Abstract
Pancreatic β-cells recognize blood glucose changes and release insulin that is a peptide hormone responsible for stable glycemia. Diabetes, a chronic disorder of insulin insufficiency, leads to disturbed glucose homeostasis and multi-organ problems. Glucose and insulin are key markers in the follow-up and control of this disease. Mitochondrial metabolism of pancreatic beta cells is a crucial part of glucose-stimulated cascade of insulin secretion. Effective factors on β-cells mitochondrial function in production of compounds such as tricarboxylic acid intermediates, glutamate, nicotinamide adenine dinucleotide phosphate, and reactive oxygen species can have great effects on the secretion of insulin under diabetes. This review enhances our knowledge of factors influencing mitochondrial function as a key mediator of glucose-induced insulin release that accordingly will be helpful to further our understanding of the mechanisms implicated in the progressive beta cell failure that results in diabetes.
Key Words: Mitochondria, glucose-sensing, insulin release, NADPH, ROS, diabetes
Introduction
Preserving glucose metabolism is essential for organismal homeostasis, for that insulin-producing pancreatic beta cells are metabolically bound to physiological glycemic homeostasis maintenance (1). Pancreatic β-cells recognize alterations in blood glucose and release insulin to create normoglycemia. Insufficient secretion of insulin causes a chronic disorder called diabetes that leads to the disturbance of glucose homeostasis and multi-organ problems. Type 1 diabetes mellitus eventuates principally from autoimmune β-cell impairment. In opposition, type 2 diabetes mellitus (T2DM) is induced by disturbance of glucose homeostasis due to insulin resistance in insulin responsive tissues and impairment of insulin secretion in pancreatic β-cells (2). Dysfunctions of β cells along with damaged glucose-stimulated insulin secretion are the principal defects in T2DM since beta cells mass reduction differs greatly between various studies. Dysfunction of mitocho-ndria is a common element of disease progression in both type 1 and type 2 diabetes mellitus.
Mitochondria play a significant role in metabolism, energy production, and redox homeostasis. Insulin release in reply to a powerful stimulation via glucose is biphasic, i.e. a fast initial peak is pursued with a slower but maintained second phase of insulin release. Mitochondria have an important role in providing signals that control these processes. Although the relationship between mitochondrial function and insulin resistance has not been determined, it has been well documented that β-cells of mitochondria have a decisive function in glucose-stimulated insulin release and contribute to the actions of insulin on its target tissues (3). Recent investigations indicate that glucose metabolism in mitochondria, in addition to the changes in adenosine triphosphate: adenosine diphosphate (ATP: ADP) ratio, generates signals that are important for control of insulin secretion (4). In this study, we review new insights into the role of mitochondrial metabolism in regulation of insulin release coupling in β-cells underlying diabetes. Based on the results of various studies, our hypothesis is that the effective factors on β-cells mitochondrial function in production of compounds such as tricarboxylic acid (TCA) interm-ediates, glutamate, nicotinamide- adenine- dinucle- otide-phosphate (NADPH), and reactive oxygen species (ROS) can have great effects on the secretion of insulin under diabetes.
Glucose-sensing pathway in β cells and glucose-dependent insulin release
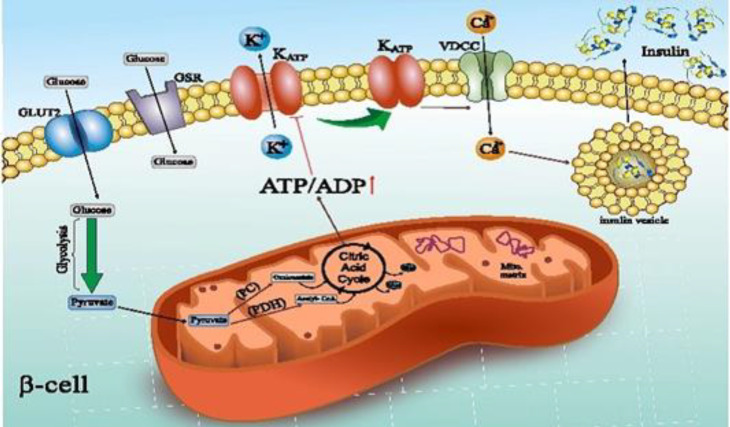
Pancreatic β cells sense blood glucose changes and secrete insulin to retain normoglycemia (5). D-glucose enters the β-cells by facilitated diffusion through a membrane bound D-glucose transporter (GLUT1 or GLUT2) (6,7). The set point of glucose-induced insulin release in pancreatic islets is about 5 mM. Decreased glucose sensitivity leads to an increased threshold for glucose-induced insulin release which eventually results in hyperglycemia (8,9). However, glucose-induced insulin release threshold is not exclusively controlled via β cell glucokinase (GCK), β cells glucose sensing is mainly controlled through GCK activity and mitochondrial metabolism, which impels the respiratory chain and ATP production by oxidative phosphorylation afterwards (OxPhos). Since oxidative phosphorylation has a fundamental function in sensory system, oxygen pressure can have an important function in glucose sensing. Available evidence indicate that tissue oxygenation plays a remarkable role in susceptibility to T2DM (10). Hexokinase 1, 2, 3, and 4 GCK enzyme kinetics and protein structure are entirely distinct. GCK has a low affinity for glucose binding and lack of considerable end-product feedback inhibition (8). In addition to GCK, taste receptor type 1 member 3 (T1R3) homodimer, a subunit of sweet taste receptor in beta cells, is another factor that acts as a glucose sensing receptor (GSR). Elimination of T1R3 gene or blocking of this receptor with guramine reduces glucose-dependent insulin secretion from beta cells. Glucose can also boost insulin release in absence of cell surface GSR; although, the beginning of secretion is delayed and the magnitude of secretory response is decelerated. Kojima et al. showed that a non-metabolizable glucose can increase metabolism and intracellular ATP (ATPi). They also indicated that T1R3 knockdown with shRNA leads to decrease of glucose-dependent raise of ATPi. This means that glucose cannot show all its capabilities in the absence of T1R3 (11-15). At the end of aerobic glycolysis, pyruvate is produced from glucose. Pyruvate can enter the mitochondrial TCA cycle in two ways, either through oxidative decarboxylation to acetyl-CoA by pyruvate dehydrogenase complex or through carboxylation to oxaloacetate by pyruvate carboxylase (16) that both of these pathways are essential for glucose-dependent insulin secretion (17). A significant amount of produced ATP is the result of mitochondrial oxidation of glucose-derived pyruvate. Elevation of ATP/ADP ratio closes ATP-sensitive K+ (K+ATP) channels, and subsequently results in depolarization of plasma membrane (18). Increasing the plasma membrane potential to -40 Mv (8) leads to voltage-dependent calcium channel opening in plasma membrane, and calcium enters the cell. An increase in cytosolic calcium leads to activation of protein kinases A and C (19), and eventually insulin secretion outside the cell (20) (Figure 1). This process is damaged in T2DM mostly due to defective mitochondrial metabolism.
