Abstract
Objectives
Most studies of aging cognition have focused on risk factors for worse performance and on either genetic or environmental factors. In contrast, we examined whether 2 factors known to individually benefit aging cognition may interact to produce better cognition: environment-based positive age beliefs and the APOE ε2 gene.
Method
The sample consisted of 3,895 Health and Retirement Study participants who were 60 years or older at baseline and completed as many as 5 assessments of cognition over 8 years.
Results
As predicted, positive age beliefs amplified the cognitive benefit of APOE ε2. In contrast, negative age beliefs suppressed the cognitive benefit of APOE ε2. We also found that positive age beliefs contributed nearly 15 times more than APOE ε2 to better cognition.
Discussion
This study provides the first known evidence that self-perceptions can influence the impact of a gene on cognition. The results underscore the importance of combined psychosocial and biological approaches to understanding cognitive function in older adults.
Keywords: Age beliefs, APOE, Cognition, Gene, Health and Retirement Study, Self-perceptions of aging
Previous studies of older persons’ cognition have focused on risk factors for worse cognitive performance and on either gene or environmental factors (e.g., Lin et al., 2017; Small et al., 2004; Wisdom et al., 2011). In contrast, the present study examined, for the first time, whether two particularly promising factors, one environmental and one genetic, may interact to benefit aging cognition: positive age beliefs and APOE ε2, respectively.To maintain and improve cognition, a need exists to examine factors that benefit cognition in later life and their interactions. Identifying these beneficial factors could lead to the development of more effective interventions (Suri et al., 2013).
The framework for the current study derives from: (a) stereotype embodiment theory, which posits positive age beliefs are an environmental factor because they are assimilated from the culture at a young age, reinforced over time, and contribute to better health outcomes, including lower stress and enhanced cognition, in later life (Levy, 2009); and (b) the bioecological model, which posits positive environmental factors can amplify beneficial genes, leading to favorable outcomes (Bronfenbrenner & Morris, 2006).
Positive age beliefs and APOE ε2 individually benefit aging cognition and reduce the risk of dementia (e.g., Lamont et al., 2015; Levy et al., 2012, 2018; Small et al., 2004; Suri et al., 2013). Experimental, longitudinal, and cross-cultural studies have found that positive age beliefs contribute to better cognition among older persons (Levy, 1996, 2009; Levy et al., 2012). An 8-year study with older persons demonstrated that the memory performance of APOE ε2 carriers improved slightly; whereas, it declined slightly for APOE ε3 carriers and declined sharply for APOE ε4 carriers (Wilson et al., 2002). Meta-analyses have also confirmed a cognitive advantage of positive age beliefs (Horton et al., 2008; Lamont et al., 2015; Meisner, 2012) and APOE ε2 (Small et al., 2004; Wisdom et al., 2011).
There have been no known previous studies of whether self-perceptions affect genetic effects on aging cognition. However, the self-perceptions of interest here, age beliefs, are a cultural construct (Levy, 2009), and there have been studies with older participants that showed culture can affect the operation of APOE ε2. For instance, researchers found that the APOE-ε2-longevity advantage was significantly greater for those with Danish ancestry than for other ethnic groups considered, and that among Danish participants this advantage was significantly greater for those living in Denmark, compared to those living in the United States (Gurinovich et al., 2019). Another study found that the contribution of APOE ε2 to lower risk for, and delayed onset of, Alzheimer’s disease was significantly greater among European than Caribbean Hispanic elders (Blue et al., 2019).
An additional reason to expect that positive age beliefs would modify the impact of APOE ε2 on better aging cognition is based on the mechanism by which APOE ε2 has been found to operate, namely clearing amyloid plaques and promoting synaptic plasticity (Suri et al., 2013). Both of these brain processes are deleteriously affected by stress (McEwen, 2017) and enhanced by favorable environmental stimulation (Birch & Kelly, 2019; Suri et al., 2013). Positive age beliefs can mitigate stress, as demonstrated by experimental (Levy et al., 2000), daily diary (Bellingtier & Neupert, 2018), and longitudinal studies (Levy & Bavishi, 2018). Also, positive age stereotypes can promote an enabling view of the environment (Levy et al., 2014). In contrast, negative age beliefs can magnify stress and promote a more helpless view of the environment (Levy & Bavishi, 2018).
