Abstract
Cannabinoids are a group of organic compounds found in cannabis. Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the two major constituents of cannabinoids, and their metabolites are contaminants of emerging concern due to the limited information on their environmental impacts. As well, their releases to the water systems and environment are expected to increase due to recent legalization. Solid-phase extraction is the most common technique for the extraction and pre-concentration of cannabinoids in water samples as well as a clean-up step after the extraction of cannabinoids from solid samples. Liquid chromatography coupled with mass spectrometry is the most common technique used for the analysis of cannabinoids. THC and its metabolites have been detected in wastewater, surface water, and drinking water. In particular, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) has been detected at concentrations up to 2590 and 169 ng L−1 in untreated and treated wastewater, respectively, 79.9 ng L−1 in surface water, and 1 ng L−1 in drinking water. High removal of cannabinoids has been observed in wastewater treatment plants; this is likely a result of adsorption due to the low aqueous solubility of cannabinoids. Based on the estrogenicity and cytotoxicity studies and modelling, it has been predicted that THC and THC-COOH pose moderate risk for adverse impact on the environment. While chlorination and photo-oxidation have been shown to be effective in the removal of THC-COOH, they also produce by-products that are potentially more toxic than regulated disinfection by-products. The potential of indirect exposure to cannabinoids and their metabolites through recreational water is of great interest. As cannabinoids and especially their by-products may have adverse impacts on the environment and public health, more studies on their occurrence in various types of water and environmental systems, as well as on their environmental toxicity, would be required to accurately assess their impact on the environment and public health.
Keywords: Cannabis, Cannabinoids, Δ9-tetrahydrocannabinol, Cannabidiol, Pharmaceuticals and personal care products
Graphical abstract
Summary of the main findings
This critical review presents the detection, occurrence, fate, toxicity, and removal of cannabinoids in the water system and the environment. Cannabinoids in wastewater are expected to increase due to legalization.
1. Introduction
Cannabis is a genus belonging to the Cannabaceae family. Cannabis includes three species, Cannabis sativa, Cannabis indica, and Cannabis ruderalis. However to date, there are still disagreements regarding whether or not cannabis is a single species (monotypic) or multiple species (polytypic) (Hartsel et al., 2016). In addition, Cannabis sativa and Cannabis indica are frequently crossbred to produce hybrid phenotypes with desired characteristics (Hartsel et al., 2016). As the focus of this review is not the taxonomy of cannabis, the monotypic name will be used for the review for the simplicity of the text.
Cannabis, which is also known as marijuana, is the most frequently used recreational/illicit drug in Asia (Dargan and Wood, 2012), Australia (AIHW, 2016), Canada (Health Canada and Canada, 2012, Health Canada and Canada, 2017), the European Union (EMCDDA, 2018), the United States (CBHSQ, 2018; Cerdá et al., 2012), or in general, the most commonly abused drug globally (UNODC, 2019). In 2017 it was estimated that the consumption of cannabis was 187 tons in the single state of Colorado (Adam et al., 2018). From March 2018 to February 2019, approximately 370 tons of cannabis were consumed nationwide in Canada (Werschler and Brennan, 2019). The cultivation and use of cannabis are prohibited in most countries, as it is classified as an illicit drug (Sharma et al., 2012). However, the shift in perspective on cannabis has resulted in the legalization of cannabis (e.g., Canada and some U.S. states) for medical use and recreational purposes. While the cultivation of Cannabis indica is illegal in many countries, the cultivation of hemp (Cannabis sativa L.), which consists of less than 0.2 or 0.3% Δ9-tetrahydrocannabinol (THC) by dried weight (Schluttenhofer and Yuan, 2017) for non-drug uses, is legal in more than 30 countries such as France and Australia (Schluttenhofer and Yuan, 2017), with China being the largest cultivator of hemp (Amaducci et al., 2015; The Economist, 2019).
It is predicted that the market share for cannabinoid-based drugs will expand up to 700% by 2020 (Saleh et al., 2019). The global cannabis market is also predicted to increase to US$ 40.6 billion by 2024 (George-Cosh, 2019). After the legalization of cannabis for medical and recreational uses in October 2018, Canada saw an increase in the consumption of cannabis in the population above the age of 15 from 14% to 18% (STATCAN, 2019). After legalization in October 2019, cannabis edibles, extracts, and topical products are estimated to have a Canadian market of CAD$ 2.7 billion (The Canadian Press, 2019). As a result of the increase in popularity of cannabidiol and hemp derived products in food and cosmetics (Hannaford, 2019; Wallace, 2019), an increase in cannabis, or more specifically cannabinoids, is expected to be released into the water systems and the environment.
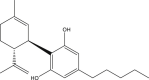
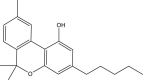
More than 421 chemicals can be extracted from cannabis (Huestis, 2007; Sharma et al., 2012). Of these, at least 113 are cannabinoids (Aizpurua-Olaizola et al., 2016). Cannabinoids are a group of diverse organic compounds that directly affect the cannabinoid receptors in the brain. Two well-known cannabinoids are THC and cannabidiol (CBD). The primary psychoactive compound, THC, is a volatile lipophilic viscous oil that contributes to the undesirable effects on the behavior caused by consumption of cannabis. CBD is credited for the medicinal properties of cannabis.
Cannabinoids can enter the body by three main routes that include inhalation, ingestion, and through the skin. After inhalation, THC is distributed to liver, spleen, adipose tissue, and lungs (Musshoff and Madea, 2006). When inhaled, THC quickly enters the bloodstream via the lungs and is then metabolized to 11-hydroxy-Δ9-tetrahydrocannabinol (THC-OH) by the liver. THC-OH is then converted into the main metabolite 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH), which is then excreted in human urine and feces, as a glucuronic acid conjugate (Castiglioni et al., 2008; Khan and Nicell, 2012). When dermally absorbed, THC enters the bloodstream through the skin. In this instance, the rate of diffusion of THC through the aqueous layer of the skin is the rate determining step for dermal THC absorption (Huestis, 2007). If ingested, the THC enters the bloodstream through the liver, resulting in delayed psychoactive effect (Huestis et al., 2006).
After excretion and during wastewater treatment, beta-glucuronidases of fecal bacteria could readily hydrolyze THC-COOH back to its free acid form (Huestis, 2005). While THC-COOH is not a psychoactive compound, it can remain in the body from several days to weeks, depending on the doses of the users (Brenneisen et al., 2010). Therefore, THC-COOH has been used in many studies as biological marker for cannabis consumption and in several sewage epidemiology studies as a surveillance tool (Balducci et al., 2016; Daughton, 2001; Khan and Nicell, 2012; Postigo et al., 2011).
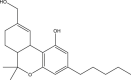
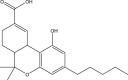
Cannabidiol (Table 1 ), the main non-psychoactive cannabinoid constituent, is another dominant cannabinoid in cannabis. Like THC, it is biogenerated from cannabigerolic acid, thus it has a similar molecular structure to THC (Table 1). With respect to the medicinal use of cannabis, CBD and synthetic cannabidiol are both used for pain relief (Hammell et al., 2016; Russo, 2008). In 2018, a cannabinoid-based pharmaceutical under the tradename of Epidiolex was approved by the US Food and Drug Administration for the treatment of seizures related to two severe types of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome (USFDA, 2018). In 2019, the sales of CBD products were legalized in Canada with restriction under the Cannabis Act (Health Canada, 2019).
Table 1.
Structures and molecular information of major cannabinoids and metabolites.
| Name |
Δ9-tetrahydrocannabinol |
11-hydroxy-Δ9-tetrahydrocannabinol |
11-nor-9-carboxy- Δ9-tetrahydrocannabinol |
Cannabidiol |
Cannabinol |
|---|---|---|---|---|---|
| Abbreviation | THC | THC-OH | THC-COOH | CBD | CBN |
| Molecular formula | C21H30O2 | C21H30O3 | C21H28O4 | C21H30O2 | C21H26O2 |
| Molar mass (g mol−1) | 314 | 330 | 344 | 314 | 310 |
| Pka | 9.81a | 9.78 ± 0.60b | 4.87/9.30a | 6.43a | |
| 9.81 ± 0.60b | 4.66 ± 0.40b | ||||
| Log Kow | 7.68a | 5.36 ± 0.42b | 6.21 | 7.03a | |
| 6.84 ± 0.35b | 5.25 ± 0.38b | ||||
| Aqueous solubility (mg L−1) | 0.040a | 7.931b | 0.230a | 0.006a | |
| 0.786b | 309b | ||||
| Structure | 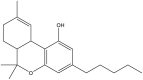 |
 |
 |
 |
 |
Cannabinol (CBN), shown in Table 1, is a mild psychoactive compound with only 10% of THC potency (Izzo et al., 2009). CBN is another common type of cannabinoid that is a major constituent in aged or cured cannabis but only a minor constituent in fresh cannabis. CBN is formed from the degradation of THC in the presence of oxygen or heat (Hartsel et al., 2016). This cannabinoid is used as a chemical indicator of improper cannabis storage and aging.
Due to the increase in the market demand for cannabis related products, there is an increase in cannabis cultivation (Chen, 2017), cannabinoids extraction (Subramaniam, 2019), and cannabis related products production. This would therefore increase the chance of cannabinoids releases into the environment through agricultural and industrial wastes. While the approval of cannabis as recreational drug might not significantly increase the cannabinoids release to the environment, the rapid increase in the demand for cannabinoid-based pharmaceuticals such as Epidiolex and the change in the perspective of the cannabis for medical uses would likely cause an increase in cannabinoids release into the environment.
With the recent change in legalization for the use and consumption of cannabis, it is timely to review the analytical methods and its occurrence in water systems. The toxicity and treatment of cannabinoids were also reviewed to understand the environmental impacts of cannabinoids and to address the lack of knowledge in the environmental field. Therefore, the objectives of this paper are to: 1) Review and summarize the analytical methods, including the sampling, sample storage, and pre-treatments available for the detection and analyses of cannabinoids; 2) Investigate the fate of cannabinoids in water systems through the review of the occurrence of cannabinoids in wastewater and the treatment methods for the removal of cannabinoids in wastewater treatment plants (WWTPs); 3) Review the occurrence of cannabinoids in the environment in both water and sediment; 4) Review the environmental toxicity of cannabinoids and their by-products or metabolites; 5) Assess the potential impacts of cannabinoids and their by-products on the environment; and 6) Discuss the impacts of cannabinoids and their by-products on public health and present future directions for relevant research.
2. Analytical methods for the determination of cannabinoids and their metabolites in wastewater and in the environment
The analysis of cannabinoids in a water system consists of three general steps: sample collection, sample pre-treatment, and identification and quantification of cannabinoids and their metabolites. A summary of the sampling, storage, extraction, and detection methods for cannabinoids is presented in Table 2 .
Table 2.
Sampling, storage, extraction, and detection methods for cannabinoids in various water and solid samples.