Fig. 1.
Glucose sensing pathway in β cells and glucose-stimulated insulin release. Glucose initially affects the cell-surface glucose sensing receptor (GSR) that produces a signal to initiate metabolism in mitochondria. Glucose then enters β cells by facilitated diffusion via D-glucose transporter 2 (GLUT2) and is metabolized to pyruvate. Pyruvate enters the TCA cycle either through oxidative decarboxylation to acetyl-CoA by pyruvate dehydrogenase (PDH) complex or through carboxylation to oxaloacetate by pyruvate carboxylase (PC). Elevation of ratio of ADP to ATP inhibits ATP-sensitive K+ (K+ATP ) channels and subsequently leads to depolarization of the plasma membrane that leads to opening of a voltage-dependent calcium channel in plasma membrane and calcium entry into the cell, and finally leading to exocytosis of insulin granules
Abnormalities in ion channels of the beta cells plasma membrane can lead to changes in glucose sensing. These ion channels are: voltage-gated potassium channels (Kv7.1), voltage dependent anion channels (VDACs), voltage dependent calcium channels (VDCCs), and KATP channels.
Mutations and abnormalities in K+ATP channels lead to an increase in the amount of ATP needed to close these channels, so normal amounts of glucose cannot produce enough ATP to close these channels. Accordingly, plasma membrane of β cells is continuously hyperpolarized, and glucose-dependent insulin secretion does not occur (21).
The K+ATP channel independent pathway of glucose-dependent insulin secretion suggests that even in the presence of K+ATP depolarizing concentrations along with diazoxide, which inhibits K+ATP channel closure, the presence of ATP and fuel metabolism is essential for glucose-dependent insulin secretion (18).
VDAC is one of the most important mitochondrial outer membrane proteins. VDAC1 and VDAC2 control the cell's life and death through controlling the transfer of metabolites, ions, as well as nucleotides, including ADP and ATP, between the mitochondria and the cytosol. Zhang et al. showed that overexpression of VDAC1 leads to malfunction of the beta cells plasma membrane in insulin secretion which is due to the absence of ATP and its role in insulin secretion. Direct VDAC1 inhibition in human T2DM β cells restitutes glucose-dependent insulin secretion, and prevents the progression of diabetes in db/db mice (22).
Insulin is secreted in two phases. A few seconds after the beta cells come in contact with the fuel secretions, a significant amount of insulin is released. This step is called the first phase. The peak of insulin secretion at this phase may be due to the presence of granules near the plasma membrane or due to increased cellular calcium. The second phase, also called the amplification phase, is more important in terms of the amount of insulin secretion because in this phase insulin is secreted for a longer period of time. The secondary phase also needs calcium and ATP. Activation of the first phase of insulin secretion and increase in intracellular calcium can occur with high potassium or arginine, non-metabolic stimulants of insulin secretion, but they cannot cause insulin secretion in the second phase.
Evidence indicates that only fuel secretions can activate both phases of insulin secretion, so the second phase, in addition to ATP, depends on other fuel metabolism products (23,24).
The role of tricarboxylic acid cycle intermediates in insulin release
Recent metabolomic investigations in models of type 1 diabetes have shown down-regulation of the key TCA cycle, mitochondrial proteins, and enzyme activities. All known TCA cycle intermediates, comprising citrate/isocitrate, α-ketoglutarate, succinate, fumarate, and malate are important components of insulin secretion, especially in the secondary phase (4). Among these intermediates, succinate and α -ketoglutarate are most closely related to insulin secretion in the secondary phase. Various studies have shown the importance of these metabolites in insulin secretion by examining the metabolic pathways associated with these compounds as well as the role of mitochondrial metabolic carriers (25,26). The performance of these intermediate compounds in insulin secretion during the first and second phases has been achieved through numerous studies on 832/13 cells (27,28).
Hals et al. showed that succinate alone could support respiration, which suggests that succinate may also be a strong inducer of phosphorylation in β-cells (29). In the fat sand rat (Psammomys obesus) islets; insulin release was not stimulated by succinate in the absence of glucose, whereas pro-insulin biosynthesis was increased five-fold. In contrast, under stimulating glucose levels, succinate doubled the insulin release, indicating the glucose dependence (30).
Studies have indicated that α-ketoglutarate can act as an insulin secretagogue, and inhibition of α-ketoglutarate-dependent hydroxylases blocks glucose stimulated insulin release. Increased malate in matrix will equilibrate with fumarate formation, decrease of succinate oxidation to fumarate, and causes subsequent increase of oxaloacetate which is a powerful inhibitor of succinate dehydrogenase (31). Reduced equivalents in mitochondrial matrix are principally generated by mitochondrial metabolite shuttles and TCA cycle (32).
Mitochondrial GTP (mtGTP), another strong signaling molecule, is created within the mitochondrion as a product of the TCA cycle in the reaction of succinyl-CoA to succinate, and is not exported to the cytosol. Two different isoforms of succinyl-CoA synthetase couple this reaction to the production of one molecule of either ATP or GTP. Silencing of the ATP-producing isoform results in a noticeable increase in glucose stimulated- insulin release, while knockdown of GTP-producing form has a negative consequence on glucose-stimulated insulin release (33). The basis of relationship between insulin secretion and mitochondrial matrix GTP requires more studies.
Matrix calcium concentration ([Ca 2+ ] mito ) in insulin release
Cellular calcium signaling has an important relationship with mitochondrial function. Only a small portion of the calcium accumulated in the mitochondria is free, and its concentration is highly regulated. Following mitochondrial stimulation, the absorption of calcium exceeds the mitochondrial capacity for calcium excretion that causesthe temporarily increase of [Ca2+]mito from 0.5 to several µM (34).
The elevation of intra mitochondrial calcium causes an increase in the activity of several matrix enzymes, including oxoglutarate dehydrogenase, isocitrate dehydrogenase, and pyruvate dehydrogenase, with the net effect of increasing NADH production. The ATP synthase is also directly activated with an increase in [Ca2+]mito (35,36).
Calcium homeostasis of mitochondria is extremely regulated by balanced calcium influx and efflux. Calcium excretion from mitochondria is performed via antiporters exchanging Ca2+ for Na+ or H+ (32).
Mitochondrial sodium calcium exchanger (NCLX) and leucine zipper-EF hand-containing transmembrane protein 1 (LETM1) are two known mitochondrial antiporters activating calcium efflux. NCLX pharmacological blocking in β cells raises [Ca2+]mito and speeds up mitochondrial oxidative metabolism and glucose-stimulated insulin release (37,38).
