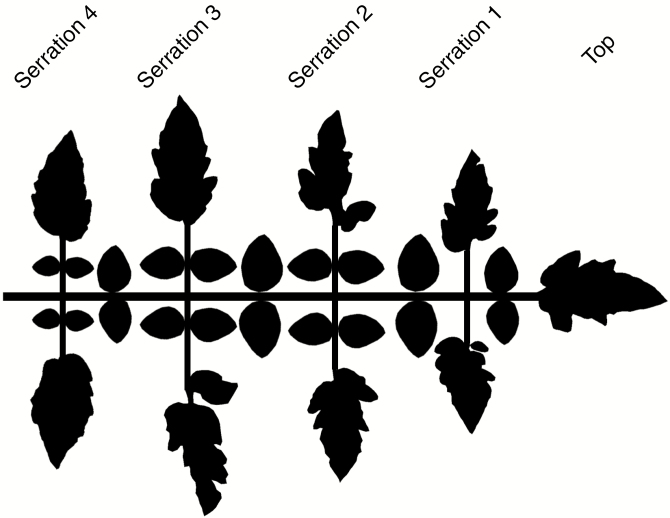
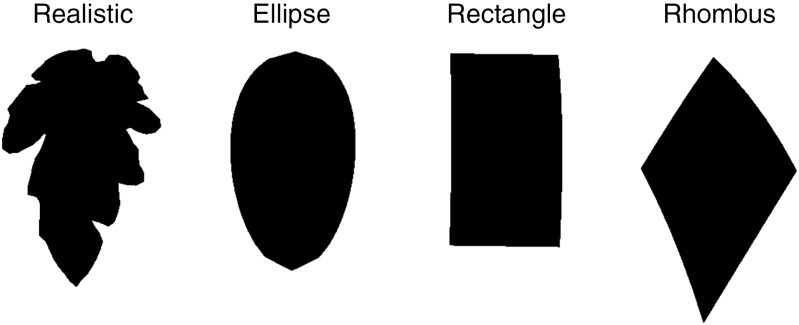
To obtain accurate descriptions of the diverse shapes of the individual leaflets, the leaflets were dissected and scanned (CanoScan LiDE 120, Canon Inc., Tokyo, Japan). The outlines of all tomato leaflets were drawn by running the scanned images through a custom ImageJ (version 1.50i) macro. Leaflet length, width and area were also automatically determined. The outlines were used as input for the ‘Triangle’ python script (Shewchuk, 1996) to create triangulation points in order to model tomato leaflets in GroIMP. In the model, four leaflet shape modelling approaches were assessed: realistic shapes using the scans, rectangles, ellipses and rhombuses, all modelled with identical leaflet surface area. The rectangles, ellipses and rhombuses all had a width/length ratio of 0.5518, which was comparable to the real tomato leaflets measured during the experiment. For the realistic shape, six different leaflet types were distinguished (i.e. top, serration 1, serration 2, serration 3, serration 4 and secondary; Fig. 1) which was assumed to be sufficient for capturing the realistic shape. All leaflet triangulation points belonging to one specific leaflet type were pooled. When a leaf was created in the model, the outline of a leaflet was randomly chosen from this pool, implemented as a meshnode and subsequently scaled to the desired size. An exception was made for secondary leaflets. The variability in their shape was very low compared to the main leaflets and thus the outline of one generic secondary leaflet was used. Real tomato leaflets show curvature and are different from the 2-D scans. Therefore, a Z-coordinate was added to the meshnode using a second-degree polynomial. Because addition of this Z-coordinate resulted in leaflet lengthening, the leaflet area increased as well. Therefore, additional scaling was performed to correct this offset. The other leaflet shapes (rectangle, ellipse and rhombus) were also modelled (Fig. 2) using meshnodes because this facilitated the implementation of curvature and scaling. Triangulation points of the original flat leaflets were also stored and used to study the influence of curvature.
Fig. 1.
A schematic overview of the tomato composite leaf structure. After the top leaflet, four different serrations can be seen at both ends a large leaflet. Smaller secondary leaflets can be found on the rachis between the different serrations, but also on the petiolules of serrations 2, 3 and 4. Terminal leaflets were randomly chosen because of their large variation. The smaller secondary leaflets were identical in shape due to a low variation. The figure is not to scale because the overlap that exists in real tomato leaves would create an unclear overview.
Fig. 2.
An overview of the different leaflet shapes that were compared in the model. All leaflets shown here have the same leaflet area. The curvature of the leaflets is also slightly visible.
The light transmission spectrum of leaflets was measured using a light source in a closed box and a spectrometer with cosine corrector (FLAME-S-VIS-NIR-ES, Ocean Optics, Largo, FL, USA). At the same leaflet position where light transmission was measured, a SPAD measurement was carried out using a SPAD-502 Plus Chlorophyll Meter (Konica Minolta, Tokyo, Japan) to obtain an indication of relative chlorophyll content. The relationship between leaf spectral characteristics and leaf chlorophyll content was established using the technique introduced by Coussement et al. (2018a). First, the light transmission measurements were used to calibrate the PROSPECT-D model (Jacquemoud and Baret, 1990; Féret et al., 2017), which models leaf reflectance and transmittance based on seven parameters representing the biochemical leaf composition in terms of four leaf pigments (i.e. chlorophyll, carotenoid, anthocyanin and relative amount of brown pigment) and three additional characteristics (i.e. the amount of photosynthetically active ‘layers’ in the leaf, the equivalent water thickness and the dry leaf mass per leaf area). Within a single plant species, the variability of several of these leaf characteristics is often limited (i.e. anthocyanin and brown pigment content), while others have little to no influence on the spectral range of interest (400–700 nm) in this study, as the model is originally designed to simulate a large variety of leaf compositions over the spectral range of 400–2500 nm. As expected, the measured SPAD values and the calibrated chlorophyll content parameters of the PROSPECT-D model, established through the measured transmission spectra, were highly correlated. Additionally, carotenoid content was found to be linearly related to leaf chlorophyll content and little variation was found within the structural parameter. As a result, the spectral characteristics of each leaf could be estimated using only the measured SPAD values as an indicator. Virtual reconstruction of the spectral characteristics was thus achieved by incorporating an allometric relationship between SPAD and leaf rank, the correlation between SPAD and the relevant PROSPECT-D parameters, and the actual PROSPECT-D model in the static FSPM. This allowed calculation of leaf absorption, transmission and reflectance of individual leaves over the entire spectral range of 400–700 nm.
