N
α,Nτ-Di-4-trifluorobenzenesulfonyl histamine inhibits α-glucosidase in vitro reversibly and selectively with a Ki value of 11.6 μM, and shows an in vivo hypoglycemic effect in mice.
N
α,Nτ-Di-4-trifluorobenzenesulfonyl histamine inhibits α-glucosidase in vitro reversibly and selectively with a Ki value of 11.6 μM, and shows an in vivo hypoglycemic effect in mice.
Abstract
A series of simple N-arylbenzenesulfonyl histamine derivatives were prepared and screened against α-glucosidase. Inhibition was in the micromolar range for several Nα,Nτ-di-arylsulfonyl compounds, with Nα,Nτ-di-4-trifluorobenzenesulfonyl histamine (IId) being the best inhibitor. Compound IId is a reversible and competitive α-glucosidase inhibitor, and presented good selectivity with respect to other target enzymes, including β-glucosidase and α-amylase, and interesting predicted physicochemical properties. Docking studies have been run to postulate ligand–enzyme interactions to account for the experimental results. In vivo, compound IId produced a similar hypoglycemic effect to acarbose with half of its dose.
Introduction
Diabetes mellitus (DM) is one of the most widespread metabolic disorders in the world.1 At present, it affects 1 in 11 adults (20–79 years old) (425 million), and it is estimated that by 2045 it will affect 1 in 10 (around 629 million).2 DM is caused by defects in insulin secretion (type 1) or insulin action (type 2).3 The disease is delineated by hyperglycemia associated with various complications such as cardiac and vascular diseases, thrombosis, cerebral and renal disorders, retinopathy and neuropathy.4,5 In humans, glucosidases are a group of enzymes that play a crucial role in carbohydrate metabolism by catalyzing the cleavage of glycosidic linkages of oligosaccharides or glycoconjugates to release monosaccharides.6 α-Glucosidase (α-Glc) is a member of this family and a key digestive enzyme. It is the most important isomaltase located in the mucous membrane of the small intestine where it hydrolyzes the non-reducing α-(1–4) glycosidic bonds. In this way, this enzyme directly controls the release of absorbable glucose in the intestine. The use of α-Glc inhibitors to control the increase in postprandial glucose level is an effective strategy for type 2 diabetes mellitus (T2DM) treatment.7
Nowadays, this T2DM management strategy is confined to three molecules: acarbose, miglitol, and voglibose. With more than 10 years in the market, these molecules are now devalued due to their efficiency problems and undesirable side effects.8 However, due to their mechanism of action, they remain a valid option for pharmacotherapy.9,10 In addition, since these drugs are poorly absorbed at the intestinal level, their adverse effects are restricted to the gastrointestinal tract (flatulence, diarrhea, and abdominal pain), preventing significant systemic effects such as hypoglycemia due to an abrupt increase in insulin release.8 Some of their gastrointestinal side effects are associated with concomitant inhibition of α-amylase (α-Amy) and the consequent accumulation of undigested carbohydrates in the large intestine.11 Therefore, specific α-Glc inhibitors may provide antidiabetic effects with fewer gastrointestinal side effects than some currently available inhibitors.12
The development of α-Glc inhibitors has attracted significant scientific attention.8,13 In the study of Ghani et al.,8 several chemotypes that inhibit α-Glc have been reported. Most research has involved sugar-mimetics14,15 that block the enzyme action by imitating its natural substrate. In particular, thiosugars like salacinol, kotalanol and their derivatives and C-branched iminosugar series have shown interesting inhibition properties.16–18 Some of these compounds show in vivo effects similar to reference drugs (miglitol or acarbose).16–18
Besides sugar-mimetics, some terpenes19 and several sulfur, nitrogen,20,21 and oxygen containing heterocyclic compounds have been reported as α-Glc inhibitors.22 Within the nitrogenated heterocycles, a few imidazole containing compounds have been studied, in particular benzimidazoles and 2,4,5-trisubstituted imidazole hybrids with pyrazoles, hydrazones and triazoles.23–27 Compounds with substitution on the nitrogen atoms of the imidazole ring are very rare in relation to this biological target.28,29 However, it has been observed that the generation of sulfonamides on the heterocyclic nitrogen atom of alkaloids30,31 and other amine compounds32–35 can increase the α-Glc inhibitory activity. This is even more noticeable when fluorinated arylsulfonyl chlorides are used in derivatization.30,35 These structural characteristics (imidazoles, sulfonamides and fluorine) have never been combined in molecules with α-Glc inhibition potential until today.
In the last few years, we have introduced chemically engineered extracts as an alternative source of bioactive compounds.36–38 As a result of the chemical diversification of an Urtica urens L. crude extract with benzenesulfonyl chloride, we identified two histamine sulfonamides (Scheme 1, Ia and IIa) that showed interesting inhibitory activity for the β-glucosidase (β-Glc) enzyme.39,40 This finding inspired the preparation of a series of Nα-arylsulfonyl and Nα,Nτ-di-arylsulfonyl histamine derivatives and their evaluation as β-Glc inhibitors40 leading to the discovery of Nα-4-fluorobenzenesulfonyl histamine as a selective, competitive β-Glc inhibitor.40
Scheme 1. Chemical structures of type I, II and III compounds. Synthesis of type III compounds. (a) Arylsulfonyl chloride, ACN, DMAP, Et3N, rt, overnight (yield: 31%, 72%, and 60% for IIIc, IIId, and IIIe, respectively).
