Abstract
Objectives
Numerous studies show benefits of mid- and late-life activity on neurocognitive health. Yet, few studies have examined how engagement in enriching activities during childhood, when the brain is most plastic, may confer long-term neurocognitive benefits that may be especially important to individuals raised in low-income settings. We examined associations between enriching early-life activities (EELAs) and hippocampal and amygdala volumes in a sample of predominantly African-American, community-dwelling older adults. We further assessed whether these associations were independent of current activity engagement.
Methods
Ninety participants from the baseline Brain Health Substudy of the Baltimore Experience Corps Trial (mean age: 67.4) completed retrospective activity inventories and an magnetic resonance imaging scan. Volumes were segmented using FreeSurfer.
Results
Each additional EELA was associated with a 2.3% (66.6 mm3) greater amygdala volume after adjusting for covariates. For men, each additional EELA was associated with a 4.1% (278.9 mm3) greater hippocampal volume. Associations were specific to these regions when compared with the thalamus, used as a control region.
Discussion
Enriching lifestyle activities during an important window of childhood brain development may be a modifiable factor that impacts lifelong brain reserve, and results highlight the importance of providing access to such activities in historically underserved populations.
Keywords: Brain aging, Health disparities, Leisure activities, Neuroimaging, Resilience
A rich literature suggests that early-life adversity (e.g., chronic stress, traumatic events) leads to poor neurocognitive outcomes later in life (Epel & Lithgow, 2014; Lupien, McEwen, Gunnar, & Heim, 2009). Marginalized populations, such as low-income African-Americans, are at higher risk of early-life adversity (Glymour & Manly, 2008) and dementia incidence (Mayeda, Glymour, Quesenberry, & Whitmer, 2016) compared to other racial groups. These differences in stress exposure may ultimately contribute to racial disparities in cognitive trajectories in later life (Zahodne, Sol, & Kraal, 2017). Furthermore, growing up in disparaged socioeconomic environments (e.g., low income, low perceived socioeconomic status [SES], etc.) leads to structural brain changes also through increased exposure to toxic stress (Brito & Noble, 2014). Participation in a variety of cognitively and socially enriching activities in mid- and late-life has been associated with better cognition among sociodeomographically at-risk older adults (Carlson et al., 2012). However, few studies have examined whether engagement in enriching activities in early life has similar neurocognitive benefits. Ultimately, we need to examine potential neuroprotective mechanisms of early-life enrichment to better understand how we can intervene within communities who are at higher risk of early adversity and poor health outcomes in later life.
To that end, lifelong engagement in physical, cognitive, and social activities has been proposed as one way to promote brain health as we age (Blazer, Yaffe, & Liverman, 2015; Gow, Pattie, & Deary, 2017). Complex, multimodal activities that incorporate varying levels of physical, cognitive, and social engagement have the potential to provide meaningful and impactful roles for older adults (Carlson et al., 2012; Fried et al., 2013), and participation in multiple activities simultaneously or sequentially has been shown to promote cognitive (Gow et al., 2017) and brain health in later life (Carlson et al., 2015; Suo et al., 2012; Valenzuela, Sachdev, Wen, Chen, & Brodaty, 2008).
Notably, multimodal lifestyle activities have been associated with increased or maintained volume of the medial temporal lobe (MTL) (Carlson et al., 2015; Valenzuela et al., 2008). Atrophy in the MTL, including the hippocampus (McKhann et al., 2011) and amygdala (Poulin, Dautoff, Morris, Barrett, & Dickerson, 2011), is associated with pathological aging. Reduced hippocampal and amygdala volumes in older age have been associated with impairments in declarative and socioemotional memory, respectively and are early markers of progression to Alzheimer’s disease (AD) (den Heijer et al., 2010). Socially, cognitively and physically enriching activity may therefore offer one means by which to mitigate age-related brain changes that adversely impact cognitive functioning.
Cognitive and Social Pathways Linking Enriching Early-Life Activities to Late-Life Brain Outcomes
Few studies have taken a life-span approach to examine how engagement in enriching early-life activities (EELAs) may impact late-life brain volumes. In a cross-sectional study of older adults, Schreiber and coworkers (2016) assessed engagement in 25 cognitive activities (e.g., reading, writing letters) across the life span. After computing composite lifestyle factor scores, they found that high lifetime cognitive activity and low vascular risk were associated with improved global cognition and episodic memory in later life, but this relationship was not mediated through hippocampal volume. However, it remains unclear whether engagement in cognitive activities during early childhood may be associated with late-life hippocampal volumes independently of engagement during other life periods. Given the substantial development of the hippocampus in early life (Epel & Lithgow, 2014) and reduced potential for neuroplasticity with age (Park & Bischof, 2013), early childhood may be a sensitive developmental window for the potential neuroprotective effects of cognitive engagement.
In addition to early-life cognitive experiences, social experiences in early life may also lead to developmental changes in brain regions that regulate stress (Epel & Lithgow, 2014), which may further impact cognition and mental health with age (Davidson & McEwen, 2012). Given that the developing brain is sensitive to early adverse exposures (Epel & Lithgow, 2014), we hypothesized that early life may also serve as a sensitive period of positive plasticity in response to engagement in socially enriching activities. For example, despite the amygdala’s role in stress reactivity, it has a role in prosocial behavior, and amygdala volume has been shown to positively correlate with social network size in adults (Bickart, Wright, Dautoff, Dickerson, & Barrett, 2011). Studying the relationships between positive, early-life social experiences and late-life brain biomarkers related to cognitive impairment and AD has the potential to improve our understanding of how the brain develops within the context of positive as well as negative life experiences.
