Abstract
Due to the healthcare burden associated with migraines, prompt and effective treatment is vital to improve patient outcomes and ED workflow. This was a prospective, randomized, double-blind trial. Adults who presented to the ED with a diagnosis of migraine from August of 2019 to March of 2020 were included. Pregnant patients, or with renal impairment were excluded. Patients were randomized to receive intravenous magnesium, prochlorperazine, or metoclopramide. The primary outcome was change in pain from baseline on a numeric rating scale (NRS) evaluated at 30 min after initiation of infusion of study drug. Secondary outcomes included NRS at 60 and 120 min, ED length of stay, necessity for rescue analgesia, and adverse effects. A total of 157 patients were analyzed in this study. Sixty-one patients received magnesium, 52 received prochlorperazine, and 44 received metoclopramide. Most patients were white females, and the median age was 36 years. Hypertension and migraines were the most common comorbidities, with a third of the patients reporting an aura. There was a median decrease in NRS at 30 min of three points across all three treatment arms. The median decrease in NRS (IQR) at 60 min was −4 (2–6) in the magnesium group, −3 (2–5) in the metoclopramide group, and −4.5 (2–7) in the prochlorperazine group (p = 0.27). There were no statistically significant differences in ED length of stay, rescue analgesia, or adverse effects. Reported adverse effects were dizziness, anxiety, and akathisia. No significant difference was observed in NRS at 30 min between magnesium, metoclopramide and prochlorperazine.
Keywords: Migraine, Metoclopramide, Magnesium, Prochlorperazine, Emergency department
1. Introduction
A migraine is a chronic neurologic disease characterized by attacks of throbbing, often unilateral headache associated with photophobia, phonophobia, nausea, vomiting, and cutaneous allodynia. It is the second most disabling neurologic condition in the United States, resulting in a $27 billion cost due to loss of productivity [1,2]. It is estimated that there are over 1.2 million visits to emergency departments (ED) in the United States are due to migraines [3]. Migraine, previously believed to be a vascular disorder, is caused by inflammation due to vasodilation in the meninges secondary to the release of vasoactive neuropeptides by stimulation of the trigeminal nerve [4]. This inflammation can result in symptoms such as headache, nausea, vomiting, dizziness, photophobia and phonophobia.
Despite migraine being a common disorder, there has yet to be a cure. Several classes of medications have been studied for the treatment of migraine. Recently, conventional therapy has shifted to the use of anti-dopaminergics which include prochlorperazine, metoclopramide and haloperidol, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and naproxen, and serotonin receptor agonists, such as sumatriptan [5]. Although intravenous (IV) opioids have historically been the most common treatment for migraines, their use has fallen out of favor due to their association with increased recurrence of headaches and ED visits, abuse potential, and most recently severe IV opiate shortage [6]. Alternative treatments include ketamine, propofol, dihydroergotamine, and magnesium [5].
Magnesium is an intracellular cation that has been associated with both the function of serotonin and regulation of vascular tone, which are both mechanisms that implicate its role in the treatment of migraine [7]. Intravenous magnesium sulfate has been studied as a treatment for migraine and has been compared to placebo, metoclopramide and prochlorperazine in previous studies [[7], [8], [9], [10], [11], [12]]. These studies have demonstrated that magnesium is well-tolerated with a good safety profile and may be efficacious in the treatment of migraine. Metoclopramide, prochlorperazine, and magnesium have been recommended in clinical practice guidelines and have commonly been used for treatment of migraine in the ED. [13,14] However, no trial has evaluated all three of these drugs within the same population. The purpose of our study was to compare the relative efficacy of magnesium, metoclopramide, and prochlorperazine for the treatment of headache and migraine in the ED.
2. Methods
2.1. Study design and setting
This study was a single-center, prospective, double-blinded, randomized-controlled, three-armed trial comparing magnesium, metoclopramide, and prochlorperazine for the treatment of migraine. This trial was conducted in a large, level 1 trauma, tertiary-care medical center ED near Chicago, Illinois from August of 2019 through March of 2020.