Calcium is transported through the mitochondrial calcium uniporter (MCU) into the mitochondria where more energy metabolism is spent on insulin secretion in second phase. Quan et al. indicated that inhibiting MCU gene expression and thus reducing the absorption of calcium by mitochondria leads to a remarkable reduction in activity of respiratory chain and increased ΔpHmito in intact and permeabilized insulin secreting cells (32). These imperfections eventuate in damaged ATP synthesis and insulin release, emphasizing the critical role of mitochondrial calcium absorption in establishment of ΔpHmito through metabolism-secretion coupling (32).
Mitochondrial calcium uptake is momentous to maintain NADPH for deletion of hydrogen peroxide (H2O2) because all enzymes that produce mitochondrial NADPH acquire their substrates from the Krebs cycle (39).
Increased insulin secretion and succinate-
dependent mitochondrial calcium is eliminated by blocking the uptake of mitochondrial calcium with ruthenium red. Studies have shown that the suggested insulin secretion KATP independent pathway is managed by mitochondrial a factor of which relies on anaplerosis and possibly calcium signaling (40).
Potential role of mitochondrial membrane and matrix pH in insulin release
The electrochemical (membrane potential Δψmito, and ΔpHmito) gradient across the inner membrane of mitochondria causes ATP synthesis (32).
The pH of matrix is a regulator of energy metabolism in beta cells. The distinguishing feature of beta cells from other cells is the presence of an acidic pHmito under resting conditions. Nutritional stimuli alkalize the matrix without significant effect on pH of the cytosol. Ionophores by stopping the nutrient-dependent ΔpHmito changes increase annihilate the proton-coupled mitochondrial ion/metabolite transport, ATP synthesis, and glucose induced insulin release regardless of increased Δψmito. Accordingly, pathogens causing a decrease of ΔpHmito can critically decline ATP generation and insulin release in pancreas β cells (41,42).
Matrix pH appears to affect metabolism–secretion coupling independent of its effect on electrochemical gradient across the inner mitochondrial membrane, as if indicated via a study using the unspecific K+ H+ ionophore nigericin to induce matrix acidification (43).
Mitochondrial membrane potential has a considerable function in glucose-stimulated insulin release in pancreatic β cells. At high concentrations of glucose, glucose oxidation is almost entirely responsible for the stabilization of hyperpolarized Δψmito whereas in low-glucose concentrations proton leak plays a more important role in the homeostatic control of Δψmito. A study indicated that increased downstream glucose oxidation by Δψmito reinforces glucose-evoked Δψmito hyperpola-rization. These investigations suggest a positive feedback loop in β cell energetics regulation as well as the possible regulatory role of proton leak in the fasting state (44).
The mitochondrial membrane potential of diabetic β-cells has been found to be a distinct response to acute inhibition of ATP synthesis during glucose stimulation. As a result, the mechanistic deficit in glucose-stimulated insulin release and mitochondrial hyperpolarization of human diabetic β-cells is located upstream of the TCA cycle, and is dampening the control of Δψmito by glucose metabolism (45).
The role of mitochondrial anaplerotic metab-olites in insulin release
In pancreatic beta cells, mitochondria has a significant function in anaplerosis that is the net synthesis of citric acid cycle intermediates that are expelled to cytosol where they are transformed to several other products that amplify insulin secretion. This pathway is termed the “metabolic amplifying pathway.”
Recent evidence indicates that classic KATP channel-dependent mechanism of glucose-dependent insulin release does not completely illustrate glucose effect on insulin secretion (46). Findings of recent studies propose that a number of metabolites produced during the TCA cycle, including NADPH, α-ketoglutarate, and GTP enhance insulin secretion in secondary phase (47). Glucose dependent pyruvate can enter the anaplerosis pathway by pyruvate carboxylase and affect insulin secretion via enhancing the production of metabolism-derived signaling molecules such as NADPH from the malate-pyruvate shuttle, citrate cataplerosis (48), glutamate (49), and signaling lipid molecules from the malonyl-CoA/LC-CoA pathway (50). After pyruvate enters the mitochondria through the pyruvate carrier, it is almost equally affected by pyruvate dehydrogenase and pyruvate carboxylase which both enzymes are essential for glucose-dependent insulin secretion (17). The β cells have a higher amount of mitochondrial enzyme pyruvate carboxylase than islet non-β-cells. Roughly 40% of pyruvate is imported into TCA cycle during glucose stimulation with its carboxylation by pyruvate carboxylase.
The products of pyruvate dehydrogenase and pyruvate carboxylase are acetyl-CoA and oxaloacetate, respectively. In pyruvate/malate shuttle, oxaloacetate is converted into malate by malate dehydrogenase of mitochondria. Malate is removed from the mitochondria, and enters cytosol where it is converted to pyruvate concomitant with the NADPH generation via cytosolic malic enzyme (ME1). Pyruvate then re-enters the mitochondria, and is again carboxylated via pyruvate carboxylase. Because this process is repeated in cyclic mode, high amounts of NADPH are produced at the expense of mitochondrial ATP and NADH.
The production of short chain acyl-CoAs derivative of glucose in cytosol from mitochondrial-derived metabolites are needed for glucose-dependent insulin release in pancreatic β cells. Another path for -producing oxaloacetate by pyruvate carboxylase is the pyruvate/citrate shuttle. In pyruvate/citrate shuttle, citrate is produced through a reaction involving citrate synthase which allows oxaloacetate and acetyl-CoA combination in mitochondria. The citrate is then transferred from the mitochondria to the cytosol, where it is converted by ATP citrate lyase into two products, acetyl-CoA and oxaloacetate.
Mitochondrial-derived citrate can be a source of short-chain acyl-CoAs production in cytosol, as acetyl-CoA carboxylase carboxylates the cytosolic acetyl-CoA to form malonyl-CoA, and malonyl-CoA is believed to be a momentous signal in insulin release or can be used for fatty acid synthesis (50-52).
In another pathway that occurs in mitochondria, pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase. From the combination of two molecules of acetyl-CoA, acetoacetyl-CoA is produced which can be converted into acetoacetate via succinyl-CoA: 3- ketoacid-CoA transferase, and released from mitochondria to cytosol where it produces acetoacetyl-CoA by acetoacetyl-CoA synthetase. Finally, cytosolic acetyl-CoA and malonyl-CoA are produced from acetoacetyl-CoA that can act as a signal to insulin release (53-56).
Free fatty acids act as signaling molecules in diverse cellular processes, including insulin secretion (57) and prepare a momentous energy source as nutrients.