Taking these various factors into account, we analyzed data from a large, nationally representative sample of older persons to examine the following hypotheses:
Positive age beliefs and APOE ε2 will individually contribute to better cognition; and
Positive age beliefs and APOE ε2 will interact to determine better cognition, so that positive age beliefs will amplify the beneficial impact of APOE ε2 on cognition.
Method
Participants
The cohort consisted of Health and Retirement Study (HRS) participants (National Institute on Aging, 2007). Among the strengths of HRS are that it is nationally representative and diverse with respect to age, sex, ethnicity, socioeconomic status, and geography (National Institute on Aging, 2007). The Institute of Social Research investigators at the University of Michigan administered the survey every 2 years, with the support of the National Institute on Aging.
In the current study, 3,895 participants met our inclusion criteria, which consisted of being at least age 60 at baseline and having baseline measures of self-perceptions of aging (SPA) and cognition, as well as at least two follow-up measures of cognition, a DNA sample, and APOE-genotype-posterior-probability scores greater than .8. These scores, calculated by HRS investigators, represent the single-nucleotide polymorphism-imputation quality for each participant; they suggested this APOE posterior probability cutoff score to meet standards in the field and to be conservative (Faul et al., 2014). In our sample, 98% of the participants had posterior-probability scores greater than .8. The pattern of significant results remained when we also included participants with a posterior probability score of .8 or lower.
In our sample, 490 (12.9%) of the study participants were APOE ε2 carriers, a percentage similar to other estimates of APOE ε2-carrier prevalence (e.g., Suri et al., 2013). The small subgroup of those who have one ε2 and one ε4 allele (n = 79), which correlate in opposite directions with cognition, was excluded from the sample to be consistent with other studies (e.g., Small et al., 2004; Wilson et al., 2002).
At baseline, the age of the sample ranged from 60 to 97 (M = 71.07, SD = 6.76) years. The sample was 50.17% female, 90.73% White, and 92.45% married. APOE ε2 carriers and non-APOE ε2 carriers did not significantly differ by age beliefs, race, marital status, and smoking status, but they significantly differed by age (APOE ε2 carriers 71.67 vs noncarriers 70.98 years) and sex (APOE ε2 carriers 45.60% vs noncarriers 50.86% female).Participants holding positive and negative age beliefs did not significantly differ by sex, race, marital status, and smoking status, but they significantly differed by age (positive 70.22 vs negative 71.95 years).
To be conservative, we included all these variables, whether or not they differed between APOE ε2 and baseline age-belief groups, as covariates in all models (along with the other covariates described in the Measures section below).
Measures
Predictor: positive age beliefs
Positive age beliefs were assessed with the widely used SPA subset of the Philadelphia Center Morale Scale (Liang & Bollen, 1983). Participants responded to five items, including “The older I get, the more useless I feel,” on a 6-point scale ranging from 1 = strongly disagree to 6 = strongly agree; with a range from 5 to 30. To make the measure reflect positive age beliefs, we reverse-scored the sum, so that a higher score indicated more positive age beliefs. A random half of the sample responded to these questions in 2008; the other half in 2010. We used the earliest measure of SPA available for each participant. SPA has been found to be reliable and valid (Levy, 2009; Levy et al., 2002; Liang & Bollen, 1983).
Predictor: APOE ε2-carrier status
Participants were divided into APOE ε2 carriers (which included ε2ε2 and ε2ε3) and non-APOE ε2 carriers (which included ε3ε3, ε3ε4, and ε4ε4). APOE genotype was assessed from saliva samples collected during home visits. A random half of the participants provided samples in 2006; the other half in 2008. Saliva-collection participation rates were 83% in 2006 and 84% in 2008. Genotyping was performed by the National Institute of Health Center for Inherited Disease Research, and then archived by the National Center for Biotechnology Information.
In a sensitivity analysis, we divided participants into three mutually exclusive APOE genotype groups: APOE ε2 carriers, APOE ε3 homozygotes (ε3ε3), and APOE ε4 carriers. As part of this procedure, we compared the APOE ε2 carriers to the APOE ε3 homozygotes.
Outcome: cognition
Participants had as many as five measures of cognition over 8 years. Cognition was assessed every 2 years with the HRS-abbreviated version of the Telephone Interview for Cognitive Status (TICS-m), a standardized and global measure of cognition that assesses orientation, immediate recall, delayed recall, and executive function. It has a total of 35 points, with a higher score indicative of better cognition (Brandt et al., 1988; Ofstedal et al., 2005). This scale has good discriminatory validity and reliability, and it assesses the types of cognition that tend to change in later life (Fisher et al., 2017). The HRS utilized versions with parallel, but different, stimuli at different waves to reduce the possibility of practice effects (Fisher et al., 2017).