Individual leaf photosynthesis rates were established by measuring light response curves on four different levels in the canopy using an LI-6400XT portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA) in March 2017. On each level, four curves (measured at 2000, 1000, 500, 250, 100, 50, 25 and 0 µmol photons m−2 s−1) were established. Eight additional shorter curves (measured at 1500, 250 and 0 µmol photons m−2 s−1) per level were measured. These light response curves were used to calibrate the combined photosynthesis – stomatal conductance – transpiration (P-SC-T) model of Kim and Lieth (2003). This model consists of three sub-models: a photosynthesis model, a stomatal conductance model and an energy balance model. Specifically, the photosynthesis sub-model is the biochemical model of photosynthetic CO2 assimilation in leaves of C3 species (Farquhar et al., 1980), with the modifications of Harley et al. (1992) and de Pury and Farquhar (1997). The stomatal conductance model is the established model of Ball et al. (1986). The leaf age parameter introduced by Kim and Lieth (2003), which incorporates a reduction in photosynthesis in very young and very old leaves, was estimated based on the different leaf levels (and thus ages) at which measurements were conducted. The P-SC-T model was implemented in the leaf organ module in the static FSPM. Based on measurements in the glasshouse, the following environmental conditions were chosen during simulations: 70 % relative humidity, 21 °C air temperature and 600 ppm CO2 concentration.
All these data were combined to recreate average tomato plants in GroIMP. Such a plant consisted of 44 internodes, 18 composite leaves, eight trusses and three flower stalks. The structure of the composite leaves (Fig. 1) consists of 43 petiole components, 20 secondary leaflets and nine irregular leaflets (i.e. top and serrations). The trusses consisted of 20 peduncle components and five tomato fruits.
Characterization and virtual re-creation of the light model
Virtual re-creation of the light conditions was achieved by constructing a virtual HPS lamp using measurements of the light spectrum with a spectrometer (Jazz spectrometer, Ocean Optics) in the glasshouse, and the light distribution file of a standard HPS lamp (1000 W GreenPower, Philips) installed in a square reflector. Using the full spectral ray tracer, called FluxLightModel, which is readily available in the GroIMP modelling software (Henke and Buck-Sorlin, 2017), an accurate representation of the light conditions was achieved. The ray tracer was set to simulate light rays between 400 and 700 nm at 5-nm intervals based on the spectrum measured in the glasshouse. For each simulation, 200 million rays were used with a maximum of ten possible reflections or transmissions.
Virtual glasshouse reconstruction
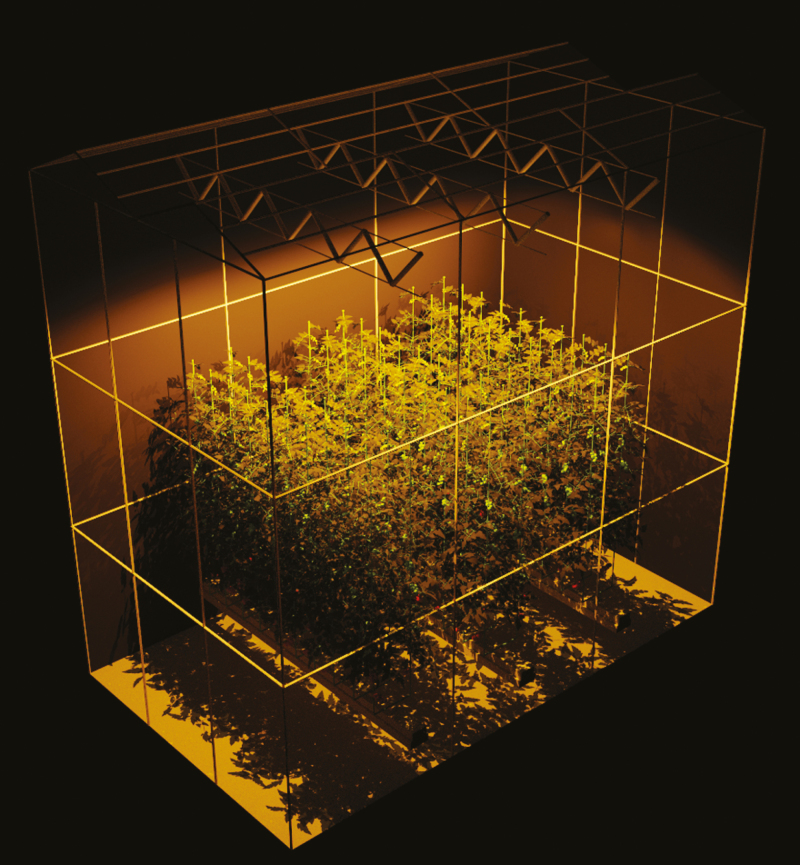
The glasshouse module contains the floor, the glass windows, the glass roof, the aluminium frames supporting the glass, the metal support structure near the top of the glasshouse, plastic support structure, rockwool slabs and rockwool blocks. The spectral properties of all glasshouse elements used in the model are summarized in Table 1. The glasshouse (3.8 × 6.5 × 6 m3) consisted of three rows of plants with a corridor in between two neighbouring rows (Fig. 3). The distance between rows was 1.6 m. In the middle of each row one HPS lamp (2100 µmol photons s−1) was mounted 5 m above the floor (1 m above the plants, which were 2.96 m tall). Plants were topped to create two stems and thus a double row of plants with a distance of 50 cm between them. The distance between different plants in one row was 33 cm. The plants were randomly rotated within the horizontal plane to create a random canopy. In total, 60 plants were simulated, but data were only used from the two plants in the centre of the glasshouse to minimize border effects.