In TCA cycle, citrate produces α-ketoglutarate by dehydrogenation and then decarboxylation. Matrix glutamate dehydrogenase aminates α-ketoglutarate and produces glutamate. Afterwards, glutamate is pushed out of the mitochondria through glutamate carrier 1. In addition, glutamate is produced by deamination of glutamine which is promoted most likely by glutaminase 2 (58-60). In beta cells, glutamate like an intracellular messenger, can enter the secretory granules, and increase glucose and incretin –dependent insulin release. Glutamate is transported via the insulin-containing secretory granules. The transport of glutamate leads to acidification of secretory granules which is necessary for both conversion of pro-insulin to mature insulin, and for insulin exocytosis through fusion of secretory granules with plasma membrane (61,62).
In mitochondria, following the oxidative deamination of glutamate by glutamate dehydrogenase, products such as NADH, NADPH, α-ketoglutarate, and ammonium ions (NH4+) are produced with ATP production by NADH and NADPH through oxidative phosphorylation leading ultimately to increased insulin secretion by closing KATP channels (63,64). As a result, decomposition of intracellular glutamate seems to increase glucose-induced insulin release by generation of various metabolic coupling factors, such as ATP, NADPH, and fatty acyl-CoA (61).
The role of nicotinamide nucleotide transhyd-rogenase (NNT) and insulin release
NADPH in mitochondria is basically generated by nicotinamide nucleotide transhydrogenase, isocitrate dehydrogenase, glutamate dehydrogenase, and malic enzyme. Nicotinamide nucleotide transhydrogenase (NNT) is placed in the mitochondrial inner membrane (65). This enzyme uses the electrochemical proton gradient to hydride transfer from NADH to NADP+, thereby increasing the ratio of NADPH/NADP+ in mitochondrial matrix (66). Because NNT uses NADH as an electron donor to produce NADPH, it can link the NAD-dependent dehydrogenases of TCA cycle to NADPH production. As NNT unites reductive potentials of mitochondrial substrates, it is considered as a high capacity source for NADPH production (67). The mitochondrial NADP-dependent isocitrate dehydrogenase converts α-ketoglutarate to isocitrate and in this way, NADPH is transferred outside of the mitochondria.
Ronchi et al. (68) indicated that inactivation of Nnt gene in C57BL/6J mice (J-mice) reduces the production of glucose-dependent insulin release, and also glucose tolerance which is the result of defects in mitochondrial oxidative stress and reduced calcium influx and production of glucose-dependent ATP.
Santos et al. (65) showed that absence of Nnt did not change the influx of glucose -induced calcium and upstream mitochondrial events, but both phases of glucose-dependent insulin secretion via calcium-dependent exocytosis, and its metabolic amplification were reduced. They demonstrated that very low concentration of glucose or absence of glucose, lead to a decrease in oxidation of cytosolic glutathione, both in presence and in absence of exogenous H2O2. This suggests that absence of NNT reverse reaction may lead to the retentionof β cells against cytosolic oxidative stress when glucose metabolism is markedly decreased.
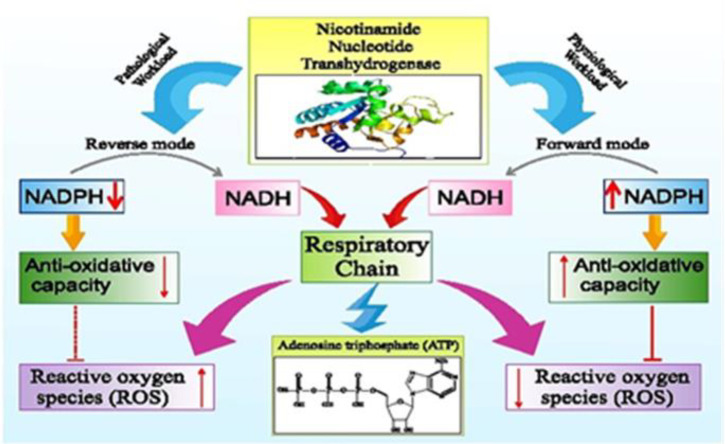
In pathophysiological circumstances, NNT catalyzes reverse reaction to produce NADH for ATP production and retain ΔΨmito by proton pumping. Moreover, the reverse mode NNT reaction can act to generate more reactive oxygen species (39,69) (Figure 2). Accordingly, one of these pathological conditions may be diabetes. Confirmation of this hypothesis, however, requires more studies.
Fig. 2.
The role of nicotinamide nucleotide transhydrogenase in physiological and pathological conditions. The reverse mode nicotinamide nucleotide transhydrogenase (NNT) reaction can act to generate more reactive oxygen species
The role of mitochondrial ROS in insulin release
Because many diabetic patients show signs of oxidative damage along with excessive ROS production, oxidative injury is believed to be the reason of diabetic complications (70,71). Also, studies have shown that using antioxidant compounds alleviates the complications of diabetes by reducing the oxidative stress (72,73).
Mitochondrial inner membrane ingredients are highly exposed to oxidative damage that results in depolarized mitochondrial membrane, induced lipid peroxidation, and perturbed ATP production which is a requisite for glucose-stimulated insulin secretion.
If mitochondrial DNA mutations accumulate, these mutations can be linked with improper performance of mitochondria, increased ROS production, and elevated oxidative damage in human diseases and aging.
Mitochondria are known to be an important regulator of apoptosis cell death. It should be noted that the onset of T2DM is accompanied by a decrease in the mass of beta cells, which can be due to increased apoptosis of beta cells (74-78). Accumulation of H2O2 leads to an increase in generation of reactive hydroxyl radical due to the reactions of fenton and Haber-Weiss of O•−2 and HO•. The increased intensity of O•−2 and HO• activate the release of mitochondrial cytochrome c which initiates the apoptosis process (79). The release of cytochrome c may be due to the direct reaction of ROS with mitochondrial cardiolipin which results in oxidation of cardiolipin and the weakening of its reaction with cytochrome c, that ultimately leads to the release of cytochrome c into the cytoplasm via outer membrane pores.
Damaged respiration capacity of mitochondria and ROS-mediated uncoupling protein-2 (UCP2) expression results in insufficient ATP production during glucose stimulation, which leads to a defect in subsequent insulin release signaling (80).
UCP2, a member of the mitochondrial anion carrier protein (MACP), reduces the synthesis of ATP by proton leak and decrease of mitochondrial membranes potential, and thus has a negative effect on insulin secretion (81).
The mitochondrial NADH/NAD + ratio and insulin release
Nicotinamide adenine dinucleotide (NAD+/ NADH) is a crucial coenzyme for oxido-reduction reactions in metabolism which has recently been known as a signaling molecule involved in calcium signaling, epigenetic regulation of catabolism gene expression, mitochondrial biogenesis and oxidative stress response (82,83).
