Dear Editor,
Coronavirus disease 2019 (COVID-19) is a newly recognized systemic condition due to the SARS-CoV-2 infection. As rheumatologists, we often found analogies between vascular inflammation, endothelial dysfunction and lung manifestations of COVID-19 and inflammatory autoimmune diseases. In the management of COVID-19 infection seems sensible to distinguish viral and host inflammatory phase since about one patient in twenty develops an uncontrolled inflammatory response with multiple organ failure. Fever, cough, dyspnoea, fatigue and myalgia are the most common symptoms and high creatine-phosphokinase, and inflammatory markers (ferritin, C-reactive protein, D-dimers, interleukin-6) are associated with a poor prognosis [1]. In this regard, several pieces of evidence point toward a central role of massive and dysfunctional endothelial activation, leading to diffuse thrombotic disease, both as a specific effect of SARS-CoV-2 and as a consequence of systemic inflammation. The so-called “cytokine storm” is the production of large amounts of mediators of inflammation that can be triggered by SARS-CoV-2 infection [2] but is also described in autoimmune and autoinflammatory diseases. Vasculopathy and thrombotic manifestations seem to characterize the more aggressive cases of COVID-19 infection [3], especially in the lungs and skin [4]. In many autoimmune diseases vascular abnormalities are associated with systemic inflammation, lung disease [5] and heart damage [6], but the clinical course is often chronic. Intriguingly, in terms of clinical picture, some epidemiologic aspects, biomarkers and pathological aspects of tissue damage, COVID-19 shows many similarities with the subset of dermatomyositis associated with anti-melanoma differentiation-associated gene 5 (MDA5) (Table 1 ). Anti-MDA5 dermatomyositis is a serologically-defined subtype of dermatomyositis that is characterized by a high risk of rapidly progressive interstitial lung disease, little evidence of clinical muscle inflammation, typical rashes and high prevalence of systemic symptoms. Viral infections have been considered as a possible trigger to the uncontrolled innate and adaptive immune response of anti-MDA5 dermatomyositis. Anti-MDA5 dermatomyositis has a poor prognosis but low recurrence rate in survivors and, while it is a very rare condition globally, it is reported much more frequently in East Asia, suggesting a genetic or environmental modulation of the onset of the disease. Even if direct evidence of a specific viral pathogen is lacking, this hypothesis is supported by the recognition of IFN induced with helicase C domain protein 1 (IFIH1) gene as a target of anti-MDA5 antibodies [7]. IFIH1 is indeed required for the normal immune response against some classes of viruses, including coronavirus, promoting the production of cytokines such as IFNγ, TNF-α, IL-1β, IL-6 and IL-18 and stimulation of TH1 cells and macrophages. In case of a defective anti-inflammatory counterbalance, the result is the development of a cytokine storm with the overexpression of pro-inflammatory mediators, sustaining rapidly progressive forms of interstitial lung disease [8]. Not surprisingly, systemic symptoms like fever are particularly frequent in these patients compared to the ones with other connective tissue diseases, and hyperferritinemia is an almost invariable finding with very high levels associated with a more severe disease course and a poor prognosis [9]. Manifestations of hypercoagulability with various degrees of thromboembolism, are also a recognized risk in inflammatory myopathies and thrombotic alterations of small and medium-sized arteries represent a histopathological hallmark in skin biopsies [10]. Radiologic appearance on chest CT of anti-MDA5 disease is very close to the one of COVID-19 (Fig. 1 ), with a bilateral distribution of ground-glass opacities with or without consolidation in posterior and peripheral lungs and - in a substantially different way from other myositis related interstitial lung disease - with prevalent peribronchovascular consolidations [11,12]. Spontaneous pneumomediastinum is not a rare finding in both severe COVID-19 and anti-MDA5 positive dermatomyositis related interstitial lung disease while is less common in anti-MDA5 negative myositis. In the above depicted context, anti-MDA5 antibodies formation may be a simple epiphenomenon due to antigen release from infected or damaged cells or may have a pathogenetic role, directly promoting tissue damage. Consistently with this last speculation, anti-MDA5 titre correlates with disease activity, prognosis and therapeutic response[13], and B-cells depletion treatment has shown to be useful in refractory cases. The significant role of humoral immunity in anti-MDA5 myositis could appear distinctive from COVID-19. To date, a pathogenetic effect of antibodies targeted against SARS-CoV-2 cannot be excluded since their neutralizing effect is still debated and the anti-IgG response has been associated with disease severity and higher proinflammatory cytokines level[14]. The possibility of