EELAs Provide a Novel Lifestyle Index of Enrichment
The few human studies that examine the neural outcomes of early-life enrichment typically focus on in-home enrichment (e.g., cognitively stimulating resources, parental social support, etc.) and examine outcomes only in adolescence and young adulthood (Rao et al., 2010; Whittle et al., 2014). For example, one prospective study of children from a low socioeconomic background found that parental care, but not environmental enrichment, at age 4 predicted hippocampal volumes in adolescence (Rao et al., 2010). Yet, the definition of environmental enrichment in this study was limited to in-home experiences (e.g., number of books, mother’s use of correct grammar). Extending upon findings summarized above among older adults, engagement in activities outside the home may provide novel social environments and a greater variety of prosocial encounters, both of which promote neurogenesis in the developing MTL regions (Bickart et al., 2011; Davidson & McEwen, 2012).
Finally, there is a need to further explore sex differences in this relationship using a life-span framework. There is little research on potential sex-specific associations between activity engagement and brain outcomes in older adulthood, despite sex differences in neurohormonal responses to social experiences (Bale & Epperson, 2015) and in the benefits of specific lifestyle activities on aging-related cognitive changes (Suo et al., 2012).
The current study addresses gaps in the literature by examining how participation in early-life activities outside the home may be associated with hippocampal and amygdala volumes in older adulthood. We selected these structures a priori, because they have shown specific regional plasticity to physical (Varma, Chuang, Harris, Tan, & Carlson, 2015; Voss, Nagamatsu, Liu-Ambrose, & Kramer, 2011), occupational (Suo et al., 2012), and social (Bickart et al., 2011; Carlson et al., 2015) engagement throughout the life span. Focusing on these specific regions also mitigated Type 1 errors from extensive multiple comparisons. We selected the thalamus as a control region, given it is also a subcortical structure and has been used previously to investigate regional specificity of activity engagement (Erickson et al., 2011; Varma et al., 2015). We thus hypothesized that thalamus volume was not associated with early-life activity.
We examined these questions using a large, cognitively healthy sample of predominantly African-American older adults living in Baltimore, Maryland. This sample may be at higher sociodemographic risk of developing dementia by virtue of their educational and racial backgrounds (Mayeda et al., 2016). We were specifically interested in the variety of activities, because previous work has suggested that variety may be more predictive of cognitive outcomes than the frequency of participation (Carlson et al., 2012). Endorsement of these activities is also designed to augment years of formal education as a lifestyle index of enrichment across socioeconomic settings. A prior study using this sample found that variety in EELAs was associated with higher educational attainment and better cognitive functioning in older age (Chan, Parisi, Moored, & Carlson, 2018). We therefore hypothesized that greater participation in early-life activities would be associated with larger hippocampal and amygdala volumes in older age. We also hypothesized that sex may moderate the relationship between EELAs and the developing brain, and we explored sex differences in the associations between EELAs and later-life hippocampal and amygdala volumes.
Method
Participants
Participants came from the baseline sample of Brain Health Study (BHS) nested within the Baltimore Experience Corps Trial (BECT), a high-intensity, intergenerational volunteering intervention. Details on the sex stratification, randomization, and sampling methodology of the BECT have been reported elsewhere (Fried et al., 2013). Eligibility criteria included: aged 60 years or older, normal cognition (≥24 on the Mini-Mental State Examination), and ability to read at a sixth grade level (M. F. Folstein, S. E. Folstein, & McHugh, 1975; Wilkinson, 1993). The BHS study had additional eligibility criteria: right-handedness; no ferrous metals in the body; and no history of stroke, brain aneurysm, or brain cancer in the past year (Carlson et al., 2015). BHS participants at baseline completed one structural magnetic resonance imaging (MRI) scan. They also completed neuropsychological tests and surveys of past and current activities (Carlson et al., 2015). All assessments were conducted by trained administrators.
There were 123 individuals enrolled in the BHS at baseline. Twenty-three were not administered the Early Life Activities Inventory, due to its development and addition after the start of the study. Eight additional participants did not complete the MRI due to claustrophobia or excessive head motion and an additional two were missing covariate measures, resulting in a final sample size of 90. Participants were predominantly African-American (91%). Participants who had missing data did not significantly differ (p > .05) in age, sex, education, and hippocampal and amygdala volumes from those with complete data. This study was approved by the Johns Hopkins School of Medicine Institutional Review Board and each participant provided written, informed consent.
Measures
EELA inventory
The Hopkins EELA inventory assessed whether participants engaged in multimodal activities in childhood or adolescence. The seven-item inventory was developed by the senior author via informal interviews with BHS participants to identify relatively common childhood activities that could be easily recalled by yes/no endorsement. Participants responded “yes” or “no” to whether they engaged in each of the following activities before age 13: (a) learning a foreign language, (b) play team sports, (c) volunteering at church, (d) took lessons (i.e., dance, choir), (e) scouting, (f) playing a musical instrument, and (g) taking vacations. Overall engagement in EELAs was computed as the sum of each binary response (yes = 1, no = 0; range: 0–7).
MRI acquisition and preprocessing
All images were collected on a 3.0T Phillips Interna scanner using a high-resolution 3D T1-weighted Magnetization Prepared Rapid Gradient Echo Imaging sequence (MPRAGE; repetition time = 8.037 ms; echo time = 3.6 ms; flip angle = 8°; 200 contiguous 1 mm sagittal slices; FOV = 200 × 256 × 200 mm; matrix size = 256 mm × 256 mm; voxel size = 1 × 1 × 1 mm). Segmentation of bilateral hippocampal and amygdala volumes was performed using FreeSurfer version 5.1.0 (Fischl, 2012). Preprocessing consisted of motion correction, non-uniform intensity normalization, and automated removal of non-brain tissue. FreeSurfer has been shown to be more reliable (Eggert, Sommer, Jansen, Kircher, & Konrad, 2012) and precise (Morey et al., 2009) than other automated techniques (e.g., FSL/FIRST) in segmenting subcortical regions and has been used in prior studies of aging populations (e.g., Beauchet, Allali, Annweiler, & Verghese, 2016; Bickart et al., 2011). Volumes for each participant were checked by at least two individual researchers and corrected manually if needed. Inter-rater reliability for images requiring manual correction was high (intraclass correlation [ICC] > 0.95). No participants were excluded due to segmentation errors. Left and right volumes were summed to produce composite bilateral measures, which were further adjusted for intracranial volume (ICV) to account for sex differences in brain size (Sanfilipo, Benedict, Zivadinov, & Bakshi, 2004). The full structural imaging and processing protocols have been described elsewhere (Carlson et al., 2015).