It has been demonstrated which TCA cycle is an oxygen-dependent pathway that produces NADH necessary to ATP synthesis by oxidizing metabolic intermediates. The ratio of NADH to NAD+ in mitochondria plays an important role in allosteric regulation of TCA cycle because it is a coupler of oxidative phosphorylation. As cell proliferation requires the proper performance of TCA cycle, the ratio of oxidized or reduced state of this coenzyme can have different effects on glucose and glutamine consumption in the TCA cycle (84-86).
Movement of NADH from cytoplasm to mitochondria is accomplished via two redox shuttles, glycerol phosphate shuttle and malate-aspartate shuttle. Nevertheless, the malate–aspartate shuttle has been suggested as the key physiological regulator of cytosolic NADH transfer to mitochondrial matrix in the beta cell by contributing to the amplifying pathway of insulin secretion (87-89).
The electron transport chain in the inner mitochondrial membrane uses NADH electrons to create proton electrochemical gradient which supplies the energy required to pump protons across the inner mitochondrial membrane, and leads to ATP synthesis.
Because β cells have very low lactate dehydrogenase activity, NADH-ubiquinone oxidoreductase (complex I), the starting point for receiving electrons from the NADH in electron transport chain, is the main enzyme responsible for oxidizing NADH to NAD+ which is essential for secretion of glucose-dependent insulin. Consequently, the re-production of NAD+ and the continuation of the glycolysis depend on the activity of complex I. Since the increase in NADH oxidation by complex I is associated with a leakage of electron and further partial oxygen reduction, the production of ROS also increases, and acts asa damaging factor in beta cells.
Pathways such as polyol, which produce NADH by consuming NAD+, increase the ratio of NADH to NAD+, thereby increasing ROS production. It is proposed that in diabetic conditions, nearly 30% of glucose is metabolized via polyol pathway. Eventually, chronic inflammation caused by chronic pseudohypoxic status due to increased ROS production can lead to β cell dysfunction (90-97).
The role of mitochondrial dynamics in pancr-eatic β- cell biology
Mitochondrial morphology plays an important role in glucose-dependent insulin release, because mitochondrial metabolism and function are influenced by the dynamics and morphology of mitochondria (98). The mitochondrial dynamics change in pancreatic β cells seems to be the starting point of the progression of T2DM (99). In mammalian cells, mitochondria are tubular networks whose life cycle is specified by fission, fusion, and autophagy. Reduced mitochondrial fusion activity has been detected to be associated with T2DM (98). Balancing between the events of fission and fusion is important for attainment of mitochondrial morphology required for a specific function (100). Mitofusins1 and 2 (MFN1/2) and 0ptic-atrophy 1(OPA1) are fusion regulators of mitochondria; whereas, mitochondrial fission is regulated via dynamic-related protein 1 (DRP1) and fission protein 1 (FIS1). GTPase gene Drp1 has a crucial function in fission because it is only able to compress mitochondria (101,102). Blocking Drp1 or Fis1 by RNA reduces autophagy of mitochondria, and causes oxidized mitochondrial proteins accumulation, decreases respiration, and induces defective release of insulin (103). Down-regulation of Drp1 in INS1 cells reduces the potential of mitochondrial membrane and ATP production. An important loss of glucose-dependent insulin secretion is shown in INS1 cells and mouse pancreatic islets. As a result, expression of Drp1 is momentous in β cells to retain the regulation of insulin release. Through acute pharmacological blocking of mitochondrial fission protein Drp1, Sesakiet al. showed that fission of mitochondria is essential for secretion of glucose-dependent insulin in the mouse and human islets (104). They corroborated that Drp1 genetic silencing increased the mitochondrial proton leak in MIN6 cells. Fis1 is an important regulator in pancreatic beta cells because both mitochondrial dynamics and release of glucose-dependent insulin correspond to accurate gene expression levels of this fission protein. Schultzet al. indicated that mitochondrial dynamics in cells with defective glucose-induced insulin release (INS1-832/2) decrease in comparison with glucose-responsive cells (INS1-832/13) (99). Blocking of Fis1 via shRNA in both INS1-832/13 cells and primary mouse beta cells causes a lack of glucose response and a remarkably number of prolonged mitochondria. Fis1 overexpression in mouse early beta cells demonstrated an upper limit at which higher Fis1 expression decreased the glucose-induced insulin release (98,99,103-107). Therefore, both mitochondrial dynamics and glucose-stimulated insulin secretion are comprehensibly adapted to levels of this fission protein.
Conclusion
The onset of glucose-dependent insulin secretion is accompanied by the transfer of glucose into the beta cell, followed by phosphorylation in the glycolysis pathway and eventually oxidation in mitochondria. Damaged activation of mitochondrial energy metabolism via glucose has been indicated in type 2 diabetic β-cells.
Glucose phosphorylation and glukokinase play highly significant roles in determining the threshold of glucose-stimulated insulin secretion. In addition to glukokinase, T1R3 homodimer is another factor that acts as a glucose sensing receptor. Elimination of T1R3 gene reduces glucose-dependent insulin secretion from beta cells. Kojima et al. indicated that T1R3 knockdown with shRNA leads to decrease of glucose-dependent raise of ATPi. This means that glucose cannot show all its capabilities in the absence of T1R3 (11). It is interesting that glucose stimulates a biphasic enhancement in ATPi. The initial peak arises around 1 min, followed by the secondary sustained phase.
Metabolic coupling factors produced by glucose oxidation enhance the effect of calcium on insulin release, which is called the booster pathway of insulin release.
Providing control signals is the significant role of mitochondria for first and second phases of insulin release.
All known TCA cycle intermediates, comprising citrate/ isocitrate, α-ketoglutarate, succinate, fumarate, and malate, are important components of insulin release, especially in the secondary phase (4). It has been demonstrated that TCA cycle is an oxygen-dependent pathway that produces NADH needed to synthesize ATP by oxidizing metabolic intermediates. The ratio of NADH to NAD+ in mitochondria plays an important role in allosteric regulation of TCA cycle because it is a coupler of oxidative phosphorylation.
The ΔΨmito and its response to glucose are principal irritants of ATP synthesis in mitochondria, and hence a central mediator of glucose-induced insulin release.
NNT catalyzes reverse reaction to produce NADH for ATP production and retain ΔΨmito by proton pumping. Accordingly, perhaps one of these pathological conditions is diabetes, the confirmation of which requires more studies. The reverse mode NNT reaction can act to produce more ROS. Mitochondrial inner membrane ingredients are highly exposed to oxidative damage that results in depolarized mitochondrial membrane, induced lipid peroxidation, and defective ATP production, which is a requisite for glucose-stimulated insulin secretion.