secondary antibody-mediated organ damage would represent another common point between COVID-19 and anti-MDA5 dermatomyositis, at least in the subgroup of patients who develop an uncontrolled immunoinflammatory response after SARS-CoV-2 infection. Notably, a cross-reactivity between anti-SARS-CoV-1 (a form of coronavirus close to the one responsible of COVID-19) antibodies and lung epithelial cells has been described[15]. The analogies between these two conditions allow speculations about the rationale of targeted therapies with promising results in anti-MDA5 positive interstitial lung disease in COVID-19. High dose corticosteroids, intravenous human immunoglobulin, JAK-inhibitors and T-cell modulating drugs reported efficacy in small case series and are currently under investigation in clinical trials for the treatment of COVID-19. A therapeutic role of direct B cells depletion seems unlikely in COVID-19 due to their crucial protective role against viral infections, unless a direct pathogenic effect of SARS-CoV-2 induced antibodies in severe COVID-19 systemic disease is proven. Other pharmacological strategies, including inhibition of IL-6 (tocilizumab, sarilumab, siltuximab and clazakizumab), IL-1 (anakinra and canakinumab), anti-GM-CSF (gimsilumab) or IFNγ (emapalumab) - rarely or neither used in the treatment of anti-MDA5 interstitial lung disease - are currently being tested for COVID-19 treatment, given the crucial role of these cytokines in the disease, but with understandable concerns on the possible interference with the host response to the virus. In COVID-19, appears crucial that a structured approach to clinical phenotyping is undertaken, in order to distinguish the phase where the viral pathogenicity is dominant by the phase in which host inflammatory response prevails.
Table 1.
Comparison between COVID-19, anti-MDA5 dermatomyositis and classic dermatomyositis.
| COVID-19 | Anti-MDA5 dermatomyositis with ILD | Classic dermatomyositis with ILD | ||
|---|---|---|---|---|
| Epidemiology | Prevalence | More than two million cases globally | Rare | Rare |
| Geographic clusters | First reports in China, (now in all continents) | Mainly reported in east Asia | None | |
| Sex predominance | None | None | Female predominance | |
| Natural history | Severe and rapidly progressive disease in about 20% of cases | Rapidly progressive | Slowly progressive | |
| Recurrence | Unknown | Rare | Relapsing-remitting | |
| Mortality rate | High | Very high | High | |
| Pathogenesis | Association with viral infection | Proven association with SARS-CoV-2 infection | Possible trigger of picoRNA- or other viruses | Debated triggering role of viruses |
| Inflammatory state | High grade systemic inflammation | High grade systemic inflammation | Low-moderate grade systemic inflammation | |
| Prothrombotic state and endothelial dysfunction | Hallmark of the disease | Hallmark of the disease | Hallmark of the disease | |
| Autoantibody mediated injury | Possible cross-reactivity of induced antibodies | Postulated direct role of anti-MDA5 | Debated direct pathogenetic role | |
| Lung histopathology | DAD and microangiopathy | DAD and microangiopathy | NSIP and OP | |
| Clinical manifestations | Lung disease | Almost always present | Almost always present | Common |
| Myositis | Mild-absent | Mild-absent | Almost always present | |
| Skin and peripheral vascular involvement | Common | Almost always present | Almost always present | |
| Fever | Almost always present | Very common | Uncommon | |
| Association with cancer | Absent | Rare | Possible | |
| Diagnosis and monitoring | CK | Mild-moderate high | Mild-moderate high | Very high |
| Ferritin | High | High | Normal or slightly increased | |
| Lymphocytes | Commonly low | Occasionally low | Occasionally low | |
| CRP | Very high | Very high | Usually normal | |
| ESR | High | High | High | |
| Antinuclear Antibodies | Unknown | Negative | Usually positive | |
| Antiphospholipid antibodies | Possibly positive | Possibly positive | Possibly positive | |
| CT scan of the chest | Bilateral GGO or consolidation in posterior and peripheral lungs | Bilateral GGO or consolidation in posterior and peripheral lungs | Bilateral peribronchovascular GGO or consolidation | |
| Nailfold capillaroscopy | Unknown | Enlarged capillaries, hemorragias, neovascularization | Enlarged capillaries, hemorragias, neovascularization | |
| Treatment | Corticosteroids | Under investigation | Commonly used | Commonly used |
| Anti-IL6 | Under investigation | Unknown efficacy | Unknown efficacy | |
| Anti-IL1 | Under investigation | Unknown efficacy | Unknown efficacy | |
| JAK-inhibitors | Under investigation | Under investigation | Under investigation | |
| Anti-CD20 | Not suitable | Rescue therapy | Rescue therapy | |
ILD interstitial lung disease, DAD diffuse alveolar damage, NSIP nonspecific interstitial pneumonia, OP organizing pneumonia, CK creatine kinase, CRP C reactive protein, ESR erythrocyte sedimentation rate, CT computed tomography, GGO ground glass opacities, IL interleukin, JAK Janus kinase.