Covariates
In all models, we included covariates that have been found to be associated with the predictors and/or the outcome of cognition (Lamont et al., 2015; Levy, 2009; Wisdom et al., 2011). These included the demographic variables of age, gender, race (White, Black, or other), and marital status. We also included baseline cognition as assessed by TICS (Brandt et al., 1988), and the health variables of smoking history, depression as assessed by the Center for Epidemiological Studies-Depression (CES-D) scale (Radloff, 1977), and whether a health care provider had told participants they have cardiovascular disease and/or diabetes. We dichotomized participants into those below and those at or above the frequently used CES-D cutoff score of 16 to identify individuals at risk of clinical depression (Lewinsohn et al., 1997). The covariate of baseline cognition was included as a continuous variable. Marital status was coded as married or not, and smoking history was coded as ever smoked or never smoked.
Analytic Plan
To examine the first hypothesis, we conducted repeated-measures generalized linear models with APOE ε2 and baseline positive age beliefs as the predictors, and cognition over the following 8 years as the outcome, adjusting for all covariates, including baseline cognition.To examine the second hypothesis, we considered whether the interaction of APOE ε2 and positive age beliefs reached significance when we conducted this same model with the addition of this interaction term. This repeated-measures model had the advantage of adjusting for time, baseline cognition, and all covariates, as well as including all available waves and individual differences in cognition over time.
To consider whether positive age beliefs amplified the beneficial impact of APOE ε2 on cognition and whether negative age beliefs suppressed the beneficial impact of APOE ε2 on cognition, we incorporated contrasts into the repeated-measures model to compare APOE ε2 carriers to noncarriers within each age-belief group, adjusting for all covariates.
In a sensitivity analysis, we divided participants into three mutually exclusive APOE groups: APOE ε2 carriers (ε22, ε23), APOE ε3 homozygotes (ε3ε3), and APOE ε4 carriers (ε34, ε44). To further consider the predicted interaction between age beliefs and APOE ε2, we examined whether the difference between the cognitive scores of APOE ε2 carriers and APOE ε3 homozygotes was significantly greater among those with positive age beliefs than among those with negative age beliefs (with the APOE ε4 carriers removed from the sample). This strategy allowed us to examine whether the interaction of APOE and age beliefs was due exclusively to the benefit of APOE ε2 or also due to the fact that APOE ε4 was included in the non-APOE ε2 carrier group. This sensitivity analysis is important because the negative impact of APOE ε4 tends to be stronger than the positive impact of APOE ε2 on cognition (Small et al., 2004; Wisdom et al., 2011).
In all models, age was incorporated as a continuous-baseline variable, as well as a time-dependent variable updated at each subsequent wave in order to take time into account. To do this, year of survey and the interaction of age and year of survey were included in the models. Additionally, we examined whether age squared added to the fit of the model and found it significantly contributed to the nonlinear progression of cognitive decline, t = 7.87, p < .001. Thus, to allow for the observed curvature of cognition over time, we included an age-squared term in the models and required a baseline cognitive assessment as well as at least two follow-up cognitive assessments. To facilitate interpretation of the interactions, SPA scores were split into below and at or above the mean of 15. The p values were presented as two-tailed tests. All analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Supporting our first hypothesis, positive age beliefs, F = 122.68, p < .001, η p2 = .007, and APOE ε2, F = 7.87, p = .005, η p2 = .0005, significantly predicted better cognition. We also found that positive age beliefs explained more of the variance in cognition than sex, race, marriage, smoking, depression, diabetes, and heart disease (see Supplementary Table 1).