Table 1.
Overview of the spectral properties (transmission, reflection and absorption) of the glasshouse structure
| Material | Transmission (%) | Reflection (%) | Absorption (%) | Source |
|---|---|---|---|---|
| Floor | 0* | 40 | 60 | de Visser et al. (2014) |
| Glass walls and roof | 80 | 10 | 10 | Kittas and Baille (1998) |
| Aluminium frames | 0* | 80 | 20 | Hasan (2006) |
| Metal structure† | 0* | 80 | 20 | — |
| Plastic support and plastic surrounding the rockwool | 0* | 75 | 25 | de Visser et al. (2014) |
All parameters were independent of wavelength.
* No light transmission was assumed for the floor, plastic, aluminium and metal.
† The properties of aluminium were used for the metal structure.
Fig. 3.
Virtual reconstruction of the glasshouse with 60 tomato plants. Three high-pressure sodium (HPS) lamps were located near the top of the glasshouse. The spectrum of the lamps only contained a small amount of blue and green photons and a large amount of red and yellow photons, causing the visual orange colour of the leaves. The starting position of each plant stem can be seen as the internodes that protrude from the rockwool blocks.
Light distribution simulations
To test the effect of leaflet shape on light distribution in the canopy, different repetitions were carried out. At first, the entire scene was constructed with fully-grown static tomato plants as described above. One repetition consisted of running the light model for plants with curved realistic leaflets, changing the shape of the leaflets without modifying anything else in the scene, and running the light model again. This process continued until light absorption and gross photosynthesis was modelled for all leaflet shapes, both flat and curved. During one repetition, the random seed for the light model was fixed. This ensured that, during one repetition, only the leaflet shape, rather than the random nature of the light model, had an influence on the results. A new random rotation within the horizontal plane was then conducted to generate a new random canopy. New realistic leaflet shapes (both curved and flat) were constructed and the entire process was repeated for the required number of repetitions. It was unnecessary to deconstruct the entire scene and general plant structure (internodes, trusses and petioles) before each new repetition because the general plant structure was constant.
Two different scenarios were tested. The first was the more simplified one and contained one individual tomato plant with one HPS lamp (2100 µmol photons s−1) located 4 m above the floor (1 m above the plant). This scene was set in an infinite black plane (without a glasshouse) and thus when a photon was reflected outside of the boundaries of the plant it was not able to reach the plant again. Results from this scenario demonstrate the effect of leaf simplifications on self-shading in a plant, without canopy interference. Seventy-five repetitions were carried out for this scenario. The second scenario was more realistic and simulated the plants inside the glasshouse as described above. Because of the strong increase in simulation time, 20 repetitions were carried out instead of 75.
Statistical analyses
Because the goal of this work was to investigate whether simplifications in leaflet shape are justified, no global statistical analyses were made where all different shapes were compared. The simplified curved leaflet shapes were statistically compared to the realistic shape using a Wilcoxon signed-rank test (R-Studio, version 1.2.1335). This test requires related samples, which as for each repetition the seed for the light model and the angles of implantation of the plants were kept constant, which led to pairing of the samples. Additionally, comparisons were made between all curved leaflet shapes and their corresponding flat shape.
RESULTS
In the following sections the different ecological scales (i.e. canopy, leaf and leaflet) will be accompanied by the shape (i.e. realistic, ellipse, rhombus and rectangle) and curvature of the leaflets used in the simulations. This was done to avoid overly complicated long treatment names (e.g. rhombus canopy refers to a canopy constructed using rhombus-shaped leaflets).