| Typea | Sample collection | Storage | Extraction | Detection | Reference |
|---|---|---|---|---|---|
| Water samples | |||||
| WW | 24 h composite | Amber glass bottles at 4 °C | Strong cation exchanger (SCX) SPE | LC-MS, ESI (−) | Castiglioni et al. (2006) |
| WW SW | 24 h composite | Amber glass bottles at 4 °C | Hydrophilic-lipophilic balance (HLB) SPE | LC-MS, ESI (+) | Boleda et al. (2007) |
| WW | 24 h composite | Amber glass bottles at −20 °C | Online HLB SPE | LC-MS, ESI (−) | Postigo et al. (2008) |
| WW SW | 24 h composite | Polyethylene high density (HDPE) bottles at −20 °C | SCX SPE | LC-MS, ESI (−) | Bijlsma et al. (2009) |
| WW SW | Grab samples | Amber glass bottles | HLB SPE | GC-MS, EI | González-Mariño et al. (2010) |
| WW | 24 h composite | Amber glass bottles at 4 °C, pH 2 | Large volume direct injection | LC-MS, ESI (+) | Berset et al. (2010) |
| WW | 24 h composite | – | HLB SPE | LC-MS, ESI (+) | Terzic et al. (2010) |
| SW | Grab samples | Amber glass bottles at −20 °C | HLB SPE | LC-MS, ESI (+) | Health Canada and Canada, 2012, Health Canada and Canada, 2017 |
| DW | – | HDPE bottles at 4 °C | HLB SPE | LC-MS, ESI (+) | Boleda et al. (2011b) |
| WW | 12 h composite | Stored at 4 °C | HLB SPE | LC-MS, ESI (+) | Gerrity et al. (2011) |
| WW SW | 24 h composite | Amber glass bottles at 4 °C, pH 3 | SCX SPE | LC-MS, ESI (−) | Pedrouzo et al. (2011) |
| WW | Grab samples 24 h composite | – | Mixed mode strong cation exchanger (MCX) SPE | LC-MS, ESI (±) | González-Mariño et al. (2012) |
| WW | Grab samples | PET bottles at 4 °C | Online HLB SPE | LC-MS, ESI (−) | Jurado et al. (2012) |
| WW SW | Grab samples | Amber glass bottles at 4 °C | Solid phase microextraction | GC-MS, EI | Racamonde et al. (2012) |
| WW | 24 h composite | Amber glass bottles at 4 °C | HLB SPE | LC-MS, ESI (+) | Health Canada and Canada, 2012, Health Canada and Canada, 2017 |
| WW | 24 h composite | HDPE bottles at 4 °C | SPE | LC-MS, ESI (+) | Nefau et al. (2013) |
| WW | 24 h composite | – | MCX SPE | LC-MS, ESI (−) | Senta et al. (2013) |
| SW | Grab samples | HDPE bottles at 4 °C | Reverse phase (RP) SPE | LC-MS, ESI (−) | Carmona et al. (2014) |
| WW | 24 h composite | HDPE bottles at −20 °C | SPE | LC-MS, ESI (−) | Devault et al. (2014) |
| WW | Polar organic chemical integrative samplers (POCIS) – exposed for 21 days | – | Online SPE | LC-MS, ESI (+) | Fedorova et al. (2014) |
| SW DW | 24 h composite Grab samples | PET bottles at −20 °C | Online HLB SPE | LC-MS, ESI (−) | Mendoza et al., (2014), 2016 |
| WW | 24 h composite | PET bottles at −20 °C | Online HLB SPE | LC-MS, ESI (+) | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| WW | 24 h composite | HDPE bottles at −20 °C, pH 3 | HLB SPE | LC-MS, ESI (−) | Östman et al., 2014 |
| WW | 24 h composite | HDPE bottles at −20 °C | HLB SPE | LC-MS, ESI (−) | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| WW | 24 h composite | PET bottles at −20 °C | SCX SPE | LC-MS, ESI (+) | Andrés-Costa et al. (2014) |
| WW | – | PET bottles at −20 °C | Large volume direct injection | LC-MS, ESI (+) | Heuett et al. (2015) |
| WW | 24 h composite | Amber glass bottles at 4 °C, pH 2 | SCX SPE | LC-MS, ESI (+) | Palardy et al. (2015) |
| WW SW | POCIS – exposed for 30 days | – | – | LC-MS, ESI (±) | Zenobio et al. (2015) |
| WW SW | Grab samples | Glass bottles at 4 °C | HLB SPE | LC-MS, ESI (±) | Zenobio et al. (2015) |
| WW | 24 h composite | Stored in −20 °C | SPE | LC-MS, ESI (±) | Gatidou et al. (2016) |
| LL | Grab samples | Amber glass bottles at 4 °C | HLB SPE | LC-MS, ESI (±) | Lu et al. (2016) |
| GW | Grab samples | PET bottles at −20 °C | Online HLB SPE | LC-MS, ESI (−) | Mastroianni et al. (2016) |
| WW SW | Grab samples | Amber glass bottles at 4 °C | Online RP SPE | LC-MS, ESI (+) | Yao et al., 2016 |
| WW SW | 24 h composite Grab samples | PET bottles at −20 °C | SCX SPE | LC-MS, ESI (+) | Andrés-Costa et al. (2016) |
| WW | Grab samples | HDPE bottles at −20 °C | SCX SPE | LC-MS, ESI (+) | Jacox et al. (2017) |
| WW | 24 h composite | Amber glass bottles at 4 °C | HLB SPE | GC-MS, EI | Chiavola et al. (2019) |
| WW | 24 h composite | Plastic bottles at −20 °C | Online RP SPE | LC-MS, ESI (+) | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| Solid | |||||
| Sludge | Grab samples | Amber glass bottles at 4 °C | Pressurize liquid extraction (PLE) followed by HLB SPE | LC-MS, ESI (+) | Mastroianni et al. (2013) |
| Sludge | Grab samples | Stored at −20 °C | PLE followed by MCX SPE | LC-MS, ESI (−) | Senta et al. (2013) |
| Sediment | Grab samples | Wrap in aluminum at −20 °C | QuEChERS | LC-MS, ESI (−) | Carmona et al. (2014) |
| Sludge | Grab samples | Stored at −20 °C | Ultrasound assisted liquid extraction | LC-MS, ESI (±) | Ivanová et al. (2018) |
| Sludge | Grab samples | Amber glass bottles at 4 °C | Mixed mode polar SPE | LC-MS, ESI (±) | Black et al. (2019) |
DW: Drinking water, WW: Wastewater, SW: Surface water, GW: Groundwater, LL: Landfill leachates.
2.1. Samples collection
Both composite samples (Bijlsma et al., 2009; Boleda et al., 2007; Castiglioni et al., 2006; Postigo et al., 2008) and grab samples (González-Mariño et al., 2010; Racamonde et al., 2012; Vazquez-Roig et al., 2010) have been used for the sampling of wastewater influent (INF) and wastewater effluent (EFF) from WWTPs. 24 h composite samples would generally be required if the samples were collected for sewage epidemiology applications. Surface water (SW) samples might also be collected to verify the impact of the EFF on the surface water. Collected water samples may be kept in either high-density polyethylene (HDPE) or amber glass bottles at −20 or 4 °C until further use. In previous studies, sludge and sediment samples were collected as grab samples and were stored at 4 °C until further processing (Black et al., 2019; Chiavola et al., 2019; Mastroianni et al., 2016).
Polar organic chemical integrative sampler (POCIS) is a passive sampling device that has been used for the sampling, the pre-concentration, and for the quantification of trace organic contaminants, such as THC-COOH. The POCIS was deployed in INF, EFF or SW for a duration of 1–4 weeks in order to cumulate sufficent concentration for analysis (Fedorova et al., 2014; Zenobio et al., 2015). POCIS can detect organic contaminants at low concentrations because of its long sampling duration and the adsorbent affinity for polar organic molecules (0 < log Kow < 4) (Alvarez et al., 2004). The commonly used sorbent materials are hydrophilic-lipophilic balance, hyper crosslinked hydroxylated polystyrene-divinylbenzene copolymer SPE resins, and the activated carbon (Alvarez et al., 2004). Silica membrane has also been suggested as an alternative sorbent for POCIS (Alvarez et al., 2004). POCIS can also spot irregular contaminant flows because of the long sampling duration, which allows the generation of time-integrated data (Arditsoglou and Voutsa, 2008). As it is challenging to convert the results from POCIS (ng POCIS−1) to concentration values (i.e., ng L−1), it was suggested that POCIS was more suitable for qualitative analysis than quantitative analysis (Fedorova et al., 2014). Currently, POCIS is generally used for risk evaluation in environmental monitoring studies (Alvarez, 2013; Martínez Bueno et al., 2016).
2.2. Samples storage
Proper storage of samples is an important aspect after sample collection to ensure the accuracy of the measured concentration. The use of proper storage conditions for cannabinoid samples is very important due to the fact that THC and its metabolites can adsorb onto the walls of glass and plastic containers (Blanc et al., 1993). As such, the stability of THC and its metabolites in wastewater has been evaluated by various research groups (McCall et al., 2016; Roth et al., 1996; Senta et al., 2014). Generally, the key parameters that influence the stability of compounds in wastewater are pH, storage temperature, and time (Baker and Kasprzyk-Hordern, 2011).
Roth et al. (1996) investigated the impacts of container material and matrix on THC-COOH losses using fluorescence polarization immunoassay and X-ray photoelectron spectroscopy. It was found that loss of THC-COOH occurred because of storage (equilibrium conditions) and/or from sample handling and pipetting (kinetic conditions). Deionized water and urine spiked with THC-COOH stored in untreated amber glass had the lowest loss of THC-COOH at temperatures between 2 and 8 °C after 16 h. The results showed that THC-COOH has a high affinity for HDPE and a lower affinity for untreated glass material. The study also found that deionized water generally resulted in higher losses compared to urine, but both had higher losses when compared to Abbott cannabinoids diluent. After the initial drop in concentration, the concentration of THC-COOH was stable for 5 h to 8 days. The loss in concentration was also found to increase with increasing temperature, where the rate of THC-COOH loss doubled when the temperature increased from 5.5 °C (k = 2.2 ± 0.4 h−1) to 22.5 °C (k = 4.1 ± 0.9 h−1). In one aliquot, approximately 8%–57% of THC-COOH could be lost due to pipetting. For the kinetic conditions, it was found that the loss of THC-COOH was highly dependent upon the solvent and pipette material used, where glass pipette resulted in the lowest loss of THC-COOH. As well, it is worth noting that the THC-COOH losses due to sample handling and pipetting were less significant that those due to equilibrium conditions because of the shorter contact time.
In a review by McCall et al. (2016), the stability of THC, its metabolites and other illicit drugs in wastewater was assessed based on the percentage of degradation of the compounds, where high stability referred to the degradation of less than 20% over 24 h. The review concluded that THC-COOH stored in amber glass bottles was the most stable, with stability up to 72 h. Heuett et al. (2015) found that filtered THC-COOH samples (1 μm GF/C glass fiber filter followed by 0.5 μm G15 glass fiber filter) stored in clear polyethylene terephthalate (PET) at −20 °C were stable for up to 3 months, with only a loss of 9% in concentration after 3 months. In contrast, THC was found to be unstable under the same storage conditions with 94% of THC loss occurring after 3 months and almost 50% of THC loss occurring within 27 days. González-Mariño et al. (2010) found that after being loaded onto HLB SPE cartridges at pH 8.5, THC-COOH was stable for up to 3 months in the dried cartridges stored at −20 °C.
Acidification of samples has been used to prevent microbial activity and growth, which sometimes also helps to preserve the sample (S˙liwka-Kaszyńska et al., 2003). However, this was not the case for THC-COOH. It was found that the loss of THC-COOH (54%) was higher at pH 2 than the loss (10%) at pH 7.4 (Senta et al., 2013, 2014). While the authors did not suggest a possible reason for the higher loss in acidic conditions, a possible explanation can be derived based on the estimated pKa of THC-COOH. The estimated pKa of THC-COOH is 4.66 ± 0.40 (Park et al., 2016) or 4.87 and 9.30 (Apul et al., 2020). Therefore, at pH 2, THC-COOH is likely to be at its neutral state and thus it is less soluble than at pH 7.4 where it would be negatively charged. This insolubility of THC-COOH at pH of 2 could result in higher loss at acidic condition (pH 2) as compared to neutral condition (pH 7.4). It was also suggested by Causanilles et al. (2017) and Khan and Nicell (2012) that the higher loss at acidic pH was due to the enhanced adsorption of THC-COOH to suspended solids. No information is available on the stability of other cannabinoids, such as CBD, in wastewater samples and thus more studies would be required to understand their stability in wastewater and water samples in general. In summary, the minimum condition for the storage of samples for cannabinoids analysis is using amber glass bottle at neutral pH, with storage temperature of ≤4 °C.
2.3. Sample pre-treatment methods
The first pre-treatment procedure for water samples is to remove the suspended solids from the samples by either centrifugation and/or filtration using different materials (e.g., glass fiber filter, nylon membrane filter, nitrocellulose filter, etc.) and retention sizes (1.6–0.2 μm) before proceeding to other procedures for the analysis of cannabinoids.
Due to the low concentrations (ng L−1 level) of cannabinoids in water systems, a pre-concentration step is generally required before analysis. Solid-phase extraction (SPE) is the most common technique used for the pre-concentration of cannabinoids from water samples because of its efficiency and availability in most analytical or environmental laboratories (Park et al., 2016). Off-line SPE required sample volumes between 50 and 500 mL (Andrés-Costa et al., 2014; Hernández et al., 2011; Jacox et al., 2017; Nefau et al., 2013; Pedrouzo et al., 2011; van Nuijs et al., 2014). Hydrophilic-lipophilic balance (HLB), mixed mode strong cation exchanger (MCX) and styrene-divinylbenzene polymer (reverse phase) have been used as the sorbents for the pre-concentration of cannabinoids from water samples, with HLB being the most commonly used sorbent (Table 2). Depending on the adopted procedures, samples were either loaded at their natural pH or acidified before loading the samples. Various organic solvents, including acetonitrile, ethyl acetate, and methanol have been used as the eluent to elute the analytes, with methanol being the most common eluent. While ammonium hydroxide is generally added into the eluent for samples that are loaded in acidic condition to enhance the analytes recovery of basic compounds, THC and its metabolites can be eluted from MCX SPE using only pure methanol (González-Mariño et al., 2012; Senta et al., 2013). The disadvantage of offline SPE is that it is time consuming because it requires multiple sample preparation steps, resulting in a decrease in the overall precision and accuracy of the analytical method (Park et al., 2016). Although with the automation of the SPE process, such error could be reduced. An additional disadvantage is the large initial volume (>50 mL) of sample required for offline SPE.