Covariates
Age, sex, education, and late-life activities were covariates in the main analyses. These measures were included in all models to adjust for potential confounding of the relationship between early-life activities and MTL volumes. Education was measured as the number of years of formal schooling. We measured depressive symptoms using the 15-item Geriatric Depression Scale (GDS) with a >5-point cut-off indicating significant depressive symptoms (D’ath, Katona, Mullan, Evans, & Katona, 1994).
The Lifestyle Activity Inventory (LAQ) was used to measure current engagement in cognitively enriching activities (Carlson et al., 2012). This inventory included a similar range of activities as others used in studies of older adults (Gow et al., 2017). Participants originally reported the frequency that they engaged in each of the activities (e.g., “doing volunteer work,” “gardening,” “cooking,” etc.) over the past year on a 6-point scale (0 = “never or less than once a month” to 5 = “every day”). A binary score (0 = “never or less than once a month,” 1 = “at least once a month”) was created for each response and responses were summed to produce a composite measure of variety of late-life activity engagement. A complete list of the LAQ items can be found elsewhere (Carlson et al., 2012). Of note, the LAQ consisted of 28 items, and the items did not directly correspond to the EELAs. However, both measures were operationalized similarly, with greater values indicating greater variety of activity engagement.
Statistical Analyses
Multiple linear regressions (MLRs) were used to assess the independent contribution of EELAs in predicting each regional volume. Model assumptions were assessed using plots of residual versus fitted values, Q-Q plots of residuals, augmented component-plus-residual (ACPR) plots to assess nonlinearities, and boxplots of highly influential observations. Each regression model was sequentially adjusted for the covariates mentioned above. Model 1 included the demographic covariates, ICV, and EELAs. Model 2 included late-life activities. Model 3 included an EELA by sex interaction term to assess sex differences in the EELA–volume associations. No correction for multiple comparisons was employed due to a priori specification of regions of interest and hypotheses. All analyses were performed in Stata version 14 (StataCorp, College Station, TX).
Results
Participant Characteristics
Table 1 presents the descriptive statistics for the total and sex-stratified samples. The mean age was 67.4 (SD = 6.1), and the sample was mostly African-American (N = 82, 91%). Participants had some college education, on average (M = 14.23, SD = 2.8). Five out of 90 participants scored >5 on the GDS (M = 1.2, SD = 1.9), suggesting low prevalence of depressive symptoms in our sample (D’ath et al., 1994). The mean hippocampal volume was 6,756 mm3 (SD = 714) and the mean amygdala volume was 2,890 mm3 (SD = 489), after adjusting for ICV. The mean LAQ score was 18.39 (SD = 3.3, range: 11–25), indicating that the participants engaged in a large variety of activities in late life, on average. A prior study with a more representative sample of women (Carlson et al., 2012) reported average participation in 11.9 (SD = 3.0) activities, suggesting women in the BHS may be more active than those in the general population. The mean EELA score was 3.02 (SD = 1.6, range: 0–7), indicating average engagement in a moderate variety of the included early activities. LAQ and EELA scores were modestly correlated (r = .27, p < .05). There were no significant sex differences in individual EELA endorsed (p > .05), except for playing a musical instrument which was endorsed more by men (χ2(1) = 4.7, p < .05). There were no significant sex differences across other measures, including brain volumes after adjusting for ICV. Zero-order correlations between all independent variables and brain volumes are provided in Supplementary Table 1.
Table 1.
Characteristics of Study Sample (N = 90)
| Total (N = 90) | Male (n = 25) | Female (n = 65) | |||
|---|---|---|---|---|---|
| Characteristics | M (SD) | Range | M (SD) | M (SD) | p-value |
| Age | 67.35 (6.1) | (60, 82) | 66.43 (6.8) | 67.71 (5.9) | .38 |
| Education | 14.23 (2.8) | (8, 20) | 15.04 (2.9) | 13.92 (2.7) | .09 |
| Race (N, % African-American) | 82 (91) | 22 (88) | 60 (94) | .53 | |
| GDS | 1.24 (1.9) | (0, 11) | 1.84 (.59) | 1.01 (.15) | .06 |
| Brain volume (mm3) | |||||
| Hippocampus | 6,756 (714) | 6,721 (785) | 6,770 (692) | .78 | |
| Amygdala | 2,890 (489) | 2,907 (477) | 2,884 (496) | .84 | |
| Thalamus | 12,273 (1,174) | 12,278 (1,400) | 12,271 (1,088) | .98 | |
| Late-life activities (count) | 18.39 (3.3) | (0, 7) | 18.04 (3.7) | 18.52 (3.2) | .54 |
| Early-life activities (count) | 3.02 (1.6) | (11, 25) | 3.20 (2.6) | 2.95 (2.5) | .53 |
Note: GDS = 15-item Geriatric Depression Scale. p-values are for t-tests/Fisher’s exact test for sex differences. Brain volumes adjusted for intracranial volume.