The morphology of mitochondria affects the function and mitochondrial metabolism. The mitochondrial dynamics change in pancreatic β cells seems to be the starting point of the progression of T2DM. This study increases our knowledge about the mechanism of action of factors affecting amplifying pathways of insulin release that accordingly will be helpful to further our understanding of mechanisms involved in beta cell defects which results in diabetes.
Acknowledgment
The financial supports of Kermanshah University of Medical Sciences (Grant No. 980130) are gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Boland BB, Rhodes CJ, Grimsby JS. The dynamic plasticity of insulin production in beta-cells. Mol Metab. 2017;6:958–73. doi: 10.1016/j.molmet.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu J, Cui Q, Yang B, et al. The impairment of glucose-stimulated insulin secretion in pancreatic beta-cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food Chem Toxicol. 2017;100:161–7. doi: 10.1016/j.fct.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol Cell Endocrinol. 2012;353:128–37. doi: 10.1016/j.mce.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Huang M, Joseph JW. Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology. 2014;155:1653–66. doi: 10.1210/en.2013-1805. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Koshkin V, Allister EM, et al. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–59. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh YS, Bae GD, Baek DJ, et al. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front Endocrinol (Lausanne) 2018;9:384. doi: 10.3389/fendo.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvucci M, Neufeld Z, Newsholme P. Mathematical model of metabolism and electrophysiology of amino acid and glucose stimulated insulin secretion: in vitro validation using a beta-cell line. PLoS One. 2013;8:e52611. doi: 10.1371/journal.pone.0052611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Li C. Nutrient sensing in pancreatic islets: lessons from congenital hyperinsulinism and monogenic diabetes. Ann N Y Acad Sci. 2018;1411:65–82. doi: 10.1111/nyas.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–85. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DF, Matschinsky FM. Oxygen dependence of glucose sensing: role in glucose homeostasis and related pathology. J Appl Physiol. 2019;126:1746–55. doi: 10.1152/japplphysiol.00047.2019. [DOI] [PubMed] [Google Scholar]
- 11.Kojima I, Nakagawa Y, Hamano K, et al. Glucose-Sensing Receptor T1R3:A New Signaling Receptor Activated by Glucose in Pancreatic beta-Cells. Biol Pharm Bull. 2015;38:674–9. doi: 10.1248/bpb.b14-00895. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa Y, Ohtsu Y, Nagasawa M, et al. Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J. 2014;61:119–31. doi: 10.1507/endocrj.ej13-0431. [DOI] [PubMed] [Google Scholar]
- 13.Medina A, Nakagawa Y, Ma J, et al. Expression of the glucose-sensing receptor T1R3 in pancreatic islet: changes in the expression levels in various nutritional and metabolic states. Endocr J. 2014;61:797–805. doi: 10.1507/endocrj.ej14-0221. [DOI] [PubMed] [Google Scholar]
- 14.Kojima I, Nakagawa Y, Ohtsu Y, et al. Return of the glucoreceptor: Glucose activates the glucose-sensing receptor T1R3 and facilitates metabolism in pancreatic beta-cells. J Diabetes Investig. 2015;6:256–63. doi: 10.1111/jdi.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraedts MC, Takahashi T, Vigues S, et al. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab. 2012;303:E464–74. doi: 10.1152/ajpendo.00163.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Krus U, Kotova O, Spegel P, et al. Pyruvate dehydrogenase kinase 1 controls mitochondrial metabolism and insulin secretion in INS-1 832/13 clonal beta-cells. Biochem J. 2010;429:205–13. doi: 10.1042/BJ20100142. [DOI] [PubMed] [Google Scholar]
- 17.Morten KJ, Potter M, Badder L, et al. Insights into pancreatic beta cell energy metabolism using rodent beta cell models. Wellcome Open Res. 2017;2:14. doi: 10.12688/wellcomeopenres.10535.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taddeo EP, Stiles L, Sereda S, et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Mol Metab. 2018;16:150–9. doi: 10.1016/j.molmet.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliasson L, Esguerra JLS, Wendt A. Lessons from basic pancreatic beta cell research in type-2 diabetes and vascular complications. Diabetol Int. 2017;8:139–52. doi: 10.1007/s13340-017-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmgren S, Nicholls DG, Taneera J, et al. Tight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretion. J Biol Chem. 2009;284:32395–404. doi: 10.1074/jbc.M109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir62 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–49. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 22.Zhang E, Mohammed Al-Amily I, Mohammed S, et al. Preserving Insulin Secretion in Diabetes by Inhibiting VDAC1 Overexpression and Surface Translocation in beta Cells. Cell Metab. 2019;29:64–77 e6. doi: 10.1016/j.cmet.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–63. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 24.Henquin JC, Ravier MA, Nenquin M, et al. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–50. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 25.Huypens P, Pillai R, Sheinin T, et al. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia. 2011;54:135–45. doi: 10.1007/s00125-010-1923-5. [DOI] [PubMed] [Google Scholar]
- 26.Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–7. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spegel P, Sharoyko VV, Goehring I, et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J. 2013;450:595–605. doi: 10.1042/BJ20121349. [DOI] [PubMed] [Google Scholar]
- 28.Huang M, Joseph JW. Metabolomic analysis of pancreatic beta-cell insulin release in response to glucose. Islets. 2012;4:210–22. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hals IK, Bruerberg SG, Ma Z, et al. Mitochondrial Respiration in Insulin-Producing beta-Cells: General Characteristics and Adaptive Effects of Hypoxia. PLoS One. 2015;10:e0138558. doi: 10.1371/journal.pone.0138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attali V, Parnes M, Ariav Y, et al. Regulation of insulin secretion and proinsulin biosynthesis by succinate. Endocrinology. 2006;147:5110–8. doi: 10.1210/en.2006-0496. [DOI] [PubMed] [Google Scholar]
- 31.Gnaiger E. Mitochondrial pathways and respiratory control: an introduction to OXPHOS analysis; mitochondr physiol network 17.18. OROBOROS INSTRUMENTS GmbH, Innsbruck, Austria: 2012. [Google Scholar]