Online SPE is an automated SPE preceding liquid chromatography, that could overcome the disadvantages of multiple sample preparation steps and the large volume of samples required for the offline SPE (Heuett et al., 2015; Postigo et al., 2008, 2010). Only 5 mL of sample were required for the online SPE, and the total time required for pre-concentrated of samples and analyzed by LC-MS/MS was only 35 min. But, low recoveries of 9%–37% for THC and its metabolites in wastewaters have been reported, which might be due to the high matrix effect of online SPE (Postigo et al., 2008). Similar trend of low recovery was also observed by Heuett et al. (2015), where the recovery of THC-COOH was only 41% using online SPE.
Berset et al. (2010) used direct injection technique to eliminate the time-consuming pre-concentration step, where the wastewater samples were directly analyzed after filtration. However, higher matrix effects and limits of quantification were observed by direct injection technique as compared with offline SPE or online SPE (Table 3 ). Solid-phase microextraction (SPME) was also used for the pre-concentration of cannabinoids for the analysis using gas chromatography coupled with mass spectrometry (GC-MS) (Jain and Singh, 2016; Racamonde et al., 2012). It was found that the divinlybenzene–carboxen–poly(dimethylsiloxane) (DVB–CAR–PDMS) fiber provided the highest recovery of more than 90% for both THC and THC-COOH (Table 3) (Racamonde et al., 2012). The advantage of SPME is that the use of organic solvent is not required, the fiber is reusable, and generally it requires smaller sample volumes (20 mL) than offline SPE. In addition, SPME can be fully automated online with a GC-MS.
Table 3.
Recovery and limit of detection (LOD) of cannabinoid and its metabolites in different matrices.
| Target analyte | Matrixa | Recovery (%)b | LOD (ng L−1) | References |
|---|---|---|---|---|
| THC-COOH | INF | 51 ± 1 | 0.5 | Castiglioni et al. (2006) |
| EFF | 61 ± 4 | 0.3 | ||
| THC | SW | 44 | 2.1 | Boleda et al. (2007) |
| INF | 42 | 2.5 | ||
| THC-COOH | SW | 86 | 3.0 | |
| INF | 96 | 3.8 | ||
| THC | INF | 9 | 3.4 | Postigo et al. (2008) |
| THC-OH | INF | 37 | 1.5 | |
| THC-COOH | INF | 13 | 1.1 | |
| THC-COOH | SW | 68 | 30 | Bijlsma et al. (2009) |
| INF | – | 2500 | ||
| EFF | – | 500 | ||
| THC | SW | – | 0.9 | González-Mariño et al. (2010) |
| INF | – | 3 | ||
| EFF | – | 3 | ||
| THC-COOH | SW | – | 1 | |
| INF | – | 1 | ||
| EFF | – | 1 | ||
| THC-COOH | INF | 81 | 20 | Terzic et al. (2010) |
| EFF | 123 | 53 | ||
| THC | SW | 60 ± 11 | 1.2 | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| THC-COOH | SW | 67 ± 13 | 1.5 | |
| THC | DW | 65 | 6 | Boleda et al. (2011b) |
| THC-OH | DW | 70 | 3 | |
| THC-COOH | DW | 73 | 15 | |
| THC | INF | – | 30 | Gerrity et al. (2011) |
| THC-COOH | INF | – | 30 | |
| THC-COOH | SW | 38 | 1 | Pedrouzo et al. (2011) |
| INF | 25 | 50 | ||
| EFF | 30 | 5 | ||
| THC | Ultrapure water | 90 ± 27 | 0.1 | González-Mariño et al. (2012) |
| INF | 105 ± 21 | 50 | ||
| EFF | 115 ± 8 | 20 | ||
| THC-COOH | Ultrapure water | 117 ± 1 | 0.1 | |
| INF | 107 ± 11 | 50 | ||
| EFF | 124 ± 8 | 20 | ||
| THC | SW INF EFF SW INF EFF | 94 104 98 96 112 92 | - | Racamonde et al. (2012) |
| INF | 104 | 1.0 | ||
| EFF | 98 | – | ||
| THC-COOH | SW | 96 | – | |
| INF | 112 | 2.5 | ||
| EFF | 92 | – | ||
| THC | INF | – | 108 | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| EFF | – | 54 | ||
| THC-OH | INF | – | 20 | |
| EFF | – | 11 | ||
| THC-COOH | INF | – | 10 | |
| EFF | – | 2 | ||
| THC THC-OH CBD CBN | Sludge | 130 | 3.1 ng g−1 | Mastroianni et al. (2013) |
| THC-OH | Sludge | 115 | 6.4 ng g−1 | |
| CBD | Sludge | 99 | 3.5 ng g−1 | |
| CBN | Sludge | 127 | 6.0 ng g−1 | |
| THC-COOH | INF | 61 | 5 | Nefau et al. (2013) |
| THC-OH | INF | 70 | 1.8 | Senta et al. (2013) |
| EFF | 63 | 1.2 | ||
| Sludge | 24 | 1.5 ng g1 | ||
| THC-COOH | INF | 31 | 2.5 | |
| EFF | 65 | 1.2 | ||
| Sludge | 30 | 0.5 ng g−1 | ||
| THC | DW | 63 | 0.03 | Carmona et al. (2014) |
| SW | 0.09 | |||
| EFF | 0.15 | |||
| Sediment | 0.24 ng g−1 | |||
| THC-COOH | DW | 0.03 | ||
| SW | 0.15 | |||
| EFF | 0.3 | |||
| Sediment | 0.4 ng g−1 | |||
| THC-COOH | INF | – | 2 | Mackuľak et al., 2014, Mackuľak et al., 2015, Mackuľak et al., 2016 |
| THC-COOH | Ultrapure water | – | 75 | Östman et al. (2014) |
| THC-COOH | INF | 66 ± 7 | 9.4 | Andrés-Costa et al. (2014) |
| THC | INF | 97 | 0.6 | Heuett et al. (2015) |
| THC-COOH | INF | 122 | 1.3 | |
| THC-COOH | INF | – | 6 | Gatidou et al. (2016) |
| THC | GW | 98 | 3.6 | Mastroianni et al. (2016) |
| THC-OH | GW | 99 | 1.6 | |
| THC-COOH | GW | 109 | 3.3 | |
| CBD | GW | 92 | 3.3 | |
| CBN | GW | 102 | 2.7 | |
| THC-COOH | SW | – | 2.5 | Yao et al. (2016) |
| INF | – | 5 | ||
| THC | SW | 72 ± 17 | 30 | Andrés-Costa et al. (2016) |
| INF | 61 ± 15 | 50 | ||
| EFF | 59 ± 15 | 35 | ||
| THC-COOH | SW | 78 ± 19 | 25 | |
| INF | 66 ± 17 | 45 | ||
| EFF | 67 ± 19 | 30 | ||
| THC | SW/INF | 25 | 11 | Jacox et al. (2017) |
| THC-COOH | SW/INF | 67 | 11 |
INF: wastewater influent; EFF: wastewater effluent; SW: surface water; GW: groundwater; DW: drinking water.
’-’ Not reported.
Pre-treatment of solid samples involved the removal of moisture from the sludge or sediment samples by freeze-drying so that the dried weight of the solid samples could be obtained (Mastroianni et al., 2013). The analytes could then be extracted from the solid phase into a liquid phase using techniques such as pressurized liquid extraction (Mastroianni et al., 2013); ultrasound assisted liquid extraction (Ivanová et al., 2018); or the quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction (Carmona et al., 2014). The extracted liquid could then be analyzed directly or concentrated using the liquid pre-treatment methods mentioned previously.
While all pre-concentration methods mentioned above have their own advantages and disadvantages, a universal challenge for all methods is the matrix effect. The matrix effect can be especially vital in wastewater, with significant ionization suppression for all analytes (Bijlsma et al., 2009). The use of isotopic compounds as surrogate standards can help to minimize the matrix effects when analyzing the analytes (Castiglioni et al., 2006; How et al., 2014). Park et al. (2016) recommended that all current pre-concentration methods would require isotopic standards to correct for matrix effects. In addition, the order of the preparatory steps was also found to play a critical role for the accurate determination of cannabinoids, especially with respect to filtration and pH adjustment (Causanilles et al., 2017). It was recommended that acidification of the samples should only occur after samples filtration and addition of the isotopic standards (Causanilles et al., 2017).
2.4. Identification and quantification of cannabinoids
The occurrence of cannabinoids and their metabolites in wastewater and/or natural water was analyzed by either liquid chromatography (LC) or gas chromatography (GC) coupled with mass spectrometry (LC-MS or GC-MS). LC-MS is more commonly used as compared to GC-MS because derivatization, typically silylation, is required for the determination of cannabinoid acid species in GC-MS, while LC-MS is comparatively more straightforward. Electrospray ionization (ESI) operated in positive and/or negative ion modes has been used for the ionization of cannabinoids when using LC-MS. Selected reaction monitoring (SRM) mode can be used to improve the selectivity and sensitivity for cannabinoids. SRM capability for trace analysis in complex samples is due to its ability to monitor only pre-selected parents and fragments ions. However, an analytical standard for each target compound is required to optimize SRM, which can be expensive and time consuming.
High-resolution mass spectrometry (HRMS) such as time-of-flight mass spectrometry (Andrés-Costa et al., 2016; Black et al., 2019) and Orbitrap mass spectrometry (Fedorova et al., 2014; Mackuľak et al., 2014) have also been utilized for the detection and analysis of cannabinoids. An advantage of using HRMS over the use of low-resolution MS (LRMS), such as tandem MS for the detection of cannabinoids, was that HRMS was able to screen suspected compounds in the samples and had comparable quantification for most of the detected compounds compared to LRMS (Andrés-Costa et al., 2016). However, a disadvantage for HRMS is that it is not readily available in many laboratories.
3. Occurrence of cannabinoids in wastewater and the environment
As cannabis is still classified as an illicit drug in most countries, the analyses of cannabinoids and their metabolites in water systems are normally part of a wider survey of illicit drug concentrations in the influent stream of WWTPs. Illicit drug concentrations in WWTPs influent could then be used to evaluate community usage. This process is named as sewage epidemiology or wastewater-based epidemiology by Daughton (2001). The intent of sewage epidemiology is to estimate the use of illicit drugs in the population by probing their concentrations in the wastewater (Chen et al., 2019; Daughton, 2001; Zuccato et al., 2005) and more recently as a potential tool for the surveillance of Covid-19 in the population (Daughton, 2020). Wastewater samples collected from specific communities, or even specific buildings, can provide a more accurate estimation of illicit drug use by the population than wastewater samples collected at the inlet of WWTPs (Chen et al., 2019; Gatidou et al., 2016; Heuett et al., 2015; Lai et al., 2011; Postigo et al., 2011). Consumed doses are then determined by the drug concentrations in the wastewater using the consumed quantity and wastewater flow rates against metabolite excretion ratios from the literature (Khan and Nicell, 2012; Postigo et al., 2011; Zhang et al., 2019; Zuccato et al., 2008). For instance, the excretion rate of THC was suggested to be 0.6% by Gracia-Lor et al. (2016), who used only the THC-COOH excretion rate, or 2.5% by Postigo et al. (2011), who used the sum of extraction rate of THC-COOH (0.5%) and THC-OH (2%).