EELAs and Hippocampal Volume
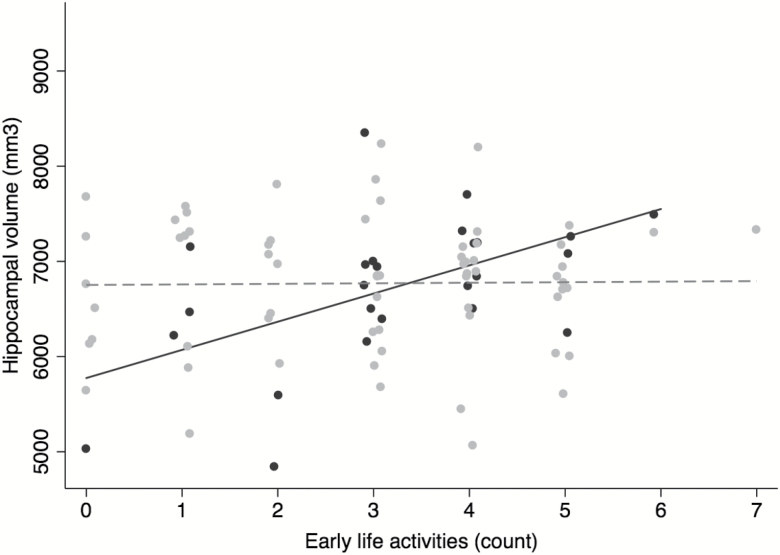
Table 2 presents the results from the MLRs of hippocampal volume on EELAs and the covariates. EELA was not associated with hippocampal volume after adjusting for covariates (Model 2: B = 57.0, SE = 47.6, p = .234). However, the EELA by sex interaction term was significant (Model 3: B = −271.4, SE = 107.8, p = .014). For men, each additional EELA was associated with an average 4.1% (278.9 mm3) greater hippocampal volume, and this difference was statistically significant (SE = 99.5, p = .006). For women, each additional EELA was associated with a nonsignificant, average 7.5 mm3 greater hippocampal volume (B = 278.9–271.4, SE = 50.1, p = .881). Figure 1 presents the sex-stratified correlations between EELAs and hippocampal volumes.
Table 2.
Multiple Linear Regressions of Early-Life Activities and Covariates on Intracranial Volume–Adjusted Hippocampal Volume (N = 90)
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | B | SE | p | B | SE | p | B | SE | p |
| Age | −35.35 | 12.03 | .004 | −35.35 | 12.10 | .004 | −31.45 | 11.83 | .009 |
| Sex (ref. = male) | |||||||||
| Female | 82.37 | 164.57 | .618 | 81.96 | 166.77 | .624 | 56.71 | 162.03 | .727 |
| Education | −22.26 | 27.56 | .421 | −22.38 | 28.34 | .432 | −30.69 | 27.68 | .271 |
| Late-life activities | 0.47 | 23.28 | .984 | −1.96 | 22.59 | .931 | |||
| Early-life activities | 57.21 | 46.14 | .218 | 57.00 | 47.56 | .234 | 278.87 | 99.49 | .006 |
| EELA × Sex | −271.36 | 107.82 | .014 | ||||||
| ΔR2 | .00 | .07 | |||||||
| R 2 | .12 | .12 | .19 | ||||||
Note: EELA = enriching early-life activity. Unstandardized beta coefficients presented. All models adjusted for age, sex, and education. Model 2 further adjusted for late-life activities. Model 3 includes the EELA by sex interaction term.
Figure 1.
Sex-stratified correlations between early-life activity engagement and hippocampal volume. Note: Greater engagement in early-life activities was associated with larger intracranial volume (ICV)–adjusted hippocampal volumes in men (r = .54), and the association remained significant after adjusting for age, education, and late-life activity engagement. The association between early-life activities and ICV-adjusted hippocampal volumes was not significant for women (r = .01). Men: black, women: gray.
EELAs and Amygdala Volume
Table 3 presents the results from the MLRs of amygdala volume on EELAs and the covariates. EELA was associated with amygdala volume after adjusting for covariates (Model 2: B = 66.6, SE = 32.4, p = .043). Each additional EELA was associated with an average 2.3% (66.6 mm3) greater amygdala volume. EELAs also explained an additional 4.7% of the variance in amygdala volumes after adjusting for late-life activities and the other covariates (ΔR2 = .132–.085). The EELA by sex interaction term was not significant (Model 3: B = −102.3, SE = 75.3, p = .178), suggesting no sex differences in the observed EELA–amygdala association.
Table 3.
Multiple Linear Regressions of Early-Life Activities and Covariates on Intracranial Volume–Adjusted Amygdala Volume (N = 90)
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | B | SE | p | B | SE | p | B | SE | p |
| Age | −20.42 | 8.19 | .015 | −20.44 | 8.23 | .015 | −18.97 | 8.26 | .024 |
| Sex (ref. = male) | |||||||||
| Female | 14.28 | 112.01 | .899 | 11.99 | 113.50 | .916 | 2.48 | 113.15 | .983 |
| Education | −4.10 | 18.76 | .828 | −4.76 | 19.29 | .806 | −7.89 | 19.33 | .684 |
| Late-life activities | 2.63 | 15.84 | .868 | 1.72 | 15.78 | .914 | |||
| Early-life activities | 67.73 | 31.41 | .034 | 66.56 | 32.37 | .043 | 150.16 | 69.48 | .034 |
| EELA × Sex | −102.25 | 75.29 | .178 | ||||||
| ΔR2 | .00 | .02 | |||||||
| R 2 | .13 | .13 | .15 | ||||||
Note: EELA = enriching early-life activity. Unstandardized beta coefficients presented. All models adjusted for age, sex, and education. Model 2 further adjusted for late-life activities. Model 3 includes the EELA by sex interaction term.
EELAs and Thalamus Volume
Table 4 presents the results from the MLRs of thalamus volume on EELAs and the covariates. EELA was not associated with thalamus volume after adjusting for covariates (Model 2: B = −73.6, SE = 82.5, p = .375). The EELA by sex interaction term was not significant (Model 3: B = −187.9, SE = 190.5, p = .327), suggesting no sex differences in the observed EELA–thalamus association.