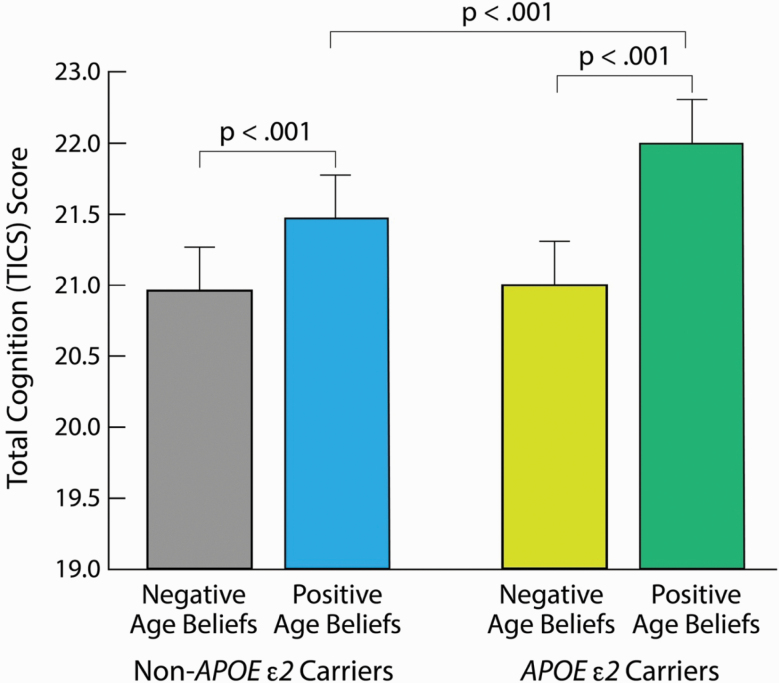
Supporting our second hypothesis, the interaction of positive age beliefs and APOE ε2 significantly predicted cognition, F = 7.74, p = .005, η p2 = .0005 (see Figure 1). Examination of the proportion of variance in cognition scores attributed to positive age beliefs and APOE ε2 (after standardizing both measures) showed that 86.15% was due to positive age beliefs; 6.15% was due to APOE ε2; and 7.69% was due to the synergistic impact of both variables.
Figure 1.
Positive age beliefs’ amplification of APOE ε2 benefit on cognition. Note: Age-belief groups were split into those below and those equal to or above the mean of 15. The total cognition scores were adjusted for all covariates, including baseline cognition, using the mean for continuous variables and the mode for categorical values.
The robustness of our findings was indicated by the interaction between positive age beliefs and APOE ε2 remaining a significant predictor of cognition with the addition of the covariate APOE ε4, a strong risk factor for dementia (Levy et al., 2018), F = 7.33, p = .007, η p2 = .0004.
In further support of the second hypothesis, we found evidence that positive age beliefs enhanced, while negative age beliefs suppressed, the beneficial impact of APOE ε2 on aging cognition. The difference between the APOE ε2 carriers and the non-APOE ε2 carriers was significantly greater for those with positive age beliefs than for those with negative age beliefs, t = 2.78, p = .005. There was no significant advantage of APOE ε2 among those with negative age beliefs, t = .04, p = .97, but there was a significant advantage of APOE ε2 among those with positive age beliefs, t = 3.95, p < .001.
Supporting our hypotheses, we found the same pattern of results in sensitivity analyses in which we conducted all of the same models, but with the APOE predictor divided into APOE ε2 carriers (ε2ε2 and ε2ε3), APOE ε3 homozygotes (ε3ε3), and APOE ε4 carriers (ε3ε4 and ε4ε4). That is, positive age beliefs, F = 127.96, p < .001, η p2 = .008, and APOE, F = 56.64, p < .001, η p2 = .007, significantly predicted better cognition. We again found that positive age beliefs explained more of the variance in cognition than sex, race, marriage, smoking, depression, diabetes, and heart disease. Supporting the second hypothesis in this sensitivity analysis, the interaction of positive age beliefs and the three-category APOE variable significantly predicted cognition, F = 3.74, p = .02, η p2 = .0005.
In a second sensitivity analysis that focused on APOE ε2 carriers and the APOE ε3 homozygotes (without APOE ε4 carriers), we found positive age beliefs, F = 117.04, p < .001, η p2 = .007, and APOE ε2, F = 56.64, p < .001, η p2 = .003, significantly predicted better cognition. We also found the interaction of positive age beliefs and the APOE variable significantly predicted cognition, F = 3.74, p = .02, η p2 = .0005. Additionally, the difference between the APOE ε2 carriers and the APOE ε3 homozygotes was significantly greater for those holding positive age beliefs than for those holding negative age beliefs, t = 2.15, p = .03.