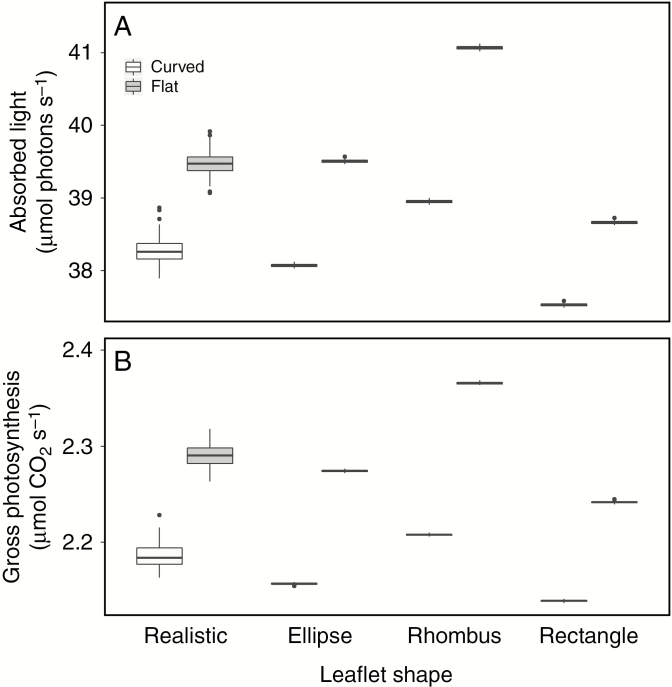
Curved leaflets: individual plant
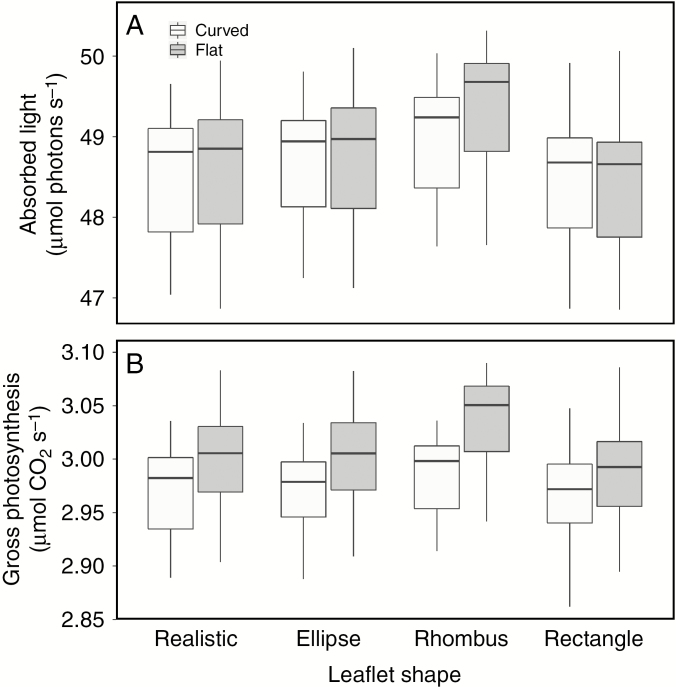
When an individual tomato plant was lighted with a single lamp in an infinite plane, the leaflet shape influenced the total light absorption by the canopy (Fig. 4A). This difference in light absorption revealed no clear trend across leaf ranks, but rather led to unpredictable differences at the individual leaflet level (Fig. 5). Overall, the rhombus canopy diverged most from the other shapes in terms of both whole-plant light absorption and whole-plant photosynthesis. The uppermost leaves of the rhombus canopy (leaf numbers 17 and 18) absorbed on average 9–10 % more light compared to the realistic leaves (P < 0.001). This led to lower absorption in the lower leaves in the rhombus canopy (leaf numbers 13 and 14) of 8–10 % less light than realistic leaves (P < 0.001). For the other leaves in the canopy, differences in captured light varied between the rhombus and realistic leaves. This effect was less relevant in relation to the total light absorption because relative light absorption in the lower half of the canopy was much lower than the top half, 4.12 % vs. 95.88 % of total light absorption, respectively (average across all different leaflet shapes). In total, the rhombus canopy captured 1.8 % more light (P < 0.001) compared to the realistic canopy. The ellipse canopy captured 0.5 % less light compared to the realistic canopy (P < 0.001), even though a higher light absorption was seen for the top two leaves (3.2–4.1 %, P < 0.001). This was compensated for by lower light absorption in the remainder of the top half of the canopy. The lowest light absorption was registered in the rectangular canopy [1.9 % lower than the realistic canopy (P < 0.001)], which was mostly attributed to a lower light capture in the top two leaves (2.6–2.7 %, P < 0.001). This effect was slightly dampened due to some lower-canopy leaves that absorbed more light than the realistic leaves. The difference in light distribution in the canopy also had an impact on total gross photosynthesis (Fig. 4B). Even though the rhombus canopy absorbed 1.8 % more light, the gross photosynthetic rate of such leaves was only 1 % higher (P < 0.001). The ellipse canopy absorbed 0.5 % less light and this translated to a 1.4 % lower gross photosynthetic rate (P < 0.001). The same effect was observed in the rectangular canopy, where a 1.8 % decrease in light absorption resulted in a 2.2 % decrease (P < 0.001) in gross photosynthesis.
Fig. 4.
Simulations of total (A) light absorption and (B) gross photosynthesis of a single plant in an infinite plane (n = 75). The four different leaflet shapes are compared, both as flat and as curved objects.
Fig. 5.
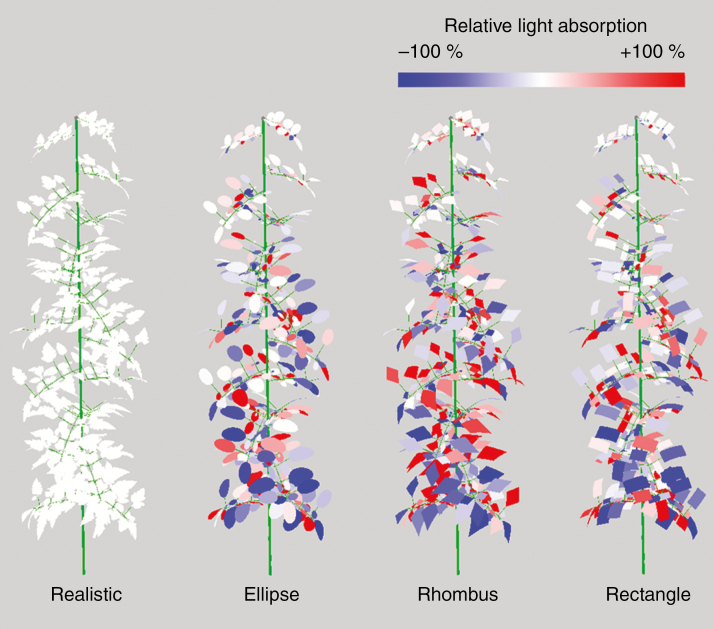
Relative light absorption of the leaflets compared to the realistic shapes of one simulation. Blue leaflets absorbed less light than their realistic counterpart and red leaflets absorbed more light. Leaflets that absorbed 100 % or more light relative to the realistic shapes were given the same red colour. Trusses were removed for the visual representation, but were present during light simulations.