Table 4.
Multiple Linear Regressions of Early-Life Activities and Covariates on Intracranial Volume–Adjusted Thalamus Volume
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | B | SE | p | B | SE | p | B | SE | p |
| Age | −45.23 | 21.18 | .036 | −44.65 | 21.26 | .039 | −42.29 | 21.40 | .052 |
| Sex (ref. = male) | |||||||||
| Female | 23.77 | 285.67 | .934 | 6.10 | 287.70 | .983 | −9.14 | 288.16 | .975 |
| Education | −0.67 | 48.65 | .989 | −7.89 | 49.90 | .875 | −14.29 | 50.33 | .777 |
| Late-life activities | 28.39 | 40.87 | .875 | 26.58 | 40.92 | .518 | |||
| Early-life activities | −62.76 | 80.77 | .439 | −73.63 | 82.52 | .375 | 78.67 | 175.11 | .654 |
| EELA × Sex | −187.91 | 190.54 | .327 | ||||||
| ΔR2 | .01 | .02 | |||||||
| R 2 | .05 | .06 | .15 | ||||||
Note: EELA = enriching early-life activity. Unstandardized beta coefficients presented. All models adjusted for age, sex, and education. Model 2 further adjusted for late life activities. Model 3 includes the EELA by sex interaction term.
Sensitivity Analyses
Sensitivity analyses were conducted to determine whether perceived early SES was driving the association between EELAs and late-life hippocampal and amygdala volumes (Supplemental Tables 2 and 3). Perceived mother’s SES was included as a proxy measure for subjective early socioeconomic experiences (Adler, Epel, Castellazzo, & Ickovics, 2000; Brito & Noble, 2014). Participants were asked to report their mother’s SES using a ladder ranging from 1 (worst off) to 10 (best off). Six participants did not report their mother’s SES. The remaining 84 participants reported their mother’s SES to be average (M = 5.57, SD = 2.29, range: 1–10). The association between EELA and amygdala volume did not change after including perceived mother’s SES in the models (Supplementary Table 3; Model 1: B = 80.24, SE = 36.48, p = .031), but the EELA by sex interaction term was no longer significant for hippocampal volume (Supplementary Table 2; Model 2: B = −230.05, SE = 127.67, p = .076). Perceived mother’s SES was not a significant predictor in any model (ps > .10), suggesting that the reduced power from including this variable and from the smaller sample size (N = 84) may be driving this change in association.
We did not have a strong rationale for investigating laterality in our analyses, given that prior work with the BHS has found that physical and social activity was related to bilateral hippocampal volumes (Carlson et al., 2015; Varma et al., 2015). However, given how prior research has found laterality differences in both conversion to MCI or AD (Tang, Holland, Dale, Younes, & Miller, 2015) and the relationship between cognitive enrichment earlier in life and later-life hippocampal volume (Suo et al., 2012), we also examined laterality differences in EELAs as sensitivity analyses (Supplementary Table 4). We found no differences in the association for left (B = 33.8, SE = 17.4, p = .06) and right (B = 32.7, SE = 17.6, p = .07) amygdala volumes. For men, EELA was significant for right (B = 186.2, SE = 51.2, p < .01), and trending for left (B = 92.6, SE = 55.1, p = .07) hippocampal volume.
Discussion
This study is the first, to our knowledge, to examine the association between early cognitive, physical, and social enrichment on late-life brain health in at-risk older adults. This sample may be considered at higher risk of poor cognitive outcomes by virtue of their racial and socioeconomic backgrounds (Mayeda et al., 2016). We found that participating in a wider variety of activities in childhood was associated with greater amygdala volumes in older adulthood and also with larger hippocampal volumes for men in our sample. These associations remained significant after adjusting for several potential confounding variables related to late-life brain and cognitive reserve, including formal education (Stern, 2009) and current lifestyle activity. Furthermore, as expected these relationships were specific to the MTL regions, compared to the thalamus, used as the control region. These have important implications for healthy aging, as the hippocampus and amygdala are integral to memory function and atrophy in these regions is an early biomarker of AD (McKhann et al., 2011; Poulin et al., 2011).
Therefore, just as early life has been shown to be a sensitive period for adverse stress exposures and subsequent cognitive and mental health outcomes (Anda et al., 2006; Epel & Lithgow, 2014), our study is the first, to our knowledge, to explore whether beneficial early enrichment may independently influence subsequent brain biomarkers related to late-life cognitive health. Evaluating early-life enrichment in conjunction with measures of early-life stressful or traumatic events (e.g., ACEs; Anda et al., 2006) provides a more holistic assessment of beneficial as well as negative early-life influences that could be leveraged in populations at risk of early-life adversity.
Our findings expand upon prior work that has examined the neurocognitive benefits of different types of early-life enrichment. Noble et al. (2012) found that those with more years of formal education had slower age-related declines in hippocampal volume. A recent study of the Lothian Birth Cohort measured multimodal engagement in lifestyle activities during several life periods and found that greater frequency of activity engagement in midlife (ages 35–50) predicted higher cognitive performance in later life (Gow et al., 2017). The current findings suggest that engagement in activities before the age of 13 may provide similar neurocognitive benefits in later life.
Our EELA inventory also complements existing measures of early enrichment. Prior studies have examined the neuroprotective effects of positive parenting (Whittle et al., 2014) or in-home enrichment (Rao et al., 2010). Years of formal education is also often used as a proxy for early-life enrichment, but reveals little about the quality of that education (Sisco et al., 2015). Combining EELAs with these existing measures may provide a more nuanced assessment of the quality of early-life cognitive and social experiences both inside and outside the home.