- 32.Quan X, Nguyen TT, Choi SK, et al. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2015;290:4086–96. doi: 10.1074/jbc.M114.632547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groschner LN, Alam MR, Graier WF. Metabolism-secretion coupling and mitochondrial calcium activities in clonal pancreatic beta-cells. Vitam Horm. 2014;95:63–86. doi: 10.1016/B978-0-12-800174-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 34.Wiederkehr A, Szanda G, Akhmedov D, et al. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011;13:601–11. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong JA, Cash NJ, Morton JC, et al. Mitochondrial Targeting of Antioxidants Alters Pancreatic Acinar Cell Bioenergetics and Determines Cell Fate. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra J, Davani AJ, Natarajan GK, et al. Cyclosporin A Increases Mitochondrial Buffering of Calcium: An Additional Mechanism in Delaying Mitochondrial Permeability Transition Pore Opening. Cells. 2019:8. doi: 10.3390/cells8091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Marchi U, Santo-Domingo J, Castelbou C, et al. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J Biol Chem. 2014;289:20377–85. doi: 10.1074/jbc.M113.540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nita , II , Hershfinkel M, Fishman D, et al. The mitochondrial Na+/Ca2+ exchanger upregulates glucose dependent Ca2+ signalling linked to insulin secretion. PLoS One. 2012;7:e46649. doi: 10.1371/journal.pone.0046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nickel AG, von Hardenberg A, Hohl M, et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015;22:472–84. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Wiederkehr A, Park KS, Dupont O, et al. Matrix alkalinization: a novel mitochondrial signal for sustained pancreatic beta-cell activation. EMBO J. 2009;28:417–28. doi: 10.1038/emboj.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan X, Das R, Xu S, et al. Mitochondrial phosphate transport during nutrient stimulation of INS-1E insulinoma cells. Mol Cell Endocrinol. 2013;381:198–209. doi: 10.1016/j.mce.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Akhmedov D, Braun M, Mataki C, et al. Mitochondrial matrix pH controls oxidative phosphorylation and metabolism-secretion coupling in INS-1E clonal beta cells. FASEB J. 2010;24:4613–26. doi: 10.1096/fj.10-162222. [DOI] [PubMed] [Google Scholar]
- 44.Gerencser AA, Mookerjee SA, Jastroch M, et al. Positive Feedback Amplifies the Response of Mitochondrial Membrane Potential to Glucose Concentration in Clonal Pancreatic Beta Cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1054–65. doi: 10.1016/j.bbadis.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerencser AA. Bioenergetic Analysis of Single Pancreatic beta-Cells Indicates an Impaired Metabolic Signature in Type 2 Diabetic Subjects. Endocrinology. 2015;156:3496–503. doi: 10.1210/en.2015-1552. [DOI] [PubMed] [Google Scholar]
- 46.Kalwat MA, Cobb MH. Mechanisms of the amplifying pathway of insulin secretion in the beta cell. Pharmacol Ther. 2017;179:17–30. doi: 10.1016/j.pharmthera.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, et al. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:1019–32. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, et al. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55 Suppl 2:S16–23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 49.Murao N, Yokoi N, Honda K, et al. Essential roles of aspartate aminotransferase 1 and vesicular glutamate transporters in beta-cell glutamate signaling for incretin-induced insulin secretion. PLoS One. 2017;12:e0187213. doi: 10.1371/journal.pone.0187213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guay C, Madiraju SR, Aumais A, et al. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem. 2007;282:35657–65. doi: 10.1074/jbc.M707294200. [DOI] [PubMed] [Google Scholar]
- 51.Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53:1007–19. doi: 10.2337/diabetes.53.4.1007. [DOI] [PubMed] [Google Scholar]
- 52.El Azzouny M, Longacre MJ, Ansari IH, et al. Knockdown of ATP citrate lyase in pancreatic beta cells does not inhibit insulin secretion or glucose flux and implicates the acetoacetate pathway in insulin secretion. Mol Metab. 2016;5:980–7. doi: 10.1016/j.molmet.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDonald MJ, Smith AD, 3rd , Hasan NM, et al. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem. 2007;282:30596–606. doi: 10.1074/jbc.M702732200. [DOI] [PubMed] [Google Scholar]
- 54.Macdonald MJ, Hasan NM, Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta. 2008;1780:966–72. doi: 10.1016/j.bbagen.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan NM, Longacre MJ, Seed Ahmed M, et al. Lower succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and ATP citrate lyase in pancreatic islets of a rat model of type 2 diabetes: knockdown of SCOT inhibits insulin release in rat insulinoma cells. Arch Biochem Biophys. 2010;499:62–8. doi: 10.1016/j.abb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panten U, Willenborg M, Schumacher K, et al. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism. 2013;62:1375–86. doi: 10.1016/j.metabol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Cen J, Sargsyan E, Bergsten P. Fatty acids stimulate insulin secretion from human pancreatic islets at fasting glucose concentrations via mitochondria-dependent and -independent mechanisms. Nutr Metab (Lond) 2016;13:59. doi: 10.1186/s12986-016-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gheni G, Ogura M, Iwasaki M, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;9:661–73. doi: 10.1016/j.celrep.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Waanders LF, Holmseth S, et al. Proteome analysis and conditional deletion of the EAAT2 glutamate transporter provide evidence against a role of EAAT2 in pancreatic insulin secretion in mice. J Biol Chem. 2014;289:1329–44. doi: 10.1074/jbc.M113.529065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenstad M, Chaudhry FA. The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion. Front Endocrinol (Lausanne) 2013;4:199. doi: 10.3389/fendo.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otter S, Lammert E. Exciting Times for Pancreatic Islets: Glutamate Signaling in Endocrine Cells. Trends Endocrinol Metab. 2016;27:177–88. doi: 10.1016/j.tem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Gammelsaeter R, Coppola T, Marcaggi P, et al. A role for glutamate transporters in the regulation of insulin secretion. PLoS One. 2011;6:e22960. doi: 10.1371/journal.pone.0022960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fahien LA, Macdonald MJ. The complex mechanism of glutamate dehydrogenase in insulin secretion. Diabetes. 2011;60:2450–4. doi: 10.2337/db10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathioudakis L, Bourbouli M, Daklada E, et al. Localization of Human Glutamate Dehydrogenases Provides Insights into Their Metabolic Role and Their Involvement in Disease Processes. Neurochem Res. 2019;44:170–87. doi: 10.1007/s11064-018-2575-y. [DOI] [PubMed] [Google Scholar]
- 65.Santos LRB, Muller C, de Souza AH, et al. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic beta-cells. Mol Metab. 2017;6:535–47. doi: 10.1016/j.molmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ronchi JA, Francisco A, Passos LA, et al. The Contribution of Nicotinamide Nucleotide Transhydrogenase to Peroxide Detoxification Is Dependent on the Respiratory State and Counterbalanced by Other Sources of NADPH in Liver Mitochondria. J Biol Chem. 2016;291:20173–87. doi: 10.1074/jbc.M116.730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopert P, Patel M. Nicotinamide nucleotide transhydr-ogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem. 2014;289:15611–20. doi: 10.1074/jbc.M113.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ronchi JA, Figueira TR, Ravagnani FG, et al. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med. 2013;63:446–56. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 69.Wolf S, Hainz N, Beckmann A, et al. Brain damage resulting from postnatal hypoxic-ischemic brain injury is reduced in C57BL/6J mice as compared to C57BL/6N mice. Brain Res. 2016;1650:224–31. doi: 10.1016/j.brainres.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Liang Q, Wang B, Pang L, et al. Application of citrate as a tricarboxylic acid (TCA) cycle intermediate, prevents diabetic-induced heart damages in mice. Iran J Basic Med Sci. 2016;19:43–8. [PMC free article] [PubMed] [Google Scholar]