Differences in light absorption and gross photosynthesis between different leaflet shapes on the leaf level were 10 % at most in the top half of the canopy. The bottom half of the canopy showed larger differences, up to 70 %, but they contributed relatively little to total light captured by the canopy. Differences at leaflet level could, however, be many times higher (Fig. 5) with large variations even within one leaf. Differences in light absorption of 50 % and higher were not rare on this scale.
Curved leaflets: glasshouse
More or less the same trends were visible in glasshouse simulations (Fig. 6A), although the differences were less pronounced, especially on the whole-plant level. The rectangular canopy had the lowest light absorption and the rhombus canopy the highest light absorption. The ellipse canopy closely matched the realistic canopy. Rhombus-shaped leaflets absorbed 0.9 % more light (P = 0.038) compared to realistic leaflets, however, gross photosynthetic rates were not significantly different (P = 0.157) (Fig. 6B). In contrast to the individual plant simulations, the glasshouse simulations showed no significant differences between the ellipse and realistic leaf shapes, with an increase of 0.3 % in light absorption (P = 0.445) and 0.03 % (P = 0.862) decrease in gross photosynthesis. The rectangular canopy also displayed non-significant differences with a decrease in light absorption of 0.3 % (P = 0.429) and a decrease in gross photosynthesis of 0.3 % (P = 0.398). However, although many differences were not statistically different at the canopy level, they were at the leaf level.
Fig. 6.
Simulated results of total per-plant (A) light absorption and (B) gross photosynthesis of the two centremost plants in the virtual glasshouse (n = 20). These two plants were surrounded by border plants in order to minimize border effects. The four different leaflet shapes are compared, both as flat and as curved objects.
Differences at the leaf level were still as large as 9 %, which is similar to individual plant simulations. In the glasshouse simulations, the bottom half of the canopy contributed 18 % to the total light absorbed (average across different leaflet shapes), contrasting the mere 4.12 % in the single plant simulations. The standard deviations in absorbed light and gross photosynthesis per plant in the glasshouse simulations were similar across all leaf simplifications (Fig. 6) in sharp contrast to the single plant simulations (Fig. 4) where leaf shape simplifications resulted in a near total loss of variance.
Flat leaflet shape approximation
The effect of discarding curvature was similar for all leaflet shapes in the individual plant simulations. The flat leaflets absorbed on average 3.0–5.4 % more light (P < 0.001) compared to their respective curved leaflet shape (Fig. 4A). Light capture in the top two leaves was higher in curved leaflets, but all other leaves showed a higher light absorption with flat leaflets. This difference in light absorption translated to an increase in gross photosynthesis by 4.8–7.2 % (P < 0.001) (Fig. 4B). The effect on light distribution was different in the glasshouse simulations (Fig. 6) where curvature had no significant effect on light absorption for the realistic, ellipse and rectangular canopy (P > 0.8). The flat rhombus canopy was statistically different from its curved counterpart and absorbed 0.7 % more light (P = 0.063). Even though three of the four flat canopies absorbed the same amount of light as the curved canopies, all of the flat canopies showed an increase in gross photosynthesis: 0.9 % (P = 0.049), 1.0 % (P = 0.040), 1.6 % (P = 0.001) and 0.8 % (P = 0.097) for the realistic, ellipse, rhombus and rectangular canopies, respectively.
Computation time
Simulations were conducted on a high-performance computing cluster (2 × 12-core Intel E5-2680v3; Haswell-EP @ 2.5 GHz) with 64 Gb RAM assigned to the simulation. The impact on computation time of using realistic leaflet shapes for generating virtual plants was limited, with a negligible effect on single-plant simulations. On larger canopies, such as those used in the glasshouse simulations, build-time for the scene was similar (1159–1179 s), but light simulation time was affected by the complexity of the leaflet shape (averaging 201.5 s for the realistic canopy, and ranging between 131 and 148 s for the simplifications). The difference in light simulation time originates from the requirement of using a far larger triangulation number for simulating the meshes of the realistic leaflet shapes (variable complexity), compared to the simple approximation (simulated with a constant triangulation of 40 triangles). If leaflet curvature is not considered, leaflet simplifications have the additional option to not use meshes at all, and use the predefined simple geometric shapes available in GroIMP. This can lead to an additional time saving in both build-time (888 s) and light simulation time (100 s). An additional time-saver would be the use of GPU-based light computing, which is compatible with the FluxLightmodel in GroIMP. As this is equally applicable to all leaflet shapes, it would thus make the absolute difference in simulation time smaller.
Discussion
In the past, there has been some research on the impact of simplifying geometric parameters (leaf position, leaflet inclination, etc.) of tree crowns in 3-D models (Parveaud et al., 2008; Da Silva et al., 2013) and the structure of composite tomato leaves (leaflet number, leaflet size, etc.) (Sarlikioti et al., 2011a). Parveaud et al. (2008) noted a strong decrease, up to 26 %, in intercepted light by the canopy when using a simplified composite leaf structure of hybrid walnut trees (Juglans regia × J. nigra). Sarlikioti et al. (2011a) reported differences in light absorption of up to 19 % for tomato. Despite this research, no studies have assessed the effect of simplifying the shape of the individual leaflets. This was unexpected, because the methods to model leaflets in this research have already been used for at least 15 years in computer imaging (Hong et al., 2005; Lu et al., 2010).