There are many potential mechanisms by which complex, “real-world” engagement across the life span may promote cognitive health. EELAs in the current study encompassed a range of social contexts and cognitive demands (e.g., high demand of learning a language vs low demand of going on trips). Cognitively, EELA may promote neurogenesis, synaptogenesis, and structural remodeling early in life that buffers against cognitive declines with age (Schreiber et al., 2016; Stern, 2009). Socially, EELAs may provide prosocial experiences and broaden social support networks, both of which may exercise and entrain prefrontal-limbic networks that regulate executive functioning and emotion regulation (Carlson, Moored, Rebok, & Eaton, in press; Carlson et al., 2012; Davidson & McEwen, 2012).
Our results also expand upon prior work examining how purposeful, prosocial experiences influence the structure and function of the amygdala, an understudied area within the context of older adult intervention studies (Davidson & McEwen, 2012). There is a rich literature on how chronic stress leads to maladaptive increases in amygdala volume early in life (Davidson & McEwen, 2012; Epel & Lithgow, 2014). Yet, the amygdala also contributes to the neurocognitive processing of emotional stimuli, and greater volumes in older adults may promote emotionally salient memories (Carlson et al., in press; Cassidy & Gutchess, 2012). One study found that increased right amygdala volume was associated with better memory for impressions in older but not younger adults (Cassidy & Gutchess, 2012). Another study using the BHS sample found that intensive volunteering led to structural changes in amygdala subregions responsible for processing emotionally salient memories (Carlson et al., under review). These shape changes were positively correlated with generativity, defined as the desire to give back to future generations (Fried et al., 2013). EELAs may provide similar purposeful, prosocial experiences that ultimately lead to a cascade of structural changes in the amygdala that benefit emotional memory in older adulthood.
We also found sex differences in the association between EELA and hippocampal volume, with men appearing to benefit more from additional early-life enrichment. This parallels previous BHS findings, in which men in the BECT had larger intervention-specific increases in hippocampal volume than women (Carlson et al., 2015). There are several potential mechanisms to explain why these sex differences are only found for the hippocampus, and not the amygdala. First, early hippocampal development in men may be more sensitive to social stress and enrichment than women, for which estrogen plays a protective role against hippocampal atrophy until later in life (Bale & Epperson, 2015). In contrast, it is not clear whether the amygdala demonstrates similar developmental sex differences (Lupien et al., 2009). Second, although there were minimal sex differences in individual EELAs endorsed, there may be sex differences in how the activities are performed, particularly in terms of frequency of engagement or physical activity. For example, African-American women tend to be less physically active during leisure time than African-American men (Azevedo et al., 2007). Physical exercise has a well-established positive association with hippocampal volume (Voss et al., 2011), and thus early-life physical activity may be an important mediator of the effect of EELA on hippocampal volume, but not necessarily amygdala volume. Nevertheless, the sex differences in the current study should be interpreted with caution due to possible selection bias. The BECT sample consisted primarily of women, and those men who enrolled were on average healthier and better educated than the women.
Preventive Intervention and Policy Impact
The current findings have intervention and policy implications for combating health disparities. African-Americans have historically faced institutionalized discriminatory policies (e.g., school segregation) that have been associated with late-life cognitive outcomes (Whitfield & Wiggins, 2003). Existing programs aimed to enrich early educational experiences, such as the Perry Preschool Program, have led to improved educational outcomes in these groups (Belfield, Nores, Barnett, & Schweinhart, 2006). Our findings provide initial evidence supporting investment in EELAs in historically underserved communities at higher risk of early-life adversity. These communities may especially benefit in later life from the provision of extracurricular activities that encourage cognitive and social engagement in early life.
Limitations and Future Directions
This study had certain limitations that can be addressed by future research. First, all analyses were cross-sectional, and we could not rule out reverse causation. For example, it is possible that older adults with larger hippocampal volumes may have better recall of early-life activities. Yet, several early studies of early-life experiences (Anda et al., 2006; Mortimer, Snowdon, & Markesbery, 2003) also examined associations with outcomes later in life using a cross-sectional design, and although prospective cohort studies are a gold standard, they are subject to cohort effects. We believe that our results provide initial evidence that warrants replication in future longitudinal studies. Future prospective neuroimaging studies can further examine whether early-life enrichment buffers against aging-related declines in MTL volume, and whether these changes mediate changes in cognition.
The EELA inventory was designed to measure easily recalled childhood activities that were common to adults in the United States, but has yet to be evaluated for generalizability to other samples. Yet, this inventory may be relatively easy to integrate into future studies of life experiences. This retrospective inventory was a simple, 7-item scale, and only required recall of having ever participated in a small range of activities. This may have mitigated recall bias by lessening the cognitive demands on participants. Furthermore, when the survey content is simple and concrete (e.g., no emotional content, no retrospective impact bias; Wilson et al., 2003), retrospective recall can be very accurate even for adults over 50 years old (Berney & Blane, 1997). Retrospectively reported early-life activity inventories have also been shown to have very high test-retest reliability (ICC > 0.9) in prior studies of older adults (Schreiber et al., 2016). We are planning to study the psychometric properties of the EELA inventory in future studies within novel older adult samples.
Finally, the sample was also comprised predominantly of community-dwelling African-Americans with high mean years of education, and the results may not be generalizable to other groups. Despite having higher education, most of the current sample attended the Baltimore City School system, which has historically underperformed academically, in part due to low financial resources to provide an enriching educational environment (Harding, Harrison-Jones, & Rebach, 2012). Such disparities in education quality, rather than quantity, may better explain racial differences in cognitive function than years of education (Sisco et al., 2015), despite years of education still being an important proxy for cognitive reserve (Stern, 2009). Furthermore, older African-Americans remain an understudied population that is at a higher sociodemographic risk of cognitive declines (Zahodne et al., 2017) and dementia (Mayeda et al., 2016). Much research among African-American youth also remains focused on environmental deprivation (but see Rao et al., 2010 for an exception). Thus, the current findings may be especially novel and important for this group. Given the importance of stressful experiences in early brain development, especially for African-Americans (Zahodne et al., 2017), future work should also attempt to disentangle the associations of both positive and negative early experiences on later-life brain volume, potentially by measuring both EELAs and ACEs (Anda et al., 2006) and including both as exposure variables in the same model.