- 71.Nasiri A, Ziamajidi N, Abbasalipourkabir R, et al. Beneficial Effect of Aqueous Garlic Extract on Inflammation and Oxidative Stress Status in the Kidneys of Type 1 Diabetic Rats. Indian J Clin Biochem. 2017;32:329–36. doi: 10.1007/s12291-016-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ziamajidi N, Nasiri A, Abbasalipourkabir R, et al. Effects of garlic extract on TNF-alpha expression and oxidative stress status in the kidneys of rats with STZ + nicotinamide-induced diabetes. Pharm Biol. 2017;55:526–31. doi: 10.1080/13880209.2016.1255978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziamajidi N, Abbasalipourkabir R, Behrouj H, et al. Effects of Aqueous Extract of Allium sativum on Biochemical Parameters and Oxidative Stress in STZ- and STZ+niacinamide-induced Diabetes Mellitus Rats. Annu Res Rev Biol. 2017;15:1–8. [Google Scholar]
- 74.Wang JP, Hsieh CH, Liu CY, et al. Reactive oxygen species-driven mitochondrial injury induces apoptosis by teroxirone in human non-small cell lung cancer cells. Oncol Lett. 2017;14:3503–9. doi: 10.3892/ol.2017.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuppen HA, Blakely EL, Turnbull DM, et al. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2010;1797:113–28. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Marchetti P, Lupi R, Del Guerra S, et al. The beta-cell in human type 2 diabetes. Adv Exp Med Biol. 2010;654:501–14. doi: 10.1007/978-90-481-3271-3_22. [DOI] [PubMed] [Google Scholar]
- 77.Orrenius S. Mitochondrial regulation of apoptotic cell death. Toxicol Lett. 2004;149:19–23. doi: 10.1016/j.toxlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 78.Kim HR, Won SJ, Fabian C, et al. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann Lab Med. 2015;35:1–14. doi: 10.3343/alm.2015.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marchi S, Giorgi C, Suski JM, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robson-Doucette CA, Sultan S, Allister EM, et al. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60:2710–9. doi: 10.2337/db11-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hershberger KA, Martin AS, Hirschey MD. Role of NAD(+) and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol. 2017;13:213–25. doi: 10.1038/nrneph.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jokinen R, Pirnes-Karhu S, Pietilainen KH, et al. Adipose tissue NAD(+)-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health. Redox Biol. 2017;12:246–63. doi: 10.1016/j.redox.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gameiro PA, Laviolette LA, Kelleher JK, et al. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem. 2013;288:12967–77. doi: 10.1074/jbc.M112.396796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin F, Sancheti H, Cadenas E. Silencing of nicotinamide nucleotide transhydrogenase impairs cellular redox homeostasis and energy metabolism in PC12 cells. Biochim Biophys Acta. 2012;1817:401–9. doi: 10.1016/j.bbabio.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.da Veiga Moreira J, Peres S, Steyaert JM, et al. Cell cycle progression is regulated by intertwined redox oscillators. Theor Biol Med Model. 2015;12:10. doi: 10.1186/s12976-015-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubi B, del Arco A, Bartley C, et al. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J Biol Chem. 2004;279:55659–66. doi: 10.1074/jbc.M409303200. [DOI] [PubMed] [Google Scholar]
- 88.Casimir M, Rubi B, Frigerio F, et al. Silencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J. 2009;424:459–66. doi: 10.1042/BJ20090729. [DOI] [PubMed] [Google Scholar]
- 89.Huypens PR, Huang M, Joseph JW. Overcoming the spatial barriers of the stimulus secretion cascade in pancreatic beta-cells. Islets. 2012;4:1–116. doi: 10.4161/isl.18338. [DOI] [PubMed] [Google Scholar]
- 90.Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb) 2013;23:266–80. doi: 10.11613/BM.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teodoro JS, Rolo AP, Palmeira CM. The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol Mech Methods. 2013;23:297–302. doi: 10.3109/15376516.2012.759305. [DOI] [PubMed] [Google Scholar]
- 92.Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286:18056–65. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braidy N, Guillemin GJ, Mansour H, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Dolle C, Rack JG, Ziegler M. NAD and ADP-ribose metabolism in mitochondria. FEBS J. 2013;280:3530–41. doi: 10.1111/febs.12304. [DOI] [PubMed] [Google Scholar]
- 95.Mouchiroud L, Houtkooper RH, Auwerx J. NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol. 2013;48:397–408. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82:551–75. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 97.Luo X, Li R, Yan LJ. Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance in beta Cell Function and Dysfunction. J Diabetes Res. 2015;2015:512618. doi: 10.1155/2015/512618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reinhardt F, Schultz J, Waterstradt R, et al. Drp1 guarding of the mitochondrial network is important for glucose-stimulated insulin secretion in pancreatic beta cells. Biochem Biophys Res Commun. 2016;474:646–51. doi: 10.1016/j.bbrc.2016.04.142. [DOI] [PubMed] [Google Scholar]
- 99.Schultz J, Waterstradt R, Kantowski T, et al. Precise expression of Fis1 is important for glucose responsiveness of beta cells. J Endocrinol. 2016;230:81–91. doi: 10.1530/JOE-16-0111. [DOI] [PubMed] [Google Scholar]
- 100.Bach D, Naon D, Pich S, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 101.Lo MC, Chen MH, Lee WS, et al. Nepsilon-(carboxymethyl) lysine-induced mitochondrial fission and mitophagy cause decreased insulin secretion from beta-cells. Am J Physiol Endocrinol Metab. 2015;309:E829–39. doi: 10.1152/ajpendo.00151.2015. [DOI] [PubMed] [Google Scholar]
- 102.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J, Chen Z, Zhang Y, et al. Rhein protects pancreatic beta-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62:3927–35. doi: 10.2337/db13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sesaki H, Adachi Y, Kageyama Y, et al. In vivo functions of Drp1: lessons learned from yeast genetics and mouse knockouts. Biochim Biophys Acta. 2014;1842:1179–85. doi: 10.1016/j.bbadis.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waterham HR, Koster J, van Roermund CW, et al. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–41. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 106.Park KS, Wiederkehr A, Kirkpatrick C, et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283:33347–56. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–15. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]