2.2. Selection of participants
Patients greater than or equal to 18 years of age presenting to the ED with a chief complaint of migraine or headache while an ED pharmacist was present were eligible for inclusion in this study. Migraine diagnosis was determined by an ED physician after thorough examination to rule out migraine mimics or headache conditions where traditional migraine therapy would be deemed inappropriate. Patients were required to have the ability to provide informed consent. Exclusion criteria included pregnancy, a stated history of renal impairment, allergy or sensitivity to any of the study drugs, or receipt of any of the study drugs prior to enrollment.
2.3. Interventions
Following assessment for study eligibility, patients were consented by the ED pharmacist. Patients were randomized to receive one of three study drugs (magnesium sulfate 2 g, metoclopramide 10 mg, or prochlorperazine 10 mg) via computer randomization. Study drug randomization and preparation was the responsibility of the IV room pharmacist who was not part of the study. The ED pharmacists, physicians, and nurses participating in administration of the medications were blinded to which drug was selected. Magnesium sulfate 2 g/50 mL D5W, prochlorperazine 10 mg/50 mL D5W, or metoclopramide 10 mg/50 mL D5W was then administered as an IV infusion over 20 min.
2.4. Measurements and outcomes
The primary outcome of this study was change in pain from baseline to 30 min after initiation of infusion. Pain was assessed by the ED pharmacist using the 11-point Numeric Rating Scale (NRS) and recorded on a data collection tool. NRS is a validated tool commonly used to measure different types of pain [15].
Secondary endpoints included change in pain score from baseline to 60 min and 120 min after initiation of infusion (as defined on a 11-point NRS), ED length of stay, and necessity for rescue analgesia at any time following the study drug administration. Safety endpoints included monitoring for common adverse effects related to the study drugs—primarily hypotension, flushing, akathisia, dystonia, nausea, vomiting, dizziness, drowsiness, or other self-reported adverse effects [[16], [17], [18]]. Patient baseline characteristics were also collected, which included patient-reported past medical history and analgesic use prior to presenting to the ED.
2.5. Analysis
A sample size of 264 subjects (88 subjects per treatment arm) was calculated to detect a difference of 1.4 points in the NRS between groups to achieve a power of 80% [19]. Statistical significance was defined a priori as p < 0.05, and normality was assessed using the Shapiro-Wilk test. Descriptive statistics were used for nominal data, while ordinal and categorical data were evaluated using Mann-Whitney U and Pearson's χ2, respectively. Between-group comparisons were made using one-way ANOVA and Kruskal-Wallis, as appropriate for all continuous data. A post hoc non-inferiority analysis of the primary endpoint was conducted using Welch's t-test with a non-inferiority margin of 1.4 [20]. Data analysis was performed through SAS software for Windows (Version 9.4) [21].
3. Results
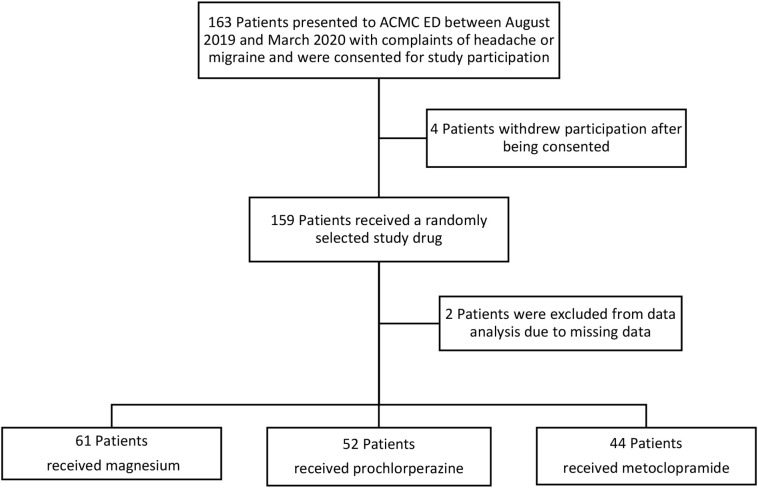
During the study period, a total of 163 patients were consented for enrollment (Fig. 1 ). Due to COVID-19, enrollment was terminated prematurely and only 157 patients were included in the final data analysis. No significant differences between baseline demographics were observed (Table 1 ). The majority of the patients were white, female, and the median age was 36 years. The most common comorbidities were hypertension and a history of migraines or headaches. Approximately one third of the patients presented with an aura, and there were no differences in baseline vital signs on presentation. (See Table 1.)
Fig. 1.