Conclusion
The approach and findings of the current study hold promise for expanding the study of early-life enrichment and later neurocognitive outcomes. The EELA inventory provides a brief tool to complement existing lifestyle indices of activity in mid- and later life. We show that, just as early-life adversity has been shown to negatively impact neural biomarkers and cognitive health, so too may early lifestyle activities leave a beneficial and lasting imprint on neurocognitive functions. While several factors influence brain health in later life, EELA may be one modifiable protective factor that leads to lifelong changes in behavioral and neural outcomes. However, EELAs may not always be accessible to those of high sociodemographic risk of pathological cognitive declines. This has implications for future policy and intervention efforts, which could focus on providing enriching activities to historically underserved children. The current findings provide novel support for this rationale and provides insight into modifiable targets to promote cognitive and brain reserve across socioeconomic and racial strata. Participating in enriching early activities appears to benefit subcortical structures that are crucial to memory function in older adulthood.
Funding
This work was supported by the National Institute on Aging (P01-AG02773503 and T32-AG000247) and the Intramural Research Program of the NIH, National Institute on Aging. Recruitment and baseline evaluation were supported by the Johns Hopkins Neurobehavioral Research Unit. Follow-up evaluation, data cleaning, and analysis were supported by the Alzheimer’s Drug Discovery Foundation.
Supplementary Material
Acknowledgments
We would like to thank the BECT BHS study participants for their critical contributions to this research, and Marian Tzuang for proof-reading and providing critical feedback.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Adler N. E. Epel E. S. Castellazzo G. & Ickovics J. R (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy White women. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 19, 586–592. doi:10.1037/0278-6133.19.6.586 [DOI] [PubMed] [Google Scholar]
- Anda R. F. Felitti V. J. Bremner J. D. Walker J. D. Whitfield C. Perry B. D. … Giles W. H (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. doi:10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo M. R. Araújo C. L. Reichert F. F. Siqueira F. V. da Silva M. C. & Hallal P. C (2007). Gender differences in leisure-time physical activity. International Journal of Public Health, 52, 8–15. doi:10.1007/s00038-006-5062-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T. L. & Epperson C. N (2015). Sex differences and stress across the lifespan. Nature Neuroscience, 18, 1413–1420. doi:10.1038/nn.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Allali G., Annweiler C., & Verghese J (2016). Association of motoric cognitive risk syndrome with brain volumes: Results from the GAIT study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 71, 1081–1088. doi:10.1093/gerona/glw012 [DOI] [PubMed] [Google Scholar]
- Belfield C. R., Nores M., Barnett S., & Schweinhart L (2006). The High/Scope Perry Preschool Program: Cost–benefit analysis using data from the age-40 followup. Journal of Human Resources, XLI, 162–190. doi:10.3368/jhr.XLI.1.162 [Google Scholar]
- Berney L. R. & Blane D. B (1997). Collecting retrospective data: Accuracy of recall after 50 years judged against historical records. Social Science & Medicine (1982), 45, 1519–1525. doi:10.1016/S0277-9536(97)00088-9 [DOI] [PubMed] [Google Scholar]
- Bickart K. C., Wright C. I., Dautoff R. J., Dickerson B. C., & Barrett L. F (2011). Amygdala volume and social network size in humans. Nature Neuroscience, 14, 163–164. doi:10.1038/nn.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D. G., Yaffe K., & Liverman C. T (2015). Cognitive aging: Progress in understanding and opportunities for action. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Brito N. H. & Noble K. G (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276. doi:10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. C., Kuo J. H., Chuang Y.-F., Varma V., Harris G., Albert M., … Fried L. P (2015). Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 11, 1340–1348. doi:10.1016/j.jalz.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. C., Moored K. D., Rebok G. W., & Eaton W. W (In press). Mental health across the lifespan and the role of brain networks. In W. W. Eaton (Ed.), Public mental health (2nd ed.). Oxford, UK: Oxford University Press. [Google Scholar]
- Carlson M. C. Parisi J. M. Xia J. Xue Q. L. Rebok G. W. Bandeen-Roche K. & Fried L. P (2012). Lifestyle activities and memory: Variety may be the spice of life. The Women’s Health and Aging Study II. Journal of the International Neuropsychological Society: JINS, 18, 286–294. doi:10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. C., Tang X., Varma V. R., Harris G., Gruenewald T., Rebok G. W., … Miller M. I. (under review). Social volunteering expands region-specific shape of the amygdala that are related to enhanced purpose. Frontiers in Aging. [Google Scholar]
- Cassidy B. S. & Gutchess A. H (2012). Structural variation within the amygdala and ventromedial prefrontal cortex predicts memory for impressions in older adults. Frontiers in Psychology, 3, 319. doi:10.3389/fpsyg.2012.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T., Parisi J. M., Moored K. D., & Carlson M. C (2018). Variety of enriching early-life activities linked to late-life cognitive functioning in urban community-dwelling African Americans. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74, 1345–1355. doi:10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’ath P., Katona P., Mullan E., Evans S., & Katona C (1994). Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Family Practice, 11, 260–266. doi:10.1093/fampra/11.3.260 [DOI] [PubMed] [Google Scholar]
- Davidson R. J. & McEwen B. S (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience, 15, 689–695. doi:10.1038/nn.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T., van der Lijn F., Koudstaal P. J., Hofman A., van der Lugt A., Krestin G. P., … Breteler M. M. B (2010). A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain, 133, 1163–1172. doi:10.1093/brain/awq048 [DOI] [PubMed] [Google Scholar]