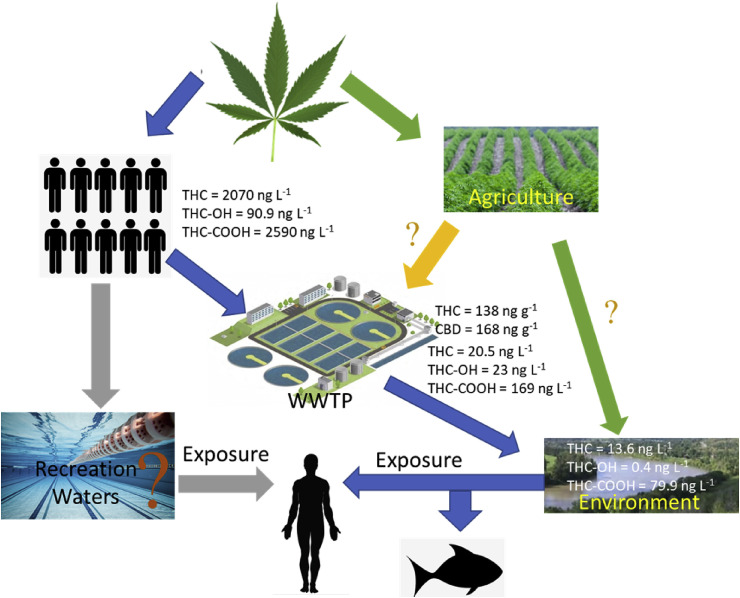
THC-COOH was generally detected at higher concentration than THC and THC-OH, which were commonly not detected (below limit of detection) or detected at concentrations below their limit of quantifications (LOQs) (Table 3, Table 4 ). The concentrations of THC, THC-OH, and THC-COOH in INF ranged from not detected to 2070, 90.9, and 2590 ng L−1, respectively (Carmona et al., 2014; Heuett et al., 2015; Postigo et al., 2010). The concentrations of THC, THC-OH, and THC-COOH in EFF ranged from not detected to 20.5, 23, and 169 ng L−1, respectively (Boleda et al., 2009; Devault et al., 2017; Gerrity et al., 2011; Postigo et al., 2008). Among the different types of natural water analyzed, THC and its metabolites were only detected in river water with the concentration of THC, THC-OH, and THC-COOH detected up to 13.6, 0.4, and 79.9 ng L−1, respectively (Catalá et al., 2015). More samples from different natural water systems would be required to confirm the trend of THC and its metabolites in natural water systems. While four studies (Boleda et al., 2011a, 2011b; Carmona et al., 2014; Mendoza et al., 2016) investigated the occurrence of THC-COOH in drinking water, only the study by Carmona et al. (2014) detected one occurrence of THC-COOH in drinking water at 1 ng L−1. As with the natural water systems, more drinking water samples would be required to confirm the general trend.
Table 4.
Occurrence of THC and its metabolites in various water systems.
INF: wastewater influent; EFF: wastewater effluent; SW: surface water; RDW: raw drinking water; TDW: treated drinking water.
’-’Not measured; N.D.: not detected.
Generally, only THC and its metabolites, THC-COOH and THC-OH, were analyzed during sewage epidemiology. This is probably due to the fact that THC is the primary psychoactive compound in cannabis. As a result, most studies on cannabinoids in the environment tend to focus on the concentration of THC and its metabolites, resulting in limited information for other cannabinoids, such as CBD, in wastewater and the environment.
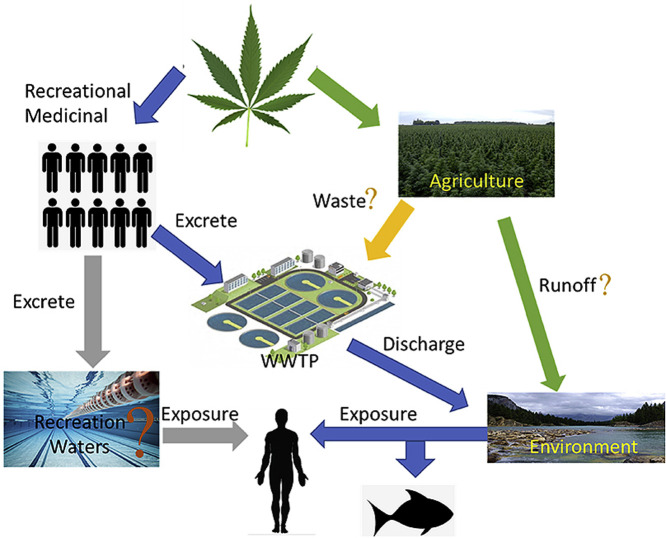
To date no information on CBD and CBN concentrations in wastewater is available, but studies have investigated the concentration of CBD and CBN in sewage sludge (Table 5 ). The study by Black et al. (2019) showed that both CBD and CBN were detected in 43% of the samples analyzed as compared to the 21%, 14%, and 7% for THC-OH, THC-COOH, and THC, respectively. Mastroianni et al. (2013) reported that THC was detected in all sewage sludge samples and CBD was detected in 80% of the sewage sludge samples. The median concentrations of THC and CBD were found to be 138 and 168 ng g−1 dried weight, respectively. In a survey study of illicit drugs in surface water, CBD was not detected in the samples, while THC was detected for up to 7% of the samples. However, the concentration of THC was below LOQ (120 ng L−1) (Mastroianni et al., 2016). CBN was not detected in a survey for pharmaceuticals in landfill leachates (Lu et al., 2016). With the legalization of cannabis or its extract for medical and recreational uses, and the rapid increase in cannabinoid-based drugs, one could predict an increase in the release of CBD into wastewater and the environment and thus more studies of its occurrence would be required. In addition, due to the high partition coefficient and low aqueous solubility of cannabinoids and their metabolites, more research on their occurrence in solid samples (sewage sludge, sediment and suspended particulate matter) would be required to gain a better understanding of the cannabinoids’ occurrence and their impact on the environment. A summary of the fate of cannabinoids and their transformation products, as well as the possible routes to human and aquatic life exposure are presented in Fig. 2.
Table 5.
Frequency of detection and median concentration of cannabinoids and its metabolites in various solid samples.
| Source | Frequency of detection (%) (Median concentration ng g−1 d.w.)a |
Reference |
||||
|---|---|---|---|---|---|---|
| THC | THC-OH | THC-COOH | CBD | CBN | ||
| Sludge | 100 (138) | 67 (78.4) | – | 80 (168) | 53 (101) | Mastroianni et al. (2013) |
| Influent particulate | – | 67 (67) | 67 (17) | – | – | Senta et al. (2013) |
| Sludge | – | 67 (7.3) | 67 (8.5) | – | – | |
| River sediment | 5 (42) | – | 1 (5) | – | – | Carmona et al. (2014) |
| Sludge | – | – | -(170) | – | -(7.1) | Ivanová et al. (2018) |
| Sludge | 7(−) | 21(−) | 14(−) | 43(−) | 43(−) | Black et al. (2019) |
’-’ Not measured.
Fig. 2.
Summary of the transportation of cannabinoids and their transformation products in the water system and the environment, and also the exposure route for human and aquatic life. Concentrations presented were the maximum detected concentrations found in the literature (Boleda et al., 2009; Health Canada and Canada, 2012, Health Canada and Canada, 2017; Heuett et al., 2015; Mastroianni et al., 2013; Postigo et al., 2008, 2010).
4. Removal of cannabinoids in water
As cannabinoids and their metabolites are found in wastewater, it is important to investigate their removal during water treatment. Their removal during water treatment would have significant impact on their occurrence in the receiving water bodies. Currently, there is no specific regulation on the maximum concentration of cannabinoids allowed in drinking water or effluent wastewater, even for cannabis cultivation and extraction, except indirectly as part of other testing requirements for effluent wastewater (AEP, 2018; Upland Agricultural Consulting, 2019). While limited work was conducted on the specific removal of cannabinoids in water, THC-COOH concentrations in the INF and EFF of WWTPs were measured in many studies, which can provide an indication on the effectiveness of wastewater treatment processes for the removal of THC-COOH. As an illustration, WWTPs in Slovakia and the Czech Republic were able to remove 84% or more of the THC-COOH in the INF, as compared with the EFF stream (Mackuľak et al., 2020). However, no details were provided on the treatment processes for the WWTPs, thus further analysis on the efficiency of each treatment method was not possible.
4.1. Biological treatment
The estimated removal rates for THC, THC-OH, and THC-COOH in WWTPs, where activated sludge was the main treatment technique, ranged from 8% to 100%, 38% to 100%, and −18.3% to 100%, respectively (Castiglioni et al., 2006; Boleda et al., 2009, Racamonde et al., 2012, Nefau et al., 2013; Carmona et al., 2014). The negative removal rate of THC-COOH during wastewater treatment has been attributed to the deconjugation of the glucuronide form of THC-COOH by the activated sludge and/or desorption from solids during treatment, or potentially from the degradation of THC in the wastewater. No temperature effect was observed for THC-COOH degradation by activated sludge between 18 and 31 °C (Devault et al., 2017). The removability of THC-COOH by algae, fungi, and enzymes was tested by exposing the organisms to water containing THC-COOH (179 ± 10 ng L−1) for an hour. The removal efficiency by these organisms was found to be 22%–36%, through a combination of sorption and biodegradation by the organisms (Mackuľak et al., 2015).
4.2. Physical removal
In the partitioning study by Senta et al. (2013), the differences in THC-OH and THC-COOH concentrations in the solid (particulate) and aqueous phases of wastewater were measured and it was found that 28% of THC-OH and 8% of THC-COOH were partitioned into the solid phase. The partitioning coefficients (Kd) were determined to be 1790 ± 930 L kg−1 for THC-OH and 357 ± 198 L kg−1 for TH-COOH in INF. In another partitioning study, the Kd and organic carbon-water partition coefficient (Koc) of THC-COOH were determined to be 1050 L kg−1 and 35100 L kg−1, respectively, using a 2-day batch equilibrium method where homogenized marine sediment was used as the solid phase (Palardy et al., 2015). The results of these studies suggested that adsorption to filter media or suspended solids is an important factor in the removal of the THC metabolites. In addition, the results also indicated that THC and its metabolites are likely to partition into the sediments within natural systems. These results were also in line with the findings reported by Carmona et al. (2014). The authors reported that THC and THC-COOH were found in the sediment at concentrations of 42 and 5 ng g−1, respectively, while THC was not detected in the water samples from the same site; THC-COOH was detected in the water sample at 7 ng L−1. Initially, it was not expected that the THC-COOH would be adsorbed onto the solids phase due to its predicted low Koc values (77.5, 147 and 174 L kg−1) that were obtained using a prediction model and software (ACD/Labs) (Khan and Nicell, 2012; Park et al., 2016). A possible reason for the difference in the experimental and predicted Koc was that THC-COOH detected on the solid phase was due to the degradation of THC at the solid phase (Carmona et al., 2014). Another plausible reason for the difference low Koc but high partition observed in experimental condition was that while the predicted Koc value was low for THC-COOH, the predicted logKow of 5.25–6.21 is similar to THC and THC-OH (6.84–7.68 and 5.36, respectively) (Apul et al., 2020), which indicated that they are hydrophobic. Thus, THC-COOH may behave similarly to THC and THC-OH and adsorb onto the hydrophobic suspended solids in wastewater and in natural water systems.
4.3. Chemical and advanced oxidation
THC-COOH was found to be very reactive with free chlorine, where the second order rate constant was 5.8 × 104 M−1 s−1 at pH 8.3 (González-Mariño et al., 2013) and 3.9 × 104 M−1 s−1 at pH 9 (Mackie et al., 2017). It was also found that the rate constant decreased with a decrease in pH (González-Mariño et al., 2013; Mackie et al., 2017). While the presence of bromide did not affect the rate of degradation of THC-COOH (González-Mariño et al., 2013). The presence of organic matter from surface water reduced the THC-COOH degradation rate (Bijlsma et al., 2009; Mackie et al., 2017). The reason for the reduced degradation rate would be that the free chlorine would preferentially react with the more reactive organic matter in surface water before reacting with the THC-COOH (González-Mariño et al., 2013). Due to the high reactivity of THC-COOH with free chlorine, it was suggested that pre-chlorination would be effective in the removal of THC-COOH in drinking water treatment; however, there was only one detection of THC-COOH in raw drinking water at a concentration of 14.7 ng L−1 (Boleda et al., 2009).
Wastewater containing cannabinoids was treated with Fenton reaction (FR) using ferric sulfate and hydrogen peroxide, and Fenton-like reaction (FLR) using zerovalent iron, hydrogen peroxide, and sulfuric acid. FR and FLR removed >98% of THC-COOH, reducing THC-COOH concentration from 179 ± 10 ng L−1 to below 3 ng L−1 (Mackuľak et al., 2015). Over 85% of THC-COOH was removed from low initial concentration of less than 20 ng L−1 by FR and FLR (Mackuľak et al., 2015, 2016). THC-COOH was degraded by the highly reactive hydroxyl radical (•OH) that was generated in both FR and FLR. The production of •OH was faster with FR than FLR, thus the degradation rate of THC-COOH was faster in FR (Mackuľak et al., 2016). The same research group also found that ferrate was able to remove >95% of THC-COOH from INF (initial concentration = 179 ± 10 ng L−1) and >82% from EFF (initial concentration = 20 ng L−1), even with the lower ferrate standard half-cell reduction potential of 0.72 V in a basic condition (Mackuľak et al., 2016). However, the mechanism of the degradation of THC-COOH by ferrate was not investigated in the study.
UV oxidation was also found to be effective in the removal of THC-COOH. 100% removal (initial concentration = 0.5 mg L−1) was achieved within 30 min after exposure to UV at 254 nm, the typical wavelength used in the WWTPs (Boix et al., 2014). In the same paper, 100% removal of THC-COOH was achieved after exposure to the equivalent of 200 h of natural sunlight (Boix et al., 2014). It was proposed that the degradation of THC-COOH occurred by electrophilic substitution at the phenol ring, which might be excited due to the UV irradiation. The higher degradation of THC-COOH in the wastewater samples by photo-oxidation might be due the high concentration of nitrate, a natural photosensitizer, in the samples (Boix et al., 2014).