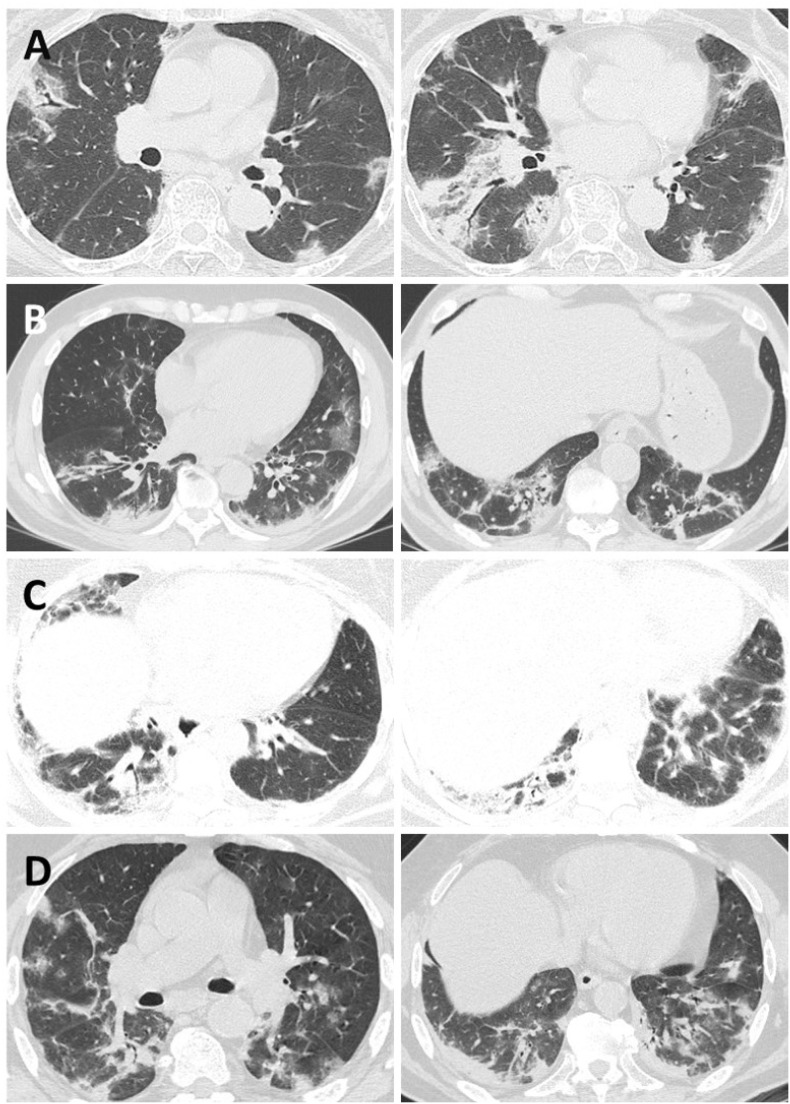
Fig. 1.
Similarities in CT scans findings of two patients with anti-MDA5 dermatomyositis (A*, C**) and two patients with COVID-19 (B, D).