Discussion
The current study found evidence of a previously unknown positive-age-belief attribute: its amplification of the APOE-ε2-cognitive benefit. The study also found evidence of a previously unknown negative-age-belief attribute: its suppression of the APOE-ε2-cognitive benefit. In addition, we found that positive age beliefs and APOE ε2 significantly interacted to predict cognition over time. These results emerged from a large and diverse sample of older persons across 8 years, adjusting for baseline cognition and other relevant covariates.
An opportunity was presented by the current study to assess the relative saliency of two disparate contributors to aging cognition. The results demonstrate the greater influence of a factor with an external origin, culture-based age beliefs, compared to a factor with an internal origin, the APOE ε2 gene. The ascendancy of the age beliefs was indicated by their contributing nearly 15 times as much as this neuroprotective gene to better cognitive performance. Nevertheless, in light of previous research focusing on risk factors for worse cognition, it is notable that we found two factors simultaneously provided a cognitive advantage.
The results also showed that both APOE ε2 carriers and non-APOE ε2 carriers significantly benefitted from positive age beliefs. This is promising, because age beliefs can be improved by intervention (Levy et al., 2014).
Our study raises the possibility that, in the future, psychological interventions aimed at preventing cognitive decline with aging could be targeted at individuals with specific genetic variants. The analyses with a neutral comparison group, APOE ε3 homozygotes, found that the beneficial effect of positive age beliefs on APOE ε2 carriers was not driven by the inclusion in the comparison group of carriers of APOE ε4, which has a detrimental impact on cognition.
Regarding the direction of the relationship, it is likely that positive age beliefs affected the influence of APOE ε2 on better cognition. First, a growing body of epigenetic research has found that environmental factors affect how genes are expressed in phenotypes, such as cognition (e.g., Cavalli & Heard, 2019). Second, studies suggest that age-belief assimilation precedes the impact of APOE on cognition because these beliefs, which tend to be internalized from childhood onwards, are resilient—even when encountering extremely stressful events (Levy et al., 2015); whereas, the influence of APOE ε2 on the brain tends to begin when old age is reached (Bunce et al., 2012).
The findings of the current study were derived from two disciplines that usually operate independently of each other: social psychology and genetics. By presenting the interrelationship of age beliefs and APOE ε2, the findings make an effective argument for integrating these complementary fields.
Supplementary Material
Acknowledgments
The survey data for the study, which was not preregistered, are publicly available (https://hrs.isr.umich.edu/data-products) and the APOE genetic files can be acquired by applying to the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/gap). The authors would like to thank the Health and Retirement Study researchers and participants for making the current study possible.
Funding
This study was supported by a National Institute on Aging grant (U01AG032284).
Conflict of Interest
None declared.
References
- Bellingtier J A, & Neupert S D (2018). Negative aging attitudes predict greater reactivity to daily stressors in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(7), 1155–1159. doi: 10.1093/geronb/gbw086 [DOI] [PubMed] [Google Scholar]
- Birch A M, & Kelly Á M (2019). Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology, 145(Pt A), 59–74. doi: 10.1016/j.neuropharm.2018.03.042 [DOI] [PubMed] [Google Scholar]
- Blue E E, Horimoto A R V R, Mukherjee S, Wijsman E M, & Thornton T A (2019). Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimer’s & Dementia, 15(12), 1524–1532. doi: 10.1016/j.jalz.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, & Folstein M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology and Behavioral Neurology, 1, 111–117. [Google Scholar]
- Bronfenbrenner U, & Morris P A (2006). The bioecological model of human development. In Lerner R M & Damon W (Eds.), Handbook of child psychology: Theoretical models of human development (pp. 793–828). John Wiley & Sons. [Google Scholar]
- Bunce D, Anstey K J, Cherbuin N, Gautam P, Sachdev P, & Easteal S (2012). APOE genotype and entorhinal cortex volume in non-demented community-dwelling adults in midlife and early old age. Journal of Alzheimer’s Disease, 30(4), 935–942. doi: 10.3233/JAD-2012-112126 [DOI] [PubMed] [Google Scholar]
- Cavalli G, & Heard E (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. doi: 10.1038/s41586-019-1411-0 [DOI] [PubMed] [Google Scholar]
- Faul J, Smith J, & Zhao W (2014). Health and Retirement Study: Candidate genes for cognition/ behavior. University of Michigan. [Google Scholar]
- Fisher G G, Hassan J, Faul J D, Rogers W, & Weir D R (2017). Health and Retirement Study. Imputation of cognitive measures: 1992–2014. University of Michigan. [Google Scholar]