Electro-oxidation achieved 76% of THC-COOH removal (initial concentration = 100 ng L−1) after 15 min and 84% removal by 60 min in INF using a boron doped diamond (BDD) electrode as the anode and a graphite electrode as the cathode at 20 mA cm−2 (Mackuľak et al., 2020). The authors also suggested that the use of BDD as the anode was estimated to be 2 times more energy efficient than using a graphite anode. The main mechanism for the degradation of THC-COOH in electro-oxidation was believed to be by the •OH generated at the BDD electrode surface.
5. Transformation by-products
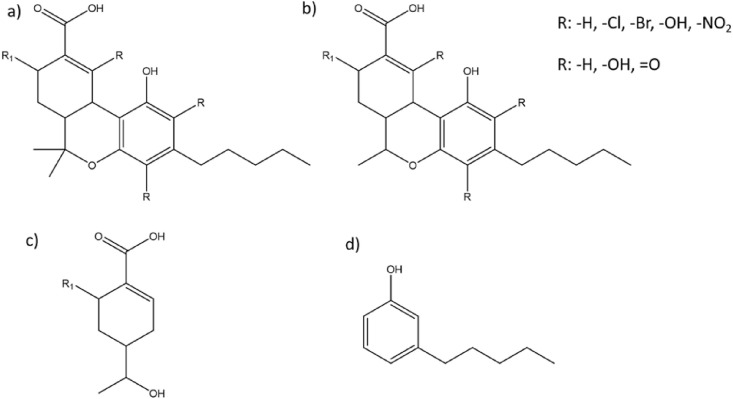
After treatment, transformation by-products (Fig. 1 ) could form and could be potentially more toxic than their parent compounds. Seven by-products, six of which were halogenated by-products, were detected after chlorination (Boix et al., 2014; González-Mariño et al., 2013; Mackie et al., 2017). The main mechanism proposed for the chlorine and THC-COOH reaction was as follows: the chlorine attached to the phenol ring of the THC-COOH by electrophilic substitution and this resulted in the formation of chlorinated by-products (González-Mariño et al., 2013). Six by-products were detected when THC-COOH was exposed to simulated sunlight (Boix et al., 2014). Thus, the result showed that THC-COOH could be naturally attenuated in the environment. However, at the same time, it produced transformation by-products of unknown properties. Less by-products were formed when THC-COOH was exposed to UV at 254 nm (Boix et al., 2014). This suggests that UV treatment could be a suitable method for the degradation of THC-COOH, especially since it produces less by-products. However, further studies regarding the toxicity of the by-products formed after treatment would be required to confirm if the UV treatment at 254 nm is indeed a more a suitable method for THC-COOH degradation. A demethyled by-product was detected when THC-COOH was allowed to hydrolyze in the dark (Boix et al., 2014). Although almost all by-products involved an electrophilic substitution at the phenol ring, there was no overlap of by-products found between the different treatments, suggesting that significantly different reaction pathways were involved in the oxidation of THC-COOH. The formation of a nitro-substituted by-product was attributed to the relatively high concentration of nitrate in the water sample used (Boix et al., 2014). Studies on the by-product formation at low nitrate concentration would be required to understand the reactivity of THC-COOH to reactive nitrogen species and the degradation rate of THC-COOH in the absence of a photosensitizer, such as nitrate.
Fig. 1.
General structure of possible by-products from the oxidation of THC-COOH. Adapted from Boix et al., (2014) and González-Mariño et al., (2013).
Being structurally similar, other cannabinoids could potentially undergo similar reactions as THC and its metabolites, resulting in the formation of transformation by-products. It was suggested that chlorination or oxidation of CBD in the presence of halogens could produce trihalomethanes, haloacetic acids, and haloacetaldehydes due to the CBD structure (Saleh et al., 2019). CBD is expected to be more reactive than THC due to its additional π-electrons in its structure and the lack of rigidity due to the cyclic group in the center (Table 1) (Saleh et al., 2019). Therefore, it is also expected that after oxidation, it will be easier for CBD to undergo fragmentation.
6. Toxicity and impact on the environment
The toxicity of THC-COOH on zebra mussels (Dreissena polymorpha) was tested at three concentrations (100, 500 and 1000 ng L−1) that were supposed to be representative of the current THC-COOH concentration in a natural water and two worst-case scenarios, assuming that the use of cannabis will continue to increase (Parolini et al., 2017). Although the study showed that only the highest concentration of THC-COOH (1000 ng L−1) caused oxidative stress to the zebra mussels, all three concentrations resulted in increased DNA fragmentation but with no specific genetic damage. Another related study showed that significant oxidative stress to zebra mussels was observed after exposure to 500 ng L−1 of THC for 14 days (Parolini and Binelli, 2014). It was found that THC at concentrations higher than 30 mg L−1 would result in increasing anxiety behaviors in zebrafish (Stewart and Kalueff, 2014). The tested concentrations used in these studies were much higher than the highest reported THC concentration (13.6 ng L−1) in surface water (Boleda et al., 2007).
Zebrafish embryos exposed to THC (6 mg L−1) or CBD (3 mg L−1) during gastrulation exhibited reduced heart rates, axial deformities, and shorter trunks (Ahmed et al., 2018). THC or CBD treatment also altered the synaptic activity at neuromuscular junctions which affected the resulting branching patterns. Ahmed et al. (2018) also observed that the number of axonal branches in the trunk musculature was reduced. Furthermore, locomotion studies also showed that the number of C-start escape responses to sound stimuli for THC and CBD exposed zebrafish larvae was severely reduced (Ahmed et al., 2018). Exposure to THC (6 mg L−1) for 5.25–10.75 h post fertilization affected the zebrafish embryos’ mircofold cell axon diameter and their escape response dynamics to touch (Amin et al., 2020). Zebrafish muscle fibers had small but significant changes in the pattern of expression of nicotinic acetylcholine receptors and were slightly disorganized even though the muscles were largely intact (Amin et al., 2020). However, qPCR results showed no obvious changes in the expression of mRNA of the nicotinic receptor subunit (Amin et al., 2020). While adverse effect on the zebrafish was observed after exposure to THC and CBD, high concentrations of THC (6 mg L−1) or CBD (3 mg L−1) used in both experiments are not likely to occur in natural water systems.
In another study, statistically significant differences (P < 0.05) were found in all the biochemical parameters of the gills, liver and serum of Cyprinus carpio fingerlings when exposed to 1.88 mg L−1 of Cannabis sativa crude leaf extract for 59 days (Audu et al., 2015). While the toxicity studies showed that cannabinoids could potentially have a negative impact on the development of aquatic animals, especially on their locomotive ability, further studies using relevant concentrations (those found in the environment, in low ng L−1) would be required to have an accurate assessment of the impact of cannabinoids on aquatic animals.
THC-COOH was found to be less toxic than THC when using a biomarker response index which was scored based on the magnitude of measured impact on an organism, i.e. zebra mussels (Parolini et al., 2017). The Hazard Quotient (HQ) method was used by Mendoza et al. (2014) to estimate the potential negative effects of THC and THC-COOH on the environment. The HQ is defined by US EPA as the ratio of the potential exposure to the substance and the concentration at which no negative effects are expected (EPA, 1997a). HQ was calculated by dividing the measured environmental concentration by a predicted no effect concentration (PNEC). The PNEC was derived from division of the available aquatic toxicity data by assessment factors. No negative effect is expected when the HQ values are below 0.1, values from 0.1 to 1 indicate low risk with potential for negative effects, values between 1.0 and 10 indicate moderate risk with some negative effects, and values that are more than 10 indicate high risk and negative effects (EPA, 1997b). The PNEC was calculated to be 200 and 29 ng L−1 for THC and THC-COOH, respectively. The calculated HQ value for THC was 0.1 and the value for THC-COOH ranged from 0.23 to 2.75, suggesting that THC-COOH posed a higher environmental risk than THC.
Computer-assisted quantitative structure–activity relationship (QSAR) models have been used for toxicity assessment when bioassay data are limited or not available (Carlsen and Kenessov, 2014; Khan and Roy, 2017; Moudgal et al., 2000). QSAR presents models that mathematically relate the chemical structural features of compounds to their definite biological activity (Yousefinejad and Hemmateenejad, 2015). Quantitative structure–toxicity relationships (QSTR), based on QSAR, provide the mathematical relationship between a given toxicity measure and numerical descriptors of a chemical structure (Can, 2014). A QSAR model with sufficient documentation to allow for an independent evaluation of the results is accepted by the European Chemical Agency as an alternative for animal testing.
Cannabinol and CBD were anticipated to interfere with estrogen receptors or other development/reproductive pathways based on the QSAR toxicity prediction model (VEGA-QSAR) (Black et al., 2019). However, in an in-vitro study, THC, CBD and CBN did not result in increased estrogenicity in MCF7-BUS cell line but marijuana smoke condensate did result in increased estrogenicity in MCF7-BUS cell line (Lee et al., 2006). The same study also suggested that the phenolic compounds in the cannabis might be the cause of the estrogenicity. It was also found that THC, CBD and CBN could result in infertility in males through endocannabinoid system (du Plessis et al., 2015). No clear conclusion could be drawn on the environmental and health impacts of cannabinoids due to the contradicting results from various studies. Therefore, more research on the toxicity would be required to better assess the risk factor of cannabinoids and their metabolites on the environment.
As cannabinoids will undergo structural transformation during water treatment, the toxicity of their by-products would also be of importance. The toxicity of by-products of THC-COOH was predicted by Toxicity Estimation Software Tool (TEST, QSTR software by US EPA) to have equal or higher toxicity than THC-COOH itself (González-Mariño et al., 2013). THC-COOH predicted 48 h Daphnia magna LC50 was 62 μg L−1, while LC50 of the chlorinated by-products ranged from 6 to 67 μg L−1 (González-Mariño et al., 2013). The 48 h Daphnia magna LC50 for common pharmaceuticals such as ibuprofen and carbamazepine, ranged from 7 to 140 mg L−1 (Han et al., 2006), thus the predicted LC50 values suggested that THC-COOH and its by-products were much more toxic than the common pharmaceuticals. It has also been predicted that the toxicity of cannabinoids and their halogenated by-products is orders of magnitude higher than that of regulated disinfection by-products such as trihalomethanes and haloacetic acids (Saleh et al., 2019). The lack of environmental toxicity data for the cannabinoids, their metabolites, and their transformation by-products necessitates further research into their toxicity to better assess their risk to the environment and the public health. The use of QSAR/QSTR could assist in the risk assessment of cannabinoids and their transformation products. However, it should be noted that the use of different QSAR software might result in different outcomes for the risk assessment.
7. Currently neglected route for cannabinoids release to environment and public exposure to cannabinoids
The legalization of cannabis for recreational and medical purposes has resulted in an increase of the use of cannabis in pharmaceutical and personal care products, such as cosmetics, bath salts, as well as, cannabis infused food, candies, and beverages. Due to the large array of products that contain cannabis, it is important to consider both the release and exposure to cannabinoids outside the traditional routes of entering the water system, which is through the municipal wastewater system.
Pharmaceutical and personal care products (PPCPs) have being detected in swimming pool water and are thought to be introduced through the immersion of the swimmer (Weng et al., 2014), or from the swimmers’ body fluids, such as urine (Jmaiff Blackstock et al., 2017), or sweat (Keuten et al., 2014). Therefore, cannabinoids and their metabolites could be introduced into the swimming pool water through use of cannabinoids-containing cosmetic products or through the body fluids of swimmers that have consumed cannabis or cannabis-infused food products. This has resulted in a ‘new’ route by which the public can be exposed to cannabinoids and their metabolites. Chlorination and UV irradiation are commonly used for the disinfection of the swimming pool input and recycled water to prevent waterborne diseases (Zwiener et al., 2007). As mention in the earlier sections, THC-COOH reacts quickly with chlorine or is degraded rapidly by UV oxidation to form by-products that are potentially more toxic than the regulated disinfection by-products. As the toxicity of the by-products from cannabinoids is unknown, swimmers may be exposed to unknown health risks and this can result in a potential public health concern. In addition, most cannabinoid toxicity studies on public health focused on the inhalation or ingestion of cannabinoids. However, dermal exposure would be the most probable route of exposure in recreation waters, like swimming pools. As such, more studies on dermal cannabinoids exposure would become more relevant. In addition, limited studies are available on the toxicity of the transformation products on public health. Thus, this would be another area of interest and possible concern.