Our results showed that a simplification of the leaflet shape in a tomato crop can lead to small, but significant, deviations in simulations of light absorption and gross photosynthesis. At the canopy level, this effect was shown to be mitigated somewhat by the canopy closure in dense canopies, such as that used in the glasshouse simulation, as overall less light escapes the canopy. At the individual leaf level, however, these differences persisted for both the individual plant and the glasshouse scenario, which can only be attributed to the geometrical properties of the shapes, as leaflet area was kept constant across different shapes. All simplified shapes were chosen to have the same width/length ratio as an average realistic leaflet. This caused the rectangles to be shorter than the realistic leaflets and the rhombuses to be longer, and the length of the ellipse-shaped leaflets was very comparable to the realistic leaflets (Fig. 2). These small differences in leaflet length might have contributed to a slightly wider or narrower canopy and thus also to a slightly lower or higher light absorption. Within-leaf shading can also partially explain the difference between leaflet shapes. In small and young leaves, the leaflets are still in close proximity to each other. The rectangular leaflets are the shortest and thus contain relatively more leaf area near the petiole and rachis, which increases the likelihood of shading other leaflets in the same leaf, thus lowering light absorption. The opposite is true for the rhombuses, which are less likely to self-shade due to their longer shape when compared to realistic leaflets.
Differences in light absorption could not be attributed to a single leaf, but rather to a complex interaction in the entire canopy. Light absorption per plant was often not statistically different in glasshouse simulations, but there were significant differences at the leaf level. This is important because the photosynthetic efficiency of leaves in a tomato canopy is not constant, but decreases with leaf age (Acock et al., 1978; Qin et al., 2011). Additionally, the non-linear response of photosynthesis to light absorption means that higher light penetration can lead to an advantage in overall photosynthesis, even with similar whole-plant light absorption. This is reflected in the results as a certain increase or decrease in light absorption did not lead to the same increase or decrease in gross photosynthesis. The individual plant ellipse canopy absorbed 0.5 % less light when compared to the realistic canopy, but the effect on gross photosynthesis was almost three times larger (i.e. 1.4 % lower). Sometimes the effect was the opposite (e.g. an increase in light absorption of 1.8 % by the individual plant rhombus canopy but only an increase of 1 % in gross photosynthesis). For the glasshouse simulations this effect was also present.
Another degree of realism that was added in comparison with previous studies is the curvature of the leaflets. Previous research has used flat leaflets, which the authors identified as a simplification in their models (Sarlikioti et al., 2011a; de Visser et al., 2014). Representation of the leaflets as triangulation points in our work made implementation of curvature straightforward, although this comes at the cost of an increase in computation time. de Visser et al. (2014) commented that curvature in leaflets might lead to a higher light use efficiency in dense canopies due to increased diffuse reflection. Our simulations did not show this result. Light absorption by plants in the glasshouse was unaffected for three out of four leaflet shapes. Leaflet curvature even decreased light absorption in the rhombus canopy. The individual plant simulations showed a decrease for all leaflet shapes. The introduction of curvature created a slightly narrower canopy as the tip of the leaflets was now pointed slightly towards the ground and not to the sides.
Overall, the simplification of leaflet shapes led to rather small differences in terms of whole-plant photosynthetic capacity. However, several arguments could be made for the inclusion of realistic leaflet shapes in models specifically aimed at assessing light efficiency. First, the results here show that deviations in light absorption do not correspond to an identical deviation in photosynthesis. This indicates that canopy structure plays an important role in light use efficiency and highlights the significance of using more accurate leaflet shapes in studying whole canopy photosynthetic efficiency.
Second, the dependency of the modelling discrepancies on canopy density is an indication that the discrepancies are primarily related to the direct component of radiation. As a result, these discrepancies may be attenuated in open canopies, in young plants and with the use of additional sources of direct radiation. The last of these is especially important when the efficiency or optimal position of specific assimilation lights such as interlights are to be evaluated with an FSPM (e.g. de Visser et al., 2014). Alternatively, the effect may be diminished when a large fraction of incoming light is diffuse, which is often the case under normal daylight conditions, which were not investigated within this study. Open canopies and young plants are of primary importance in dynamic FSPMs, where the growth of plants is simulated over their entire lifetime. An over- or underestimation of assimilated carbon can lead to a positive or negative feedback loop, respectively, with under- or overproduction of leaf area, leading to increasingly large modelling errors. As a result, the modelling discrepancy related to leaf shape simplifications can be higher in a dynamic model, as exemplified by Schmidt and Kahlen (2018).
Third, it could be argued that the use of realistic leaflet shapes has relatively little drawbacks. An increase in measurement time is to be expected due to the need for a leaflet scan database, rather than the faster alternative of a leaf area meter. Post-processing and virtual reconstruction also take longer, but can be completely automated and scripts can be reused. Additionally, these two tasks would only need to be conducted once to establish a reusable database for this purpose. An increase in light computation time is an inevitable drawback, but the effect is quite limited. Taking these drawbacks into account, a decrease in model uncertainty due to the incorporation of realistic leaf shape may be of value, as uncertainty in complex models such as FSPMs is often difficult to assess (Ford and Kennedy, 2011). The inherent impossibility of exactly replicating the crop canopy and light conditions complicates direct comparison of model simulation with experimental measurements. Within dense canopies, with a large degree of canopy closure, actual light measurements in the canopy can be used for validation and compared to virtual sensor results (e.g. Buck-Sorlin et al., 2011; Coussement et al., 2018a) due to the large spatial homogeneity of light conditions below the canopy. In relatively open canopies this becomes problematic because direct, non-intercepted light contributes to the vast majority of overall sensed light below the canopy, leading to highly heterogenic light conditions with clear shadow and light spots.