CONSORT (Consolidated standards of reporting trials) Diagram
Table 1.
Patient Baseline Demographics
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| Age, median (IQR) | 34 (27–48) | 37.5 (29.5–48) | 37.5 (27–47.5) | 0.67 |
| BMI, median (IQR) | 31.2 (25.5–35.9) | 30.1 (26.2–34.4) | 33.4 (26.5–36.2) | 0.52 |
| Female sex, No. (%) | 44 (72) | 33 (75) | 46 (88) | 0.09 |
| Race, No.(%) | ||||
| White | 30 (49) | 23 (52) | 14 (27) | 0.07 |
| Black | 25 (41) | 16 (36) | 28 (54) | 0.19 |
| Hispanic | 6 (10) | 5 (11) | 9 (17) | 0.47 |
| Comorbidities, No. (%) | ||||
| Migraine/Headache | 17 (28) | 13 (29.5) | 19 (36.5) | 0.59 |
| Hypertension | 15 (25) | 10 (23) | 15 (29) | 0.78 |
| Diabetes | 8 (13) | 7 (16) | 5 (10) | 0.65 |
| Depression/Anxiety | 7 (11.5) | 5 (11) | 3 (6) | 0.63 |
| Asthma | 4 (6.5) | 6 (14) | 7 (13) | 0.39 |
| Gastrointestinal disorders | 5 (8) | 5 (11) | 4 (8) | 0.79 |
| Aneurysm/ICH | 3 (5) | 3 (7) | 3 (6) | 0.92 |
| Arthritis/MSK pain | 4 (6.5) | 3 (7) | 3 (6) | 0.98 |
| Presence of aura, No. (%) | 18 (29.5) | 14 (32) | 20 (38.5) | 0.52 |
Abbreviations: BMI, body mass index; ICH, intercranial hemorrhage; IQR, interquartile range; MSK, musculoskeletal.
Approximately 50% of patients reported taking medications to abort their migraine prior to presenting to the ED. The most common medications taken prior to presentation to the ED were acetaminophen and NSAIDs (Table A1). Upon admission to the ED, more than two-thirds of the patients received medications prior to receiving the study medication (Table 3 ). Over 70% of patients received a dose of IV diphenhydramine prior to the study medication to prevent potential adverse effects such as akathisias. Other concomitant therapies included acetaminophen, IV NSAIDs, dexamethasone, and ondansetron (Table A2).
Table 3.
Concomitant Medication Administration
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| Patient reported medication administration prior to admission, No. (%) | 29 (47.5) | 19 (43) | 24 (46) | <0.01 |
| Received medications in ED prior to study drug, No. (%) | 49 (80) | 38 (86) | 40 (77) | 0.50 |
| Need for rescue analgesia, No. (%) | 26 (43) | 15 (34) | 17 (33) | 0.50 |
| Rescue analgesia between 30 and 120 minutes, No. (%) | 19 (31) | 11 (25) | 14 (27) | 0.85 |
Abbreviations: ED, emergency department.
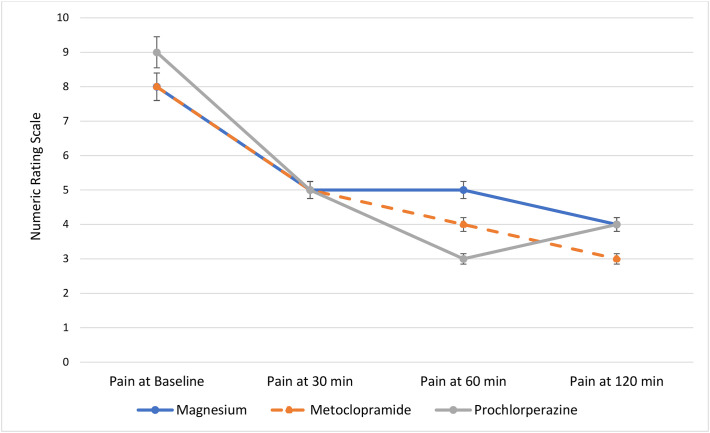
The median (IQR) baseline pain score for patients in the magnesium group was 8 (7–10), 8 (7–9.5) in the metoclopramide group, and 9 (7–10) in the prochlorperazine group. Although the baseline pain scores were slightly higher in the prochlorperazine group, this was not statistically significant (p = 0.52). Fig. 2 highlights the median pain scores for patients at baseline, 30, 60, and 120 min after the study drug infusion. Based on the results of the Mann-Whitney U analysis, the changes in pain scores from baseline to 30 min within each group were statistically significant (p < 0.01). However, the Kruskal-Wallis one-way analysis for variance demonstrated that differences in median pain scores between each treatment arm were not statistically significant (Table 2). While prochlorperazine did appear to have a greater effect on pain reduction at one hour, this was not statistically significant (p = 0.27). It is worth noting that the sample sizes of each group decreased as time progressed, presumably due to patients being discharged prior to the full duration of data collection. Interestingly, there was no difference in ED length of stay between groups (Table 4 ).