With the recent changes in cannabis regulations and the shifts in public perspective on the use of cannabis, research on the occurrence of cannabinoids in recreation water systems, such as swimming pools and spa water, and in cannabis related agricultural and industrial solid wastes and wastewaters should be included in the future studies. In addition, their impact on public health should also be considered.
8. Conclusions and recommendations
While cannabinoids have been released into the environment for decades, with the current trends in the legalization of cannabis for medical and recreational uses, the release of cannabinoids and their metabolites, especially CBD, the main cannabinoid responsible for the medicinal properties of cannabis, can be expected to increase. Therefore, more studies are required to assess the fate, transformation, and removal of cannabinoids from domestic and industrial wastewater, drinking water, recreational water, and natural water systems.
The analysis of cannabinoids is a critical part in the study of the occurrence and removal of cannabinoids from the water system and environment. As such, many studies have been conducted on the analytical procedures used for the detection of cannabinoids. The use of glass amber bottles, no pH adjustment, and storage temperature of ≤4 °C were found to be the most suitable methods for the storage of water samples. Analysis of cannabinoids in water samples within 3 days has also been recommended. Storage of SPE cartridges loaded with samples at −20 °C could be used if samples need to be stored for up to 3 months before analysis. SPE was the most common pre-concentration method with LC-MS being the most common detection technique for the cannabinoids. The use of isotopic standards was also recommended for the analysis of cannabinoids to reduce the error due to recovery and matrix effects. Acidification of the samples should be done only after filtration and addition of isotopic standards. As it was also found that cannabinoids have high affinity for the solid phase, more validation and optimization on the extraction of cannabinoids from solid samples (sewage sludge, sediment, and suspended solids) would be required to improve the analysis of cannabinoids and their transformation products in the solid samples.
The majority of occurrence studies focused on THC, the main psychoactive cannabinoid, and its metabolites in wastewaters where their concentrations ranged from not detected to more than 2400 ng L−1. While CBD was not found in wastewater, it was found in up to 80% of the sewage sludge samples with concentrations as high as 168 ng g−1 dried weight. Therefore, more emphasis on the occurrence and removal of cannabinoids in sewage sludge and sediment would be required to understand the fate of the cannabinoids in the environment and water systems.
While current studies show that WWTPs were generally effective in the removal of cannabinoids from the wastewater, more studies on the degradation of cannabinoids in the sludge samples would be required due to the high partitioning coefficient of the cannabinoids. In addition, studies on the occurrences of cannabinoids in soil sediments would also be recommended to better understand the impact of cannabinoids on the environment. Treatment methods such as chlorination, photo-oxidation, and electro-oxidation were found to be effective in the removal of THC-COOH. However, by-products that are potentially more toxic than their parent compounds, common pharmaceuticals, and regulated disinfection by-products are formed. Therefore, more studies would be recommended to understand the transformation pathways of cannabinoids and the environmental toxicity of these transformation products.
Although some studies have found that cannabinoids and THC’s metabolites have negative impacts on aquatic life, the concentrations used in those studies were much higher than those detected in natural water systems. Therefore, no clear conclusions could be made with regard to the environmental impacts of cannabinoids due to the lack of toxicity assays conducted at relevant concentrations, or concentrations more representative of those found in natural water systems. In addition, the synergetic effects with other micropollutants present in natural systems have not been reported. To overcome the lack of bioassay data for the cannabinoids, toxicity modelling, such as QSTR, has been used to assist in the risk assessment of cannabinoids to the environment. Toxicity modelling has predicted that cannabinoids and THC’s metabolites have potential to cause adverse effects to the environment.
Finally, because of the change in the regulations of cannabis, recreation waters, such as swimming pools, could be a potential source for public exposure to cannabinoids and their transformation products. Therefore, studies of the occurrence and health impact of cannabinoids and their transformation products in recreation water would be of great interest.
CRediT author statement
Zuo Tong How: Investigation, writing. Mohamed Gamal El-Din: Funding acquisition, Review and editing
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by research grants from a Natural Sciences and Engineering Research Council of Canada (NSERC) Senior Industrial Research Chair (IRC) in Oil Sands Tailings Water Treatment. As a part of the University of Alberta’s Future Energy Systems research initiative, this research was made possible in part thanks to funding from the Canada First Research Excellence Fund. The authors would also like to acknowledge Dr. Chelsea Benally and Dr. Pamela Chelme-Ayala for their proofreading and suggestions of the manuscript.
Footnotes
This paper has been recommended for acceptance by Charles Wong.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2020.115642.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adam O., Miles L., Brian L., Jacob R., Clinton S. Marijunana Policy Group; Colorado: 2018. Market Size and Demand for Marijuana in Colorado: 2017 Market Update. [Google Scholar]
- AEP . In: Cannabis Wastewater Fact Sheet. Alberta G.o., editor. Alberta Environmental and Parks, Government of Alberta; Alberta: 2018. [Google Scholar]
- Ahmed K.T., Amin M.R., Shah P., Ali D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (Δ9-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018;8(1):10518. doi: 10.1038/s41598-018-28689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIHW . In: National Drug Strategy Household Survey 2016. Health. D o., editor. 2016. [Google Scholar]
- Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., Etxebarria N., Usobiaga A. Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016;79(2):324–331. doi: 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- Alvarez D.A. Development of semipermeable membrane devices (SPMDs) and polar organic chemical integrative samplers (POCIS) for environmental monitoring. Environ. Toxicol. Chem. 2013;32(10):2179–2181. doi: 10.1002/etc.2339. [DOI] [PubMed] [Google Scholar]
- Alvarez D.A., Petty J.D., Huckins J.N., Jones-Lepp T.L., Getting D.T., Goddard J.P., Manahan S.E. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. 2004;23(7):1640–1648. doi: 10.1897/03-603. [DOI] [PubMed] [Google Scholar]
- Amaducci S., Scordia D., Liu F.H., Zhang Q., Guo H., Testa G., Cosentino S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015;68:2–16. [Google Scholar]
- Amin M.R., Ahmed K.T., Ali D.W. Early exposure to THC alters M-cell development in zebrafish embryos. Biomedicines. 2020;8(1):5. doi: 10.3390/biomedicines8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés-Costa M.J., Andreu V., Picó Y. Analysis of psychoactive substances in water by information dependent acquisition on a hybrid quadrupole time-of-flight mass spectrometer. J. Chromatogr. A. 2016;1461:98–106. doi: 10.1016/j.chroma.2016.07.062. [DOI] [PubMed] [Google Scholar]
- Andrés-Costa M.J., Rubio-López N., Morales Suárez-Varela M., Pico Y. Occurrence and removal of drugs of abuse in wastewater treatment plants of valencia (Spain) Environ. Pollut. 2014;194:152–162. doi: 10.1016/j.envpol.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Apul Onur G., Rowles Lewis Stetson, III, Khalid Arsalan, Karanfil Tanju, Richardson Susan D., Saleh Navid B. Transformation potential of cannabinoids during their passage through engineered water treatment systems: A perspective. Environ. Int. 2020;137 doi: 10.1016/j.envint.2020.105586. [DOI] [PubMed] [Google Scholar]
- Arditsoglou A., Voutsa D. Passive sampling of selected endocrine disrupting compounds using polar organic chemical integrative samplers. Environ. Pollut. 2008;156(2):316–324. doi: 10.1016/j.envpol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Audu B.S., Ajima M.N.O., Ofojekwu P.C. Enzymatic and biochemical changes in common carp, Cyprinus carpio (L.) fingerlings exposed to crude leaf extract of Cannabis sativa (L.) Asian Pacific Journal of Tropical Disease. 2015;5(2):107–115. [Google Scholar]
- Baker D.R., Kasprzyk-Hordern B. Critical evaluation of methodology commonly used in sample collection, storage and preparation for the analysis of pharmaceuticals and illicit drugs in surface water and wastewater by solid phase extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A. 2011;1218(44):8036–8059. doi: 10.1016/j.chroma.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Balducci C., Green D.C., Romagnoli P., Perilli M., Johansson C., Panteliadis P., Cecinato A. Cocaine and cannabinoids in the atmosphere of Northern Europe cities, comparison with Southern Europe and wastewater analysis. Environ. Int. 2016;97:187–194. doi: 10.1016/j.envint.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Berset J.-D., Brenneisen R., Mathieu C. Analysis of llicit and illicit drugs in waste, surface and lake water samples using large volume direct injection high performance liquid chromatography – electrospray tandem mass spectrometry (HPLC–MS/MS) Chemosphere. 2010;81(7):859–866. doi: 10.1016/j.chemosphere.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Bijlsma L., Sancho J.V., Pitarch E., Ibáñez M., Hernández F. Simultaneous ultra-high-pressure liquid chromatography–tandem mass spectrometry determination of amphetamine and amphetamine-like stimulants, cocaine and its metabolites, and a cannabis metabolite in surface water and urban wastewater. J. Chromatogr. A. 2009;1216(15):3078–3089. doi: 10.1016/j.chroma.2009.01.067. [DOI] [PubMed] [Google Scholar]
- Black G.P., Anumol T., Young Thomas M. Analyzing a broader spectrum of endocrine active organic contaminants in sewage sludge with high resolution LC-QTOF-MS suspect screening and QSAR toxicity prediction. Environ. Sci. J. Integr. Environ. Res.: Processes & Impacts. 2019;21(7):1099–1114. doi: 10.1039/c9em00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc J.A., Manneh V.A., Ernst R., Berger D.E., de Keczer S.A., Chase C., Centofanti J.M., DeLizza A.J. Adsorption losses from urine-based cannabinoid calibrators during routine use. Clin. Chem. 1993;39(8):1705–1712. [PubMed] [Google Scholar]
- Bodík Igor, Tomáš Mackuľak, Milota Fáberová, Lucia Ivanová. Occurrence of illicit drugs and selected pharmaceuticals in Slovak municipal wastewater. Environ. Sci. Pollut. Res. 2016;23(20):21098–21105. doi: 10.1007/s11356-016-7415-5. [DOI] [PubMed] [Google Scholar]
- Boix C., Ibáñez M., Bijlsma L., Sancho J.V., Hernández F. Investigation of cannabis biomarkers and transformation products in waters by liquid chromatography coupled to time of flight and triple quadrupole mass spectrometry. Chemosphere. 2014;99:64–71. doi: 10.1016/j.chemosphere.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Boleda M.R., Galceran M.T., Ventura F. Trace determination of cannabinoids and opiates in wastewater and surface waters by ultra-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2007;1175(1):38–48. doi: 10.1016/j.chroma.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Boleda M.R., Galceran M.T., Ventura F. Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain. Water Res. 2009;43(4):1126–1136. doi: 10.1016/j.watres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Boleda M.R., Galceran M.T., Ventura F. Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environ. Pollut. 2011;159(6):1584–1591. doi: 10.1016/j.envpol.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Boleda M.R., Huerta-Fontela M., Ventura F., Galceran M.T. Evaluation of the presence of drugs of abuse in tap waters. Chemosphere. 2011;84(11):1601–1607. doi: 10.1016/j.chemosphere.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Brenneisen R., Meyer P., Chtioui H., Saugy M., Kamber M. Plasma and urine profiles of Δ9-tetrahydrocannabinol and its metabolites 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes. Anal. Bioanal. Chem. 2010;396(7):2493–2502. doi: 10.1007/s00216-009-3431-3. [DOI] [PubMed] [Google Scholar]