Compared to the study of Sarlikioti et al. (2011a), who simulated a number of different scenarios with different leaflet arrangements, including internode lengths, leaf elevation and angles, it is clear that realistic leaflet shapes play a relatively less important role in capturing canopy shape compared to other topological characteristics of the plant. With reported differences in light absorption of 2–11 % compared to a reference structure, it is clear that the main focus of plant characterization should be diverted to these components.
Translation of these results to other FSPMs would probably lead to parallel conclusions. Leaflet shape simplifications are expected to exhibit the largest deviations in open canopies, highly irregular leaflet types and dynamic models which rely heavily on early light competition. Additionally, our results show that, if an approximation is to be chosen, it is best to opt for an approximate shape in which the overlap between simplified and realistic shape is maximal, as exemplified by the far lower deviations of the elliptic approximation compared to the rhombic.
Conclusions
In this study, we have shown that using simplified leaflet shapes to represent tomato leaves resulted in significant, but relatively small, deviations from the realistic shape, with changes in individual leaflet light interception, gross photosynthesis and canopy light distribution. However, assessment of the cost–benefit of realistic shape inclusion shows relatively little drawbacks for a decrease in model uncertainty. Especially when static FSPMs, such as those used in this paper, are specifically developed to obtain realistic simulations of light interception or lamp placement, there seems to be few arguments for simplifications. On top of this, the relatively small deviations obtained in this research might lead to larger deviations in dynamic FSPMs. The main workload for re-creating realistic shapes is a one-time investment to create a shape database and automated scripts for shape extraction and reconstruction. Additionally, the cost in simulation time is only marginally increased.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Material S1: allometric relationships in the static tomato model.
ACKNOWLEDGEMENTS
We thank Fran Lauriks and Sarah Verbeke for their help with the collection of data and Gerhard H. Buck-Sorlin for his GroIMP implementation of the Kim–Lieth model.
Funding
This work was supported by Flanders Innovation & Entrepreneurship [IWT140979, LightMan]. The fund supported the PhD of J.V. and research for Ghent University, Research Station for Vegetable Production, Research Centre Hoogstraten, and Knowledge Centre for Energy-related Research, Thomas More.
LITERATURE CITED
- Acock B, Charles-Edwards DA, Fitter DJ, et al. . 1978. The contribution of leaves from different levels within a tomato crop to canopy net photosynthesis: an experimental examination of two canopy models. Journal of Experimental Botany 29: 815–827. doi:10.1093/jxb/29.4.815. [Google Scholar]
- Ball JT, Woodrow IE, Berry JA. 1986. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggins J, ed. Progress in photosynthesis research. Dordrecht: Springer, 221–224. doi:10.1007/978-94-017-0519-6. [Google Scholar]
- Buck-Sorlin GH, de Visser PHB, Henke M, et al. . 2011. Towards a functional–structural plant model of cut-rose: simulation of light environment, light absorption, photosynthesis and interference with the plant structure. Annals of Botany 108: 1121–34. doi:10.1093/aob/mcr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Nguyen TMN, Kahlen K, Stützel H. 2014. Quantification of the effects of architectural traits on dry mass production and light interception of tomato canopy under different temperature regimes using a dynamic functional–structural plant model. Journal of Experimental Botany 65: 6399–6410. doi:10.1093/jxb/eru356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JM, Heuvelink E. 2005. Introduction: the tomato crop and industry. In: Heuvelink E, ed. Tomatoes. Wallingford: CABI Publishing, 1–20. [Google Scholar]
- Coussement JR, Henke M, Lootens P, Roldán-Ruiz I, Steppe K, De Swaef T. 2018a. Modelling leaf spectral properties in a soybean functional–structural plant model by integrating the prospect radiative transfer model. Annals of Botany 122: 669–696. doi:10.1093/aob/mcy105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussement JR, Steppe K, Lootens P, Roldán-Ruiz I, De Swaef T. 2018b. A flexible geometric model for leaf shape descriptions with high accuracy. Silva Fennica 51: 1–14. [Google Scholar]
- Dorais M, Gosselin A, Trudel MJ. 1991. Annual greenhouse tomato production under a sequential intercropping system using supplemental light. Scientia Horticulturae 45: 225–234. doi:10.1016/0304-4238(91)90067-9. [Google Scholar]
- FAO 2017. Worldwide tomato production 2017. Rome: FAO. [Google Scholar]
- Farquhar GD, Von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 90: 78–90. [DOI] [PubMed] [Google Scholar]
- Féret JB, Gitelson AA, Noble SD, Jacquemoud S. 2017. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sensing of Environment 193: 204–215. doi:10.1016/j.rse.2017.03.004. [Google Scholar]
- Ford ED, Kennedy MC. 2011. Assessment of uncertainty in functional–structural plant models. Annals of Botany 108: 1043–53. doi:10.1093/aob/mcr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier C, Pradal C. 2012. A plastic, dynamic and reducible 3D geometric model for simulating gramineous leaves. In Proceedings - 2012 IEEE 4th International Symposium on Plant Growth Modeling, Simulation, Visualization and Applications, PMA 2012. Shanghai, China: IEEE press, 125–132. doi:10.1109/PMA.2012.6524823. [Google Scholar]