Fig. 2.
Median pain scores at baseline, 30 min, 60 min, and 120 min.
Table 2.
Change in Median Pain Scores
| Magnesium | Metoclopramide | Prochlorperazine | p Value | |
|---|---|---|---|---|
| Change in pain score at 30 min, median (IQR) | (n = 61) | (n = 44) | (n = 52) | 0.71 |
| −3 (1–4.25) | −3 (1–4) | −3 (1–5) | ||
| Change in pain score at 60 min, median (IQR) | (n = 56) | (n = 37) | (n = 39) | 0.27 |
| −4 (2–6) | −3 (2–5) | −4.5 (2–7) | ||
| Change in pain score at 120 min, median (IQR) | (n = 30) | (n = 16) | (n = 25) | 0.66 |
| −4 (2.25–7) | −4 (2–7.25) | −3 (3–7.75) |
Abbreviations: IQR, interquartile range.
Table 4.
ED Length of Stay
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| ED LOS (min), median (IQR) | 325 (274–410) | 308 (263.5–403) | 332 (268.5–391) | 0.84 |
| Time from study drug administration to discharge (min), median (IQR) | 139 (98–208) | 122.5 (93–175) | 140.5 (92.8–230.3) | 0.39 |
Abbreviations: ED, emergency department; IQR, interquartile range; LOS, length of stay.
More patients in the magnesium group required rescue analgesia, defined as the necessity for additional medications following the administration of the study drug. Approximately one third of patients received a dose of rescue analgesia between 30 and 120 min after receiving the study drug (Table 3). The most commonly administered rescue analgesics were IV NSAIDs, anti-dopaminergic agents, and acetaminophen. Other agents included opiates, ondansetron, and the combination of acetaminophen, butalbital, and aspirin (Table A3). Finally, adverse events were reported in 5% of patients in the magnesium group, 4.5% in the metoclopramide group, and 11.5% in the prochlorperazine group (p = 0.51). The most commonly reported adverse effects were dizziness, akathisias, and anxiety. The akathisias occurred in the prochlorperazine group, requiring treatment with diphenhydramine.
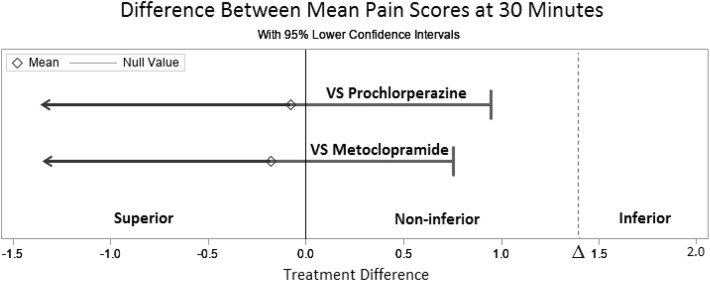
No statistically significant differences in change in pain scores were found between all three treatment arms; however, a post hoc noninferiority analysis revealed that when compared to prochlorperazine and metoclopramide, IV magnesium was non-inferior (Fig. 3 ). The difference between the mean pain score in the magnesium group compared to the prochlorperazine group was 0.08, which was well below the non-inferiority margin (p < 0.01). Similarly, the difference between the mean pain score in the magnesium group compared to the metoclopramide group was 0.18—also below the noninferiority margin (p < 0.01).
Fig. 3.
Magnesium versus prochlorperazine and metoclopramide noninferiority experiment outcomes.