The images show bilateral subpleural areas of patchy ground glass opacities and consolidation accompanied by traction bronchiectasis and perilobular linear opacities.
*Courtesy of Prof. Noriho Sakamoto, Nagasaki University Graduate School of Biomedical Sciences.
**Courtesy of Prof. Juan González-Moreno, Internal Medicine Department, Hospital Son Llàtzer, Palma.
In conclusion - in early phases of COVID-19 - the eradication of SARS-CoV-2 should be the goal to prevent the subsequent inflammatory storm while in the established phases of the inflammatory response the aim should be to extinguish effectively the inflammatory-immune response. In a context where the key therapeutic targets have to be fully understood, we believe that looking at the experience with autoimmune diseases of rheumatological interest, such as anti-MDA5 related lung disease could guide and stimulate the development of useful therapeutic strategies. Moreover, even if the long-term impact of COVID-19 is not yet established, as rheumatologist we deeply expect that the medical efforts to extinguish the burden of inflammation in severe COVID-19 as soon as this occurs, may help to contain the number of patients that will develop chronic damage and functional impairment, especially in the respiratory compartment.
Funding info
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Ethical approval information
Non applicable.
Data sharing statement
There are no data in this work (letter to the Editor).
Contributorship
All the authors gave substantial contributions to the conception or design of the work, acquisition, analysis or interpretation of data, drafting the work or revising it critically for important intellectual content and final approval of the version published.
Declaration of Competing Interest
There are no competing interests for any author.
Acknowledgements
We thank Prof. Noriho Sakamoto from Nagasaki University Graduate School of Biomedical Sciences and Prof. Juan González-Moreno from Internal Medicine Department, Hospital Son Llàtzer, Palma for the permission to reprint the CT images of anti-MDA5 dermatomyositis.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;(102537) doi: 10.1016/j.autrev.2020.102537. published online ahead of print, 2020 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox E.S., Akmatbekov A., Harbert J.L. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16387. published online ahead of print, 2020 Mar 26. [DOI] [PubMed] [Google Scholar]
- 5.Marigliano B., Soriano A., Margiotta D., Vadacca M., Afeltra A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun Rev. 2013;12(11):1076–1084. doi: 10.1016/j.autrev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzis E., Gremese E., Bosello S., Nurmohamed M.T., Sinagra G., Ferraccioli G. Microvascular heart involvement in systemic autoimmune diseases: the purinergic pathway and therapeutic insights from the biology of the diseases. Autoimmun Rev. 2019;18(4):317–324. doi: 10.1016/j.autrev.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Sato S., Hoshino K., Satoh T. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60(7):2193–2200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 8.Gono T., Kaneko H., Kawaguchi Y. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford) 2014;53(12):2196–2203. doi: 10.1093/rheumatology/keu258. [DOI] [PubMed] [Google Scholar]
- 9.Fujiki Y., Kotani T., Isoda K. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28(1):133–140. doi: 10.1080/14397595.2017.1318468. [DOI] [PubMed] [Google Scholar]
- 10.Pau-Charles I., Moreno P.J., Ortiz-Ibáñez K. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28(8):1097–1102. doi: 10.1111/jdv.12300. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto N., Ishimoto H., Nakashima S. Clinical features of anti-MDA5 antibody-positive rapidly progressive interstitial lung disease without signs of dermatomyositis. Intern Med. 2019;58(6):837–841. doi: 10.2169/internalmedicine.1516-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Moreno J., Raya-Cruz M., Losada-Lopez I., Cacheda A.P., Oliver C., Colom B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int. 2018;38(7):1293–1296. doi: 10.1007/s00296-018-3991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato S., Kuwana M., Fujita T. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23(3):496–502. doi: 10.1007/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020:ciaa344. doi: 10.1093/cid/ciaa344. published online ahead of print, 2020 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y.S., Lin C.F., Fang Y.T. Antibody to severe acute respiratory syndrome (SARS)-associated coronavirus spike protein domain 2 cross-reacts with lung epithelial cells and causes cytotoxicity. Clin Exp Immunol. 2005;141(3):500–508. doi: 10.1111/j.1365-2249.2005.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]