- Godin C, Sinoquet H. 2005. Functional – structural plant modelling. New Phytologist 166: 705–708. doi:10.1111/j.1469-8137.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- Harley PC, Thomas RB, Reynolds JF, Strain BR. 1992. Modelling photosynthesis of cotton grown in elevated CO2. Plant, Cell & Environment 15: 271–282. doi:10.1111/j.1365-3040.1992.tb00974.x. [Google Scholar]
- Hasan H. 2006. Aluminium - Understanding the elements of the periodic table. New York: Rosen Publishing Group. [Google Scholar]
- Henke M, Buck-Sorlin GH. 2017. Using a full spectral raytracer for calculating light microclimate in functional–structural plant modelling. Computing and Informatics 36: 887–907. doi:10.4149/cai. [Google Scholar]
- Henke M, Huckemann S, Kurth W, Sloboda B. 2014. Reconstructing leaf growth based on non-destructive digitizing and low-parametric shape evolution for plant modelling over a growth cycle. Silva Fennica 48: 1–23. doi:10.14214/sf.1019. [Google Scholar]
- Hong SM, Simpson B, Baranoski GVG. 2005. Interactive venation-based leaf shape modeling. Computer Animation and Virtual Worlds 16: 415–427. doi:10.1002/cav.88. [Google Scholar]
- Iwata H, Nesumi H, Ninomiya S, Takano Y, Ukai Y. 2002. Diallel analysis of leaf shape variations of citrus varieties based on elliptic Fourier descriptors. Breeding Science 52: 89–94. doi:10.1270/jsbbs.52.89. [Google Scholar]
- Jacquemoud S, Baret F. 1990. PROSPECT : a model of leaf optical properties spectra. Remote Sensing of Environment 34: 75–91. [Google Scholar]
- Kim SH, Lieth JH. 2003. A coupled model of photosynthesis, stomatal conductance and transpiration for a rose leaf (Rosa hybrida L.). Annals of Botany 91: 771–781. doi:10.1093/aob/mcg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittas C, Baille A. 1998. Determination of the spectral properties of several greenhouse cover materials and evaluation of specific parameters related to plant response. Journal of Agricultural Engineering Research 71: 193–202. doi:10.1006/jaer.1998.0310. [Google Scholar]
- Kniemeyer O. 2008. Design and implementation of a graph-grammar based language for functional-structural plant modelling. PhD Thesis, Brandenburg University of Technology. [Google Scholar]
- Lu S, Guo X, Zhao C, Li C. 2010. Shape modeling of organs and structures generating for crops. In: Cao W, White JW, Wand E, eds. Crop modeling and decision support. Heidelberg: Springer-Verlag, 99–108. doi:10.1007/978-3-642-01132-0_12. [Google Scholar]
- Marcelis LFM, Maas FM, Heuvelink E. 2002. The latest developments in the lighting technologies in Dutch horticulture. Acta Horticulturae 580: 35–42. doi:10.17660/ActaHortic.2002.580.3. [Google Scholar]
- McAvoy RJ, Janes HW. 1984. The use of high pressure sodium lights in greenhouse tomato crop production. Acta Horticulturae 148: 877–888. [Google Scholar]
- Moerkens R, Vanlommel W, Vanderbruggen R, Van Delm T. 2016. The added value of LED assimilation light in combination with high pressure sodium lamps in protected tomato crops in Belgium. Acta Horticulturae 1134: 119–124. [Google Scholar]
- Morrow RC. 2008. LED lighting in horticulture. HortScience 43: 1947–1950. [Google Scholar]
- Neto JC, Meyer GE, Jones DD, Samal AK. 2006. Plant species identification using elliptic Fourier leaf shape analysis. Computers and Electronics in Agriculture 50: 121–134. doi:10.1016/j.compag.2005.09.004. [Google Scholar]
- Parveaud C-E, Chopard J, Dauzat J, Courbaud B, Auclair D. 2008. Modelling foliage characteristics in 3D tree crowns: influence on light interception and leaf irradiance. Trees 22: 87–104. doi:10.1007/s00468-007-0172-9. [Google Scholar]
- de Pury DGG, Farquhar GD. 1997. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell & Environment 20: 537–557. [Google Scholar]
- Qin FF, Du FL, Xu HL, Xu QC. 2011. Light penetration and leaf photosynthesis in canopy of tomato and Aralia cordata in comparison with wheat. Acta Horticulturae 907: 355–358. doi:10.17660/ActaHortic.2011.907.58. [Google Scholar]
- Sarlikioti V, de Visser PHB, Buck-Sorlin GH, Marcelis LFM. 2011a How plant architecture affects light absorption and photosynthesis in tomato: towards an ideotype for plant architecture using a functional-structural plant model. Annals of Botany 108: 1065–73. doi:10.1093/aob/mcr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlikioti V, de Visser PHB, Marcelis LFM. 2011b Exploring the spatial distribution of light interception and photosynthesis of canopies by means of a functional-structural plant model. Annals of Botany 107: 875–883. doi:10.1093/aob/mcr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Kahlen K. 2018. Towards more realistic leaf shapes in functional-structural plant models. Symmetry 10: 278. doi:10.3390/sym10070278. [Google Scholar]
- Shewchuk JR. 1996. Triangle: engineering a 2D quality mesh generator and delaunay triangulator. Applied Computational Geometry: Towards Geometry Engineering 1148: 203–222. [Google Scholar]
- da Silva D, Han L, Costes E. 2013. Light interception efficiency of apple trees: a multiscale computational study based on MAppleT. Ecological Modelling 290: 1–9. doi:10.1016/j.ecolmodel.2013.12.001. [Google Scholar]
- Tewolde FT, Lu N, Shiina K, et al. . 2016. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Frontiers in Plant Science 7: 1–10. doi:10.3389/fpls.2016.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst G, Oosterkamp J, Hogewoning SW, Harbinson J, van Ieperen W. 2010. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiologia Plantarum 138: 289–300. doi:10.1111/j.1399-3054.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- de Visser PHB, Buck-Sorlin GH, van der Heijden GWAM. 2014. Optimizing illumination in the greenhouse using a 3D model of tomato and a ray tracer. Frontiers in Plant Science 5: 48. doi:10.3389/fpls.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.