4. Discussion
The results of the MAGraine study demonstrated that IV magnesium sulfate, metoclopramide, and prochlorperazine were effective in decreasing pain scores for migraines at 30, 60, and 120 min, however one agent was not superior to the rest. Although prochlorperazine trended towards a greater decrease in pain scores at 60 min, this potential benefit may be offset by the increased adverse effects, mainly akathisias. Our results suggest that magnesium may have had a faster onset, which is consistent with the results of Shahrami and colleagues [12]. However, Shahrami compared magnesium sulfate to concomitant administration of metoclopramide and dexamethasone, and therefore the dexamethasone may have contributed to the delayed effects of metoclopramide. Corbo and colleagues reported that combination therapy of magnesium sulfate with metoclopramide resulted in decreased efficacy of metoclopramide, however the results of our non-inferiority analysis demonstrated that magnesium sulfate was non-inferior to metoclopramide [8].
Limitations of the study include the unexpected premature termination of recruitment, which ultimately lead to unequal treatment arms and the study being underpowered. This makes it difficult to draw conclusions with regards to whether one agent fared better for migraine abortion. Furthermore, there was no uniform protocol for time to initiation of medications in the ED prior to study drug administration or for rescue therapy. This ultimately could have confounded the results of this study since it is unknown if pain relief was related to the administration of the study drug versus adjunctive therapies. Additionally, the choice of adjunctive therapies was at the physician's discretion. Approximately one third of patients received additional therapies prior to 120 min, which may have also confounded migraine relief. Finally, although there was no difference in ED length of stay between groups, the length of stay may have varied due to the timing of presentation to the ED, and prioritization for high acuity patients.
5. Conclusion
There was no statistically significant difference in change in median pain scores between IV magnesium, metoclopramide, and prochloperazine. However, IV magnesium was not inferior to prochlorperazine or metoclopramide at 30 min when treating headaches and migraines in the ED—despite patients requiring greater rescue analgesia. Although prochlorperazine may be more effective at controlling pain at one hour, it may also result in greater adverse effects. IV magnesium may be used as an adjunctive agent for the treatment of migraines, or may serve as a safe alternative when agents such as prochloperazine or metoclopramide are not appropriate.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgements
We thank Hannah Henderson, PharmD Candidate and Elishka Braun, PharmD Candidate for their assistance with data collection. We also thank Kuntal Patel, PharmD, BCPS for suggesting the name of this study.
A. Appendix
Table A1.
Medications administered prior to admission.a
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| Acetaminophen | 12 (41) | 9 (47) | 10 (42) | 0.99 |
| NSAIDs | 15 (52) | 13 (68) | 9 (37.5) | 0.36 |
| APAP/ASA/caffeine | 10 (34.5) | 2 (10.5) | 3 (12.5) | 0.07 |
| Opiates | 19 (31) | 11 (25) | 14 (27) | 0.85 |
| Triptans | 0 (0) | 1 (5) | 2 (8) | 0.52 |
| APAP/butalbital/caffeine | 0 (0) | 1 (5) | 2 (8) | 0.32 |
Abbreviations: APAP, acetaminophen; ASA, aspirin.
Patient-reported.
Table A2.
Concomitant medications administered in the ED.
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| Acetaminophen | 7 (14) | 7 (18) | 8 (20) | 0.76 |
| NSAIDs | 9 (18) | 11 (29) | 6 (15) | 0.19 |
| Diphenhydramine | 41 (84) | 30 (79) | 29 (72.5) | 0.35 |
| Opiates | 1 (2) | 2 (5) | 1 (2.5) | 0.61 |
| Dexamethasone | 9 (18) | 11 (29) | 6 (15) | 0.81 |
| Ondansetron | 8 (13) | 6 (16) | 8 (20) | 0.94 |
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs.
Table A3.
Rescue analgesia.
| Magnesium (n = 61) | Metoclopramide (n = 44) | Prochlorperazine (n = 52) | p Value | |
|---|---|---|---|---|
| Acetaminophen | 4 (15) | 6 (40) | 4 (24) | 0.42 |
| NSAIDs | 11 (42) | 6 (40) | 5 (29) | 0.44 |
| Metoclopramide | 4 (15) | 0 (0) | 2 (12) | 0.22 |
| Opiates | 6 (23) | 5 (33) | 5 (29) | 0.44 |
| Prochlorperazine | 5 (19) | 0 (0) | 2 (12) | 0.22 |
| APAP/ butalbital/ ASA | 2 (8) | 0 (0) | 0 (0) | 0.20 |
| Ondansetron | 4 (7) | 2 (5) | 0 (0) | 0.19 |
| Antidopaminergicsa | 12 (20) | 6 (14) | 8 (15) | 0.69 |
Abbreviations: APAP, acetaminophen; ASA, aspirin; NSAIDs, nonsteroidal anti-inflammatory drugs.