- Gurinovich A., Andersen S. L., Puca A., Atzmon G., Barzilai N., & Sebastiani P. (2019). Varying effects of APOE alleles on extreme longevity in European ethnicities. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 74(S1), 45–51, doi: 10.1093/gerona/glz179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton S, Baker J, Pearce G W, & Deakin J M (2008). On the malleability of performance: Implications for seniors. Journal of Applied Gerontology, 27, 446–465. doi: 10.1177/0733464808315291 [DOI] [Google Scholar]
- Lamont R A, Swift H J, & Abrams D (2015). A review and meta-analysis of age-based stereotype threat: Negative stereotypes, not facts, do the damage. Psychology and Aging, 30(1), 180–193. doi: 10.1037/a0038586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. (1996). Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology, 71(6), 1092–1107. doi: 10.1037//0022-3514.71.6.1092 [DOI] [PubMed] [Google Scholar]
- Levy B. (2009). Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science, 18(6), 332–336. doi: 10.1111/j.1467-8721.2009.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B R, & Bavishi A (2018). Survival advantage mechanism: Inflammation as a mediator of positive self-perceptions of aging on longevity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73(3), 409–412. doi: 10.1093/geronb/gbw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B R, Hausdorff J M, Hencke R, & Wei J Y (2000). Reducing cardiovascular stress with positive self-stereotypes of aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 55(4), 205–213. doi: 10.1093/geronb/55.4.p205 [DOI] [PubMed] [Google Scholar]
- Levy B R, Pilver C, Chung P H, & Slade M D (2014). Subliminal strengthening: Improving elders’ physical function over time through an implicit-age-stereotype intervention. Psychological Science, 25, 2127–2135. doi: 10.1177/0956797614551970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B R, Slade M D, Chung P H, & Gill T M (2015). Resiliency over time of elders’ age stereotypes after encountering stressful events. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70(6), 886–890. doi: 10.1093/geronb/gbu082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B R, Slade M D, Kunkel S R, & Kasl S V (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261–270. doi: 10.1037//0022-3514.83.2.261 [DOI] [PubMed] [Google Scholar]
- Levy B R, Slade M D, Pietrzak R H, & Ferrucci L (2018). Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One, 13(2), e0191004. doi: 10.1371/journal.pone.0191004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B R, Zonderman A B, Slade M D, & Ferrucci L (2012). Memory shaped by age stereotypes over time. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67(4), 432–436. doi: 10.1093/geronb/gbr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P M, Seeley J R, Roberts R E, & Allen N B (1997). Center for Epidemiologic Studies Depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging, 12(2), 277–287. doi: 10.1037//0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- Liang J, & Bollen K A (1983). The structure of the Philadelphia Geriatric Center Morale scale: A reinterpretation. Journal of Gerontology, 38(2), 181–189. doi: 10.1093/geronj/38.2.181 [DOI] [PubMed] [Google Scholar]
- Lin C H, Lin E, & Lane H Y (2017). Genetic biomarkers on age-related cognitive decline. Frontiers in Psychiatry, 8, 247. doi: 10.3389/fpsyt.2017.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, doi: 10.1177/2470547017692328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner B A. (2012). A meta-analysis of positive and negative age stereotype priming effects on behavior among older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67(1), 13–17. doi: 10.1093/geronb/gbr062 [DOI] [PubMed] [Google Scholar]
- National Institute on Aging (2007). Growing older in America: The Health and Retirement Study. Author. [Google Scholar]
- Ofstedal M B, Fisher G, & Herzog A R (2005). Documentation of cognitive functioning measures in the Health and Retirement Study. University of Michigan. [Google Scholar]
- Radloff L S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Small B J, Rosnick C B, Fratiglioni L, & Bäckman L (2004). Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging, 19, 592–600. doi: 10.1037/0882-7974.19.4.592 [DOI] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg A J, & Mackay C E (2013). The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ɛ2. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2878–2886. doi: 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Wilson R, Bienias J, Kravis E B, Evans D, & Bennett D (2002). The apolipoprotein E ε2 allele and decline in episodic memory. Journal of Neurology, Neurosurgery and Psychiatry. 73, 672–677. doi: 10.1136/jnnp.73.6.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom N M, Callahan J L, & Hawkins K A (2011). The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiology of Aging, 32(1), 63–74. doi: 10.1016/j.neurobiolaging.2009.02.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.