- Can A. Quantitative structure–toxicity relationship (QSTR) studies on the organophosphate insecticides. Toxicol. Lett. 2014;230(3):434–443. doi: 10.1016/j.toxlet.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Carlsen L., Kenessov B. In: Transformation Products of Emerging Contaminants in the Environment. Lambropoulou D.A., Nollet L.M.L., editors. 2014. pp. 859–876. [Google Scholar]
- Carmona E., Andreu V., Picó Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci. Total Environ. 2014;484:53–63. doi: 10.1016/j.scitotenv.2014.02.085. [DOI] [PubMed] [Google Scholar]
- Castiglioni S., Zuccato E., Chiabrando C., Fanelli R., Bagnati R. Mass spectrometric analysis of illicit drugs in wastewater and surface water. Mass Spectrom. Rev. 2008;27(4):378–394. doi: 10.1002/mas.20168. [DOI] [PubMed] [Google Scholar]
- Castiglioni S., Zuccato E., Crisci E., Chiabrando C., Fanelli R., Bagnati R. Identification and measurement of illicit drugs and their metabolites in urban wastewater by liquid Chromatography−Tandem mass spectrometry. Anal. Chem. 2006;78(24):8421–8429. doi: 10.1021/ac061095b. [DOI] [PubMed] [Google Scholar]
- Catalá M., Domínguez-Morueco N., Migens A., Molina R., Martínez F., Valcárcel Y., Mastroianni N., López de Alda M., Barceló D., Segura Y. Elimination of drugs of abuse and their toxicity from natural waters by photo-Fenton treatment. Sci. Total Environ. 2015;520:198–205. doi: 10.1016/j.scitotenv.2015.03.042. [DOI] [PubMed] [Google Scholar]
- Causanilles A., Baz-Lomba J.A., Burgard D.A., Emke E., González-Mariño I., Krizman-Matasic I., Li A., Löve A.S.C., McCall A.K., Montes R., van Nuijs A.L.N., Ort C., Quintana J.B., Senta I., Terzic S., Hernandez F., de Voogt P., Bijlsma L. Improving wastewater-based epidemiology to estimate cannabis use: focus on the initial aspects of the analytical procedure. Anal. Chim. Acta. 2017;988:27–33. doi: 10.1016/j.aca.2017.08.011. [DOI] [PubMed] [Google Scholar]
- CBHSQ . In: Results from the 2018 National Survey on Drug Use and Health. Services. U S D o H H, editor. 2018. Key substance use and mental health indicators in the United States. [Google Scholar]
- Cerdá M., Wall M., Keyes K.M., Galea S., Hasin D. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120(1):22–27. doi: 10.1016/j.drugalcdep.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Venkatesan A.K., Halden R.U. Alcohol and nicotine consumption trends in three U.S. communities determined by wastewater-based epidemiology. Sci. Total Environ. 2019;656:174–183. doi: 10.1016/j.scitotenv.2018.11.350. [DOI] [PubMed] [Google Scholar]
- Chen S. South China Morning Post; Beijing: 2017. Green Gold: How China Quietly Grew into a Cannabis Superpower. [Google Scholar]
- Chiavola A., Tedesco P., Boni M.R. Fate of selected drugs in the wastewater treatment plants (WWTPs) for domestic sewage. Environ. Sci. Pollut. Control Ser. 2019;26(2):1113–1123. doi: 10.1007/s11356-017-9313-x. [DOI] [PubMed] [Google Scholar]
- Dargan P.I., Wood D.M. Recreational drug use in the Asia Pacific region: improvement in our understanding of the problem through the UNODC programmes. J. Med. Toxicol. 2012;8(3):295–299. doi: 10.1007/s13181-012-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. American Chemical Society; 2001. Pharmaceuticals and Care Products in the Environment; pp. 348–364. [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736:139631. doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault D.A., Néfau T., Levi Y., Karolak S. The removal of illicit drugs and morphine in two waste water treatment plants (WWTPs) under tropical conditions. Environ. Sci. Pollut. Control Ser. 2017;24(33):25645–25655. doi: 10.1007/s11356-015-6032-z. [DOI] [PubMed] [Google Scholar]
- Devault Damien A, Néfau Thomas, Pascaline Hélène, Karolak Sara, Levi Yves. First evaluation of illicit and licit drug consumption based on wastewater analysis in Fort de France urban area (Martinique, Caribbean), a transit area for drug smuggling. Sci. Total Environ. 2014;490:970–978. doi: 10.1016/j.scitotenv.2014.05.090. [DOI] [PubMed] [Google Scholar]
- du Plessis S.S., Agarwal A., Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015;32(11):1575–1588. doi: 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA . In: Trends and Developments. Addition. E M C f D.a.D., editor. Publications Office of the European Union; Lisbon: 2018. The European drug report 2018. [Google Scholar]
- Epa U. Interim Final; 1997. Ecological Risk Assessment Guidance for Superfund: Process for Designing and Conducting Ecological Risk Assessments. [Google Scholar]
- Epa U. 1997. Terms of environment: glossary, abbreviations and acronyms. [Google Scholar]
- Fedorova G., Randak T., Golovko O., Kodes V., Grabicova K., Grabic R. A passive sampling method for detecting analgesics, psycholeptics, antidepressants and illicit drugs in aquatic environments in the Czech Republic. Sci. Total Environ. 2014;487:681–687. doi: 10.1016/j.scitotenv.2013.12.091. [DOI] [PubMed] [Google Scholar]
- Gatidou G., Kinyua J., van Nuijs A.L.N., Gracia-Lor E., Castiglioni S., Covaci A., Stasinakis A.S. Drugs of abuse and alcohol consumption among different groups of population on the Greek Island of Lesvos through sewage-based epidemiology. Sci. Total Environ. 2016;563–564:633–640. doi: 10.1016/j.scitotenv.2016.04.130. [DOI] [PubMed] [Google Scholar]
- George-Cosh D. 2019. Global cannabis market will nearly triple to US$40. 6B by 2024: Study. [Google Scholar]
- Gerrity D., Trenholm R.A., Snyder S.A. Temporal variability of pharmaceuticals and illicit drugs in wastewater and the effects of a major sporting event. Water Res. 2011;45(17):5399–5411. doi: 10.1016/j.watres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- González-Mariño I., Quintana J.B., Rodríguez I., Cela R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. J. Chromatogr. A. 2010;1217(11):1748–1760. doi: 10.1016/j.chroma.2010.01.046. [DOI] [PubMed] [Google Scholar]
- González-Mariño I., Quintana J.B., Rodríguez I., González-Díez M., Cela R. Screening and selective quantification of illicit drugs in wastewater by mixed-mode solid-phase extraction and quadrupole-time-of-flight liquid chromatography–mass spectrometry. Anal. Chem. 2012;84(3):1708–1717. doi: 10.1021/ac202989e. [DOI] [PubMed] [Google Scholar]
- González-Mariño I., Rodríguez I., Quintana J.B., Cela R. Investigation of the transformation of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol during water chlorination by liquid chromatography–quadrupole-time-of-flight-mass spectrometry. J. Hazard Mater. 2013;261:628–636. doi: 10.1016/j.jhazmat.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Gracia-Lor E., Zuccato E., Castiglioni S. Refining correction factors for back-calculation of illicit drug use. Sci. Total Environ. 2016;573:1648–1659. doi: 10.1016/j.scitotenv.2016.09.179. [DOI] [PubMed] [Google Scholar]
- Hammell D.C., Zhang L.P., Ma F., Abshire S.M., McIlwrath S.L., Stinchcomb A.L., Westlund K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain. 2016;20(6):936–948. doi: 10.1002/ejp.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.H., Hur H.G., Kim S.D. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: occurrence and toxicity to Daphnia magna. Environ. Toxicol. Chem. 2006;25(1):265–271. doi: 10.1897/05-193r.1. [DOI] [PubMed] [Google Scholar]
- Hannaford A. British Broadcasting Corporation; 2019. What’s behind the Rise in Cannabis-Infused Products? [Google Scholar]
- Hartsel J.A., Eades J., Hickory B., Makriyannis A. In: Nutraceuticals. Gupta R.C., editor. Academic Press; Boston: 2016. pp. 735–754. [Google Scholar]
- Health Canada . In: Canadian Alcohol and Drug Use Monitoring Survey. Canada H., editor. 2012. [Google Scholar]
- Health Canada . In: Canadian Tobacco, Alcohol and Drugs Survey (CTADS): Summary of Results for 2017. Canada H., editor. 2017. [Google Scholar]
- Health Canada . In: Cannabidiol (CBD) Canada H., editor. Government of Canada; 2019. [Google Scholar]
- Hernández F., Bijlsma L., Sancho J.V., Díaz R., Ibáñez M. Rapid wide-scope screening of drugs of abuse, prescription drugs with potential for abuse and their metabolites in influent and effluent urban wastewater by ultrahigh pressure liquid chromatography–quadrupole-time-of-flight-mass spectrometry. Anal. Chim. Acta. 2011;684(1):96–106. doi: 10.1016/j.aca.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Heuett N.V., Ramirez C.E., Fernandez A., Gardinali P.R. Analysis of drugs of abuse by online SPE-LC high resolution mass spectrometry: communal assessment of consumption. Sci. Total Environ. 2015;511:319–330. doi: 10.1016/j.scitotenv.2014.12.043. [DOI] [PubMed] [Google Scholar]
- How Z.T., Busetti F., Linge K.L., Kristiana I., Joll C.A., Charrois J.W.A. Analysis of free amino acids in natural waters by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2014;1370:135–146. doi: 10.1016/j.chroma.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Huestis M.A. In: Cannabinoids. Pertwee R.G., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2005. pp. 657–690. [Google Scholar]
- Huestis M.A., ElSohly M., Nebro W., Barnes A., Gustafson R.A., Smith M.L. Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther. Drug Monit. 2006;28(4):540–544. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- Huestis M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanová L., Mackuľak T., Grabic R., Golovko O., Koba O., Staňová A.V., Szabová P., Grenčíková A., Bodík I. Pharmaceuticals and illicit drugs – a new threat to the application of sewage sludge in agriculture. Sci. Total Environ. 2018;634:606–615. doi: 10.1016/j.scitotenv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30(10):515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jacox A., Wetzel J., Cheng S.-Y., Concheiro M. Quantitative analysis of opioids and cannabinoids in wastewater samples. Forensic Sciences Research. 2017;2(1):18–25. doi: 10.1080/20961790.2016.1270812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Singh R. Microextraction techniques for analysis of cannabinoids. Trac. Trends Anal. Chem. 2016;80:156–166. [Google Scholar]
- Jmaiff Blackstock L.K., Wang W., Vemula S., Jaeger B.T., Li X.-F. Sweetened swimming pools and hot tubs. Environ. Sci. Technol. Lett. 2017;4(4):149–153. [Google Scholar]
- Jurado A., Mastroianni N., Vàzquez-Suñé E., Carrera J., Tubau I., Pujades E., Postigo C., de Alda M.L., Barceló D. Drugs of abuse in urban groundwater. A case study: Barcelona. Sci. Total Environ. 2012;424:280–288. doi: 10.1016/j.scitotenv.2012.02.074. [DOI] [PubMed] [Google Scholar]
- Keuten M.G.A., Peters M.C.F.M., Daanen H.A.M., de Kreuk M.K., Rietveld L.C., van Dijk J.C. Quantification of continual anthropogenic pollutants released in swimming pools. Water Res. 2014;53:259–270. doi: 10.1016/j.watres.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Khan K., Roy K. Ecotoxicological modelling of cosmetics for aquatic organisms: a QSTR approach. SAR QSAR Environ. Res. 2017;28(7):567–594. doi: 10.1080/1062936X.2017.1352621. [DOI] [PubMed] [Google Scholar]
- Khan U., Nicell J.A. Sewer epidemiology mass balances for assessing the illicit use of methamphetamine, amphetamine and tetrahydrocannabinol. Sci. Total Environ. 2012;421–422:144–162. doi: 10.1016/j.scitotenv.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Lai F.Y., Ort C., Gartner C., Carter S., Prichard J., Kirkbride P., Bruno R., Hall W., Eaglesham G., Mueller J.F. Refining the estimation of illicit drug consumptions from wastewater analysis: Co-analysis of prescription pharmaceuticals and uncertainty assessment. Water Res. 2011;45(15):4437–4448. doi: 10.1016/j.watres.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Oh S.M., Chung K.H. Estrogenic effects of marijuana smoke condensate and cannabinoid compounds. Toxicol. Appl. Pharmacol. 2006;214(3):270–278. doi: 10.1016/j.taap.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lu M.-C., Chen Y.Y., Chiou M.-R., Chen M.Y., Fan H.-J. Occurrence and treatment efficiency of pharmaceuticals in landfill leachates. Waste Manag. 2016;55:257–264. doi: 10.1016/j.wasman.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Mackie A.L., Park Y.R., Gagnon G.A. Chlorination kinetics of 11-Nor-9-carboxy-Δ9-tetrahydrocannabinol: effects of pH and humic acid. Environ. Sci. Technol. 2017;51(18):10711–10717. doi: 10.1021/acs.est.7b02234. [DOI] [PubMed] [Google Scholar]
- Mackuľak T., Birošová L., Bodík I., Grabic R., Takáčová A., Smolinská M., Hanusová A., Híveš J., Gál M. Zerovalent iron and iron(VI): effective means for the removal of psychoactive pharmaceuticals and illicit drugs from wastewaters. Sci. Total Environ. 2016;539:420–426. doi: 10.1016/j.scitotenv.2015.08.138. [DOI] [PubMed] [Google Scholar]