Diphenhydramine, droperidol, haloperidol.
References
- 1.QuickStats Percentage of adults aged ≥18 years who reported having a severe headache or migraine in the past 3 months, by sex and age group — National Health Interview Survey, United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:654. doi: 10.15585/mmwr.mm6624a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman B.W., West J., Vinson D.R. Current management of migraine in US emergency departments: An analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2015;35:301–309. doi: 10.1177/0333102414539055. [DOI] [PubMed] [Google Scholar]
- 4.Charles A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018 Feb;17(2):174–182. doi: 10.1016/S1474-4422(17)30435-0. Epub 2017 Dec 8. [DOI] [PubMed] [Google Scholar]
- 5.Friedman B.W. Managing migraine. Ann Emerg Med. 2017 Feb;69(2):202–207. doi: 10.1016/j.annemergmed.2016.06.023. Epub 2016 Aug 7. [DOI] [PubMed] [Google Scholar]
- 6.Friedman B.W., Vinson D.R. Convincing the skeptic. How to fix emergency department headache management. Cephalalgia. 2015;35:641–643. doi: 10.1177/0333102414557704. [DOI] [PubMed] [Google Scholar]
- 7.Corbo J., Esses D., Bijur P.E. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001 Dec;38(6):621–627. doi: 10.1067/mem.2001.119424. [DOI] [PubMed] [Google Scholar]
- 8.Ginder S., Oatman B., Pollack M. A prospective study of i.v. magnesium and i.v. prochlorperazine in the treatment of headaches. J Emerg Med. 2000 Apr;18(3):311–315. doi: 10.1016/s0736-4679(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 9.Demirkaya S., Vural O., Dora B. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache. 2001 Feb;41(2):171–177. doi: 10.1046/j.1526-4610.2001.111006171.x. [DOI] [PubMed] [Google Scholar]
- 10.Bigal M.E., Bordini C.A., Tepper S.J., Speciali J.G. Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia. 2002 Jun;22(5):345–353. doi: 10.1046/j.1468-2982.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 11.Cete Y., Dora B., Ertan C. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs metoclopramide in the management of acute migraine attacks in the emergency department. Cephalalgia. 2005;25:199–204. doi: 10.1111/j.1468-2982.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 12.Shahrami A., Assarzadegan F., Hatamabadi H.R. Comparison of therapeutic effects of magnesium sulfate vs. dexamethasone/metoclopramide on alleviating acute migraine headache. J Emerg Med. 2015;48:69–76. doi: 10.1016/j.jemermed.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Marmura M.J., Silberstein S.D., Schwedt T.J. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015 Jan;55(1):3–20. doi: 10.1111/head.12499. [DOI] [PubMed] [Google Scholar]
- 14.Loder E., Burch R., Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: A summary and comparison with other recent clinical practice guidelines. Headache. 2012 Jun;52(6):930–945. doi: 10.1111/j.1526-4610.2012.02185.x. [DOI] [PubMed] [Google Scholar]
- 15.Haefeli M., Elfering A. Pain assessment. Eur Spine J. 2006;15(Suppl. 1):S17–S24. doi: 10.1007/s00586-005-1044-x.REDCAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnesium sulfate. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com.
- 17.Metoclopramide. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com.
- 18.Prochlorperazine. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com.
- 19.Flight L., Julious S.A. Practical guide to sample size calculations: Non-inferiority and equivalence trials. Pharmaceut Statist. 2016;15:80–89. doi: 10.1002/pst.1716. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2020. SAS system for windows, version 9.4. [Google Scholar]
- 21.D’Agostino R.B., Massaro J.M., Sullivan L.M. Non-inferiority trials: Design concepts and issues—the encounters of academic consultants in statistic. Stat Med. 2003;22(2):169–186. doi: 10.1002/sim.1425. [DOI] [PubMed] [Google Scholar]