- Eggert L. D. Sommer J. Jansen A. Kircher T. & Konrad C (2012). Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS ONE, 7, e45081. doi:10.1371/journal.pone.0045081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S., & Lithgow G. J (2014). Stress biology and aging mechanisms: Toward understanding the deep connection between adaptation to stress and longevity. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 69(Suppl. 1), S10–S16. doi:10.1093/gerona/glu055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., … Kramer A. F (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108, 3017–3022. doi:10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62, 774–781. doi:10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F. Folstein S. E. & McHugh P. R (1975). “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fried L. P. Carlson M. C. McGill S. Seeman T. Xue Q. L. Frick K. … Rebok G. W (2013). Experience Corps: A dual trial to promote the health of older adults and children’s academic success. Contemporary Clinical Trials, 36, 1–13. doi:10.1016/j.cct.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour M. M. & Manly J. J (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. doi:10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Gow A. J. Pattie A. & Deary I. J (2017). Lifecourse activity participation from early, mid, and later adulthood as determinants of cognitive aging: The Lothian Birth Cohort 1921. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 72, 25–37. doi:10.1093/geronb/gbw124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H. R., Harrison-Jones L., & Rebach H. M (2012). A study of the effectiveness of supplemental educational services for Title I students in Baltimore city public schools. The Journal of Negro Education, 81, 52–66. https://doi.org/10.7709/jnegroeducation.81.1.0052 [Google Scholar]
- Lupien S. J. McEwen B. S. Gunnar M. R. & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10, 434–445. doi:10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Mayeda E. R. Glymour M. M. Quesenberry C. P. & Whitmer R. A (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 216–224. doi:10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. M. Knopman D. S. Chertkow H. Hyman B. T. Jack C. R. Jr, Kawas C. H. … Phelps C. H (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 263–269. doi:10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R. A., Petty C. M., Xu Y., Hayes J. P., Wagner H. R., Lewis D. V., … McCarthy G (2009). A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage, 45, 855–866. doi:10.1016/j.neuroimage.2008.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J. A. Snowdon D. A. & Markesbery W. R (2003). Head circumference, education and risk of dementia: Findings from the Nun Study. Journal of Clinical and Experimental Neuropsychology, 25, 671–679. doi:10.1076/jcen.25.5.671.14584 [DOI] [PubMed] [Google Scholar]
- Noble K. G., Grieve S. M., Korgaonkar M. S., Engelhardt L. E., Griffith E. Y., Williams L. M., & Brickman A. M (2012). Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience, 6, 1–10. doi:10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C. & Bischof G. N (2013). The aging mind: Neuroplasticity in response to cognitive training. Dialogues in Clinical Neuroscience, 15, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin S. P. Dautoff R. Morris J. C. Barrett L. F. & Dickerson B. C; Alzheimer’s Disease Neuroimaging Initiative (2011). Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Research, 194, 7–13. doi:10.1016/j.pscychresns.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H. Betancourt L. Giannetta J. M. Brodsky N. L. Korczykowski M. Avants B. B., … Farah M. J (2010). Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage, 49, 1144–1150. doi:10.1016/j.neuroimage.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilipo M. P., Benedict R. H. B., Zivadinov R., & Bakshi R (2004). Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: The proportion vs. residual method. NeuroImage, 22, 1732–1743. doi:10.1016/j.neuroimage.2004.03.037 [DOI] [PubMed] [Google Scholar]
- Schreiber S. Vogel J. Schwimmer H. D. Marks S. M. Schreiber F. & Jagust W (2016). Impact of lifestyle dimensions on brain pathology and cognition. Neurobiology of Aging, 40, 164–172. doi:10.1016/j.neurobiolaging.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco S. Gross A. L. Shih R. A. Sachs B. C. Glymour M. M. Bangen K. J., … Manly J. J (2015). The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 557–567. doi:10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C. León I. Brodaty H. Trollor J. Wen W. Sachdev P. & Valenzuela M. J (2012). Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. NeuroImage, 63, 1542–1551. doi:10.1016/j.neuroimage.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Tang X. Holland D. Dale A. M. Younes L. & Miller M. I (2015). Baseline shape diffeomorphometry patterns of subcortical and ventricular structures in predicting conversion of mild cognitive impairment to Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 44, 599–611. doi:10.3233/JAD-141605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M. J. Sachdev P. Wen W. Chen X. & Brodaty H (2008). Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE, 3, e2598. doi:10.1371/journal.pone.0002598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma V. R., Chuang Y., Harris G. C., Tan E. J., & Carlson M. C (2015). Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus, 25, 605–615. doi:10.1002/hipo.22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M. W. Nagamatsu L. S. Liu-Ambrose T. & Kramer A. F (2011). Exercise, brain, and cognition across the life span. Journal of Applied Physiology (Bethesda, Md.: 1985), 111, 1505–1513. doi:10.1152/japplphysiol.00210.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield K. E., & Wiggins S. A (2003). The impact of desegregation on cognition among older African Americans. Journal of Black Psychology, 29, 275–291. doi:10.1177/0095798403254209 [Google Scholar]
- Whittle S. Simmons J. G. Dennison M. Vijayakumar N. Schwartz O. Yap M. B., … Allen N. B (2014). Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. doi:10.1016/j.dcn.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. S. (1993). WRAT-3: Wide range achievement test administration manual. Wilmington, DE: Wide Range, Inc. [Google Scholar]
- Wilson R. S., Bennett D. A., Bienias J. L., Mendes de Leon C. F., Morris M. C., & Evans D. A (2003). Cognitive activity and cognitive decline in a biracial community population. Neurology, 61, 812–816. doi:10.1212/01.WNL.0000083989.44027.05 [DOI] [PubMed] [Google Scholar]
- Zahodne L. B., Sol K., & Kraal Z (2017). Psychosocial pathways to racial/ethnic inequalities in late-life memory trajectories. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74, 409–418. doi:10.1093/geronb/gbx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.