- Mackuľak T., Medvecká E., Vojs Staňová A., Brandeburová P., Grabic R., Golovko O., Marton M., Bodík I., Medveďová A., Gál M., Planý M., Kromka A., Špalková V., Škulcová A., Horáková I., Vojs M. Boron doped diamond electrode – the elimination of psychoactive drugs and resistant bacteria from wastewater. Vacuum. 2020;171:108957. [Google Scholar]
- Mackuľak T., Mosný M., Grabic R., Golovko O., Koba O., Birošová L. Fenton-like reaction: a possible way to efficiently remove illicit drugs and pharmaceuticals from wastewater. Environ. Toxicol. Pharmacol. 2015;39(2):483–488. doi: 10.1016/j.etap.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Mackuľak T., Škubák J., Grabic R., Ryba J., Birošová L., Fedorova G., Špalková V., Bodík I. National study of illicit drug use in Slovakia based on wastewater analysis. Sci. Total Environ. 2014;494–495:158–165. doi: 10.1016/j.scitotenv.2014.06.089. [DOI] [PubMed] [Google Scholar]
- Martínez Bueno M.J., Herrera S., Munaron D., Boillot C., Fenet H., Chiron S., Gómez E. POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Environ. Sci. Pollut. Control Ser. 2016;23(6):5019–5029. doi: 10.1007/s11356-014-3796-5. [DOI] [PubMed] [Google Scholar]
- Mastroianni N., Bleda M.J., López de Alda M., Barceló D. Occurrence of drugs of abuse in surface water from four Spanish river basins: spatial and temporal variations and environmental risk assessment. J. Hazard Mater. 2016;316:134–142. doi: 10.1016/j.jhazmat.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Mastroianni N., Postigo C., de Alda M.L., Barcelo D. Illicit and abused drugs in sewage sludge: method optimization and occurrence. J. Chromatogr. A. 2013;1322:29–37. doi: 10.1016/j.chroma.2013.10.078. [DOI] [PubMed] [Google Scholar]
- McCall A.-K., Bade R., Kinyua J., Lai F.Y., Thai P.K., Covaci A., Bijlsma L., van Nuijs A.L.N., Ort C. Critical review on the stability of illicit drugs in sewers and wastewater samples. Water Res. 2016;88:933–947. doi: 10.1016/j.watres.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Mendoza A., Rodríguez-Gil J.L., González-Alonso S., Mastroianni N., López de Alda M., Barceló D., Valcárcel Y. Drugs of abuse and benzodiazepines in the Madrid Region (Central Spain): seasonal variation in river waters, occurrence in tap water and potential environmental and human risk. Environ. Int. 2014;70:76–87. doi: 10.1016/j.envint.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Mendoza A., Zonja B., Mastroianni N., Negreira N., López de Alda M., Pérez S., Barceló D., Gil A., Valcárcel Y. Drugs of abuse, cytostatic drugs and iodinated contrast media in tap water from the Madrid region (central Spain):A case study to analyse their occurrence and human health risk characterization. Environ. Int. 2016;86:107–118. doi: 10.1016/j.envint.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Moudgal C.J., Lipscomb J.C., Bruce R.M. Potential health effects of drinking water disinfection by-products using quantitative structure toxicity relationship. Toxicology. 2000;147(2):109–131. doi: 10.1016/s0300-483x(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Musshoff F., Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther. Drug Monit. 2006;28(2):155–163. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- Nefau T., Karolak S., Castillo L., Boireau V., Levi Y. Presence of illicit drugs and metabolites in influents and effluents of 25 sewage water treatment plants and map of drug consumption in France. Sci. Total Environ. 2013;461–462:712–722. doi: 10.1016/j.scitotenv.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Östman Marcus, Fick Jerker, Näsström Elin, Lindberg Richard H. A snapshot of illicit drug use in Sweden acquired through sewagewater analysis. Sci. Total Environ. 2014;472:862–871. doi: 10.1016/j.scitotenv.2013.11.081. [DOI] [PubMed] [Google Scholar]
- Palardy A., Gagné J.-P., Tremblay L. Presence of illicit drugs and pharmaceutical residues in the wastewaters of an eastern Canadian city. Journal of xenobiotics. 2015;5(2):5773. doi: 10.4081/xeno.2015.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.R., Mackie A.L., Gagnon G.A. A critical review of the occurrence, detection, and treatment of Δ9-tetrahydrocannabinol in aquatic environments. Environ. Rev. 2016;25(3):255–268. [Google Scholar]
- Parolini M., Binelli A. Oxidative and genetic responses induced by Δ-9-tetrahydrocannabinol (Δ-9-THC) to Dreissena polymorpha. Sci. Total Environ. 2014;468–469:68–76. doi: 10.1016/j.scitotenv.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Parolini M., Castiglioni S., Magni S., Della Torre C., Binelli A. Increase in cannabis use may indirectly affect the health status of a freshwater species. Environ. Toxicol. Chem. 2017;36(2):472–479. doi: 10.1002/etc.3575. [DOI] [PubMed] [Google Scholar]
- Pedrouzo M., Borrull F., Pocurull E., Marcé R.M. Drugs of abuse and their metabolites in waste and surface waters by liquid chromatography-tandem mass spectrometry. J. Separ. Sci. 2011;34(10):1091–1101. doi: 10.1002/jssc.201100043. [DOI] [PubMed] [Google Scholar]
- Postigo C., de Alda M.L., Barceló D. Evaluation of drugs of abuse use and trends in a prison through wastewater analysis. Environ. Int. 2011;37(1):49–55. doi: 10.1016/j.envint.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Postigo C., Lopez de Alda M.J., Barceló D. Fully automated determination in the low nanogram per liter level of different classes of drugs of abuse in sewage water by on-line solid-phase extraction-liquid Chromatography−Electrospray-tandem mass spectrometry. Anal. Chem. 2008;80(9):3123–3134. doi: 10.1021/ac702060j. [DOI] [PubMed] [Google Scholar]
- Postigo C., López de Alda M.J., Barceló D. Drugs of abuse and their metabolites in the Ebro River basin: occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environ. Int. 2010;36(1):75–84. doi: 10.1016/j.envint.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Racamonde I., Villaverde-de-Sáa E., Rodil R., Quintana J.B., Cela R. Determination of Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in water samples by solid-phase microextraction with on-fiber derivatization and gas chromatography–mass spectrometry. J. Chromatogr. A. 2012;1245:167–174. doi: 10.1016/j.chroma.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Roth K.D.W., Siegel N.A., Johnson R.W., Jr., Litauszki L., Salvati L., Jr., Harrington C.A., Wray L.K. Investigation of the effects of solution composition and container material type on the loss of 11-nor-Δ9-THC-9-Carboxylic acid. J. Anal. Toxicol. 1996;20(5):291–300. doi: 10.1093/jat/20.5.291. [DOI] [PubMed] [Google Scholar]
- Russo E.B. Cannabinoids in the management of difficult to treat pain. Therapeut. Clin. Risk Manag. 2008;4(1):245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S˙liwka-Kaszyńska M., Kot-Wasik A., Namieśnik J. Preservation and storage of water samples. Crit. Rev. Environ. Sci. Technol. 2003;33(1):31–44. [Google Scholar]
- Saleh N.B., Apul O., Karanfil T. The genesis of a critical environmental concern: cannabinoids in our water systems. Environ. Sci. Technol. 2019;53(4):1746–1747. doi: 10.1021/acs.est.8b06999. [DOI] [PubMed] [Google Scholar]
- Schluttenhofer C., Yuan L. Challenges towards revitalizing hemp: a multifaceted crop. Trends Plant Sci. 2017;22(11):917–929. doi: 10.1016/j.tplants.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Senta I., Krizman I., Ahel M., Terzic S. Integrated procedure for multiresidue analysis of dissolved and particulate drugs in municipal wastewater by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2013;405(10):3255–3268. doi: 10.1007/s00216-013-6720-9. [DOI] [PubMed] [Google Scholar]
- Senta I., Krizman I., Ahel M., Terzic S. Assessment of stability of drug biomarkers in municipal wastewater as a factor influencing the estimation of drug consumption using sewage epidemiology. Sci. Total Environ. 2014;487:659–665. doi: 10.1016/j.scitotenv.2013.12.054. [DOI] [PubMed] [Google Scholar]
- Sharma P., Murthy P., Bharath M.M.S. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran. J. Psychiatry. 2012;7(4):149–156. [PMC free article] [PubMed] [Google Scholar]
- STATCAN . In: National Cannabis Survey. 1st quarter. Canada S., editor. 2019. p. 2019. [Google Scholar]
- Stewart A.M., Kalueff A.V. The behavioral effects of acute Δ9-tetrahydrocannabinol and heroin (diacetylmorphine) exposure in adult zebrafish. Brain Res. 2014;1543:109–119. doi: 10.1016/j.brainres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Subramaniam V. National Post; 2019. Why Business Is Booming for Cannabis Extraction Companies, Despite the Supply Shortages. [Google Scholar]
- The Canadian Press . Canadian Broadcasting Corporation; Canada: 2019. Cannabis Edibles and Infused Products Could Be $2.7B Market, Deloitte Estimates. [Google Scholar]
- The Economist . The Economist; Kuming: 2019. Industrial cannabis is booming in China. [Google Scholar]
- Terzic S., Senta I., Ahel M. Illicit drugs in wastewater of the city of Zagreb (Croatia) – estimation of drug abuse in a transition country. Environ. Pollut. 2010;158(8):2686–2693. doi: 10.1016/j.envpol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- UNODC . United Nations Office on Drugs and Crime (UNODC); Vienna: 2019. World Drug Report 2019. [Google Scholar]
- Upland Agricultural Consulting . 2019. Report to Government of British Columbia Canada ’Commercial Cannabis Production in British Columbia: Best Available Control Technologies and Regulatory Oversight of Environmental Considerations British Columbia. [Google Scholar]
- USFDA . US Food and Drug Administation; 2018. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. [Google Scholar]
- van Nuijs A.L.N., Gheorghe A., Jorens P.G., Maudens K., Neels H., Covaci A. Optimization, validation, and the application of liquid chromatography-tandem mass spectrometry for the analysis of new drugs of abuse in wastewater. Drug Test. Anal. 2014;6(7–8):861–867. doi: 10.1002/dta.1460. [DOI] [PubMed] [Google Scholar]
- Vazquez-Roig P., Andreu V., Blasco C., Picó Y. SPE and LC-MS/MS determination of 14 illicit drugs in surface waters from the Natural Park of L’Albufera (València, Spain) Anal. Bioanal. Chem. 2010;397(7):2851–2864. doi: 10.1007/s00216-010-3720-x. [DOI] [PubMed] [Google Scholar]
- Wallace A. CNN; San Francisco: 2019. CBD Product Sales Are Booming. Now the FDA Needs to Weigh in. [Google Scholar]
- Weng S., Sun P., Ben W., Huang C.-H., Lee L.T., Blatchley E.R., Iii The presence of pharmaceuticals and personal care products in swimming pools. Environ. Sci. Technol. Lett. 2014;1(12):495–498. [Google Scholar]
- Werschler T., Brennan A. In: Wastewater-based Estimates of Cannabis and Drug Use in Canada: Pilot Test Detailed Results. Canada S., editor. 2019. [Google Scholar]
- Yao Bo, Lian Lushi, Pang Weihai, Yin Daqiang, Chan Shen-An, Song Weihua. Determination of illicit drugs in aqueous environmental samples by online solid-phase extraction coupled to liquid chromatography–tandem mass spectrometry. Chemosphere. 2016;160:208–215. doi: 10.1016/j.chemosphere.2016.06.092. [DOI] [PubMed] [Google Scholar]
- Yousefinejad S., Hemmateenejad B. Chemometrics tools in QSAR/QSPR studies: a historical perspective. Chemometr. Intell. Lab. Syst. 2015;149:177–204. [Google Scholar]
- Zenobio J.E., Sanchez B.C., Leet J.K., Archuleta L.C., Sepúlveda M.S. Presence and effects of pharmaceutical and personal care products on the Baca National Wildlife Refuge, Colorado. Chemosphere. 2015;120:750–755. doi: 10.1016/j.chemosphere.2014.10.050. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang R., Li P., Ren Y., Gao J., Mueller J.F., Thai P.K. Temporal profile of illicit drug consumption in Guangzhou, China monitored by wastewater-based epidemiology. Environ. Sci. Pollut. Control Ser. 2019;26(23):23593–23602. doi: 10.1007/s11356-019-05575-3. [DOI] [PubMed] [Google Scholar]
- Zuccato E., Castiglioni S., Bagnati R., Chiabrando C., Grassi P., Fanelli R. Illicit drugs, a novel group of environmental contaminants. Water Res. 2008;42(4):961–968. doi: 10.1016/j.watres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Zuccato E., Chiabrando C., Castiglioni S., Calamari D., Bagnati R., Schiarea S., Fanelli R. Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environ. Health. 2005;4(1):14. doi: 10.1186/1476-069X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiener C., Richardson S.D., De Marini D.M., Grummt T., Glauner T., Frimmel F.H. Drowning in disinfection byproducts? Assessing swimming pool water. Environ. Sci. Technol. 2007;41(2):363–372. doi: 10.1021/es062367v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.