Here we report the preparation of new analogues of histamine containing three sulfonyl moieties and the in vitro α-Glc inhibitory evaluation of the series of Nα-arylsulfonyl, Nα,Nτ-di-arylsulfonyl, and Nα,Nα,Nτ-tri-arylsulfonyl histamine derivatives (compounds of types I, II and III, Scheme 1). This screening led to the identification of Nα,Nτ-di-4-(trifluoromethyl)benzenesulfonyl histamine (IId), a selective reversible inhibitor with a Ki value significantly lower than that of acarbose. Binding interactions with the enzyme were studied by computational modeling and molecular docking. Finally, compound IId was able to decrease the post prandial glucose levels in vivo.
Results and discussion
α-Glc inhibition properties
Sulfonamides have been a leading constituent in the molecular scaffold of a large number of biologically active molecules.41 In a recent report, some Nα-arylsulfonyl histamines (type I compounds, Scheme 1) showed interesting and selective inhibition properties for β-Glc (almonds).40 These compounds showed inhibitory properties similar to those of the reference inhibitor, 1-deoxynojirimycin (1-DNJ), and in contrast, Nα,Nτ-di-arylbenzenesulfonyl derivatives (type II compounds, Scheme 1) did not produce significant inhibition of this enzyme.
Unexpectedly, the opposite outcome was observed for α-Glc inhibition. When tested at 100 μM, the concentration similar to the IC50 value of the reference inhibitor acarbose,42 type I compounds produced inhibition values below 50% (Fig. 1, bottom). In contrast, at the same concentration, several type II compounds showed inhibition values higher than 50% (Fig. 1, top). According to these results, the presence of an arylsulfonyl substituent in the imidazole ring increases the α-Glc inhibitory properties.
Fig. 1. % α-Glc inhibition of 100 μM type I (bottom) and II (top) histamine derivatives. Data are mean ± SD of the experiment conducted in triplicate. *Turbidity was observed at the tested concentration.
The series IIa–j were tested at different concentrations to determine their IC50 values, showing good to excellent α-Glc inhibition properties (Table 1). Two library members, IId and IIe (Fig. 2), presented IC50 values of 10.38 and 16.31 μM, respectively, around one order of magnitude below that of acarbose (Table 1).
Table 1. In vitro α-Glu inhibitory activity of Nα,Nτ-di-arylsulfonyl histamine derivatives. Data are mean ± SD of the experiment conducted in triplicate.
| Compound | IC50 (μM) |
| IIa | >200 |
| IIb | >200 |
| IIc | >200 |
| IId | 10.38 ± 1.05 |
| IIe | 16.31 ± 1.21 |
| IIf | 53.49 ± 1.33 |
| IIg | 60.88 ± 1.04 |
| IIh | 66.94 ± 1.08 |
| IIi | 67.07 ± 1.05 |
| IIj | 88.32 ± 1.07 |
| Acarbose | 122.70 ± 1.06 |
Fig. 2. Structure of the two best α-Glu inhibitors of the library, IId and IIe.
Inhibition results suggest a significant influence of the substituent present at the aromatic moiety. Compound IId, with a p-CF3 substituent, showed the best inhibitory effect. Removal of the CF3 substituent or its replacement with a p-CH3 or a p-fluorine atom led to inactive compounds IIa–c (IC50 higher than 200 μM). Interestingly, when CF3 is removed, and a CH3 group and a fluorine atom are introduced in positions 5 and 2, respectively, the second compound of the series is obtained (IIe). Other substitutions with chlorine, fluorine, CF3 and –OCH3 in different positions of the aromatic rings led to compounds with intermediate inhibition properties (IC50 values between 53 and 88 μM, IIf–j).
The differences observed in α-Glc inhibition between type I and type II series indicate that the interaction with the enzyme can be strongly influenced by N-arylsulfonylation. Introduction of a third arylsulfonyl moiety into the Nα of the two best inhibitors (IId and IIe) and the inactive IIc led to compounds IIIc–e. These type III compounds were prepared from the corresponding type II compounds (IIc–e) in the presence of Et3N/DMAP (Scheme 1).
For these three examples, α-Glc inhibition evaluation showed that this transformation did not improve the activity: IIIc is inactive (IC50 > 200 μM, the same as its precursor), IIId showed an IC50 value of 20.23 μM that is interesting but higher than that of its precursor, and IIIe (IC50 > 200 μM) did not show the inhibition properties displayed by its precursor. However, it must be noted that the number of compounds tested is too low to establish any SAR thus type III compounds cannot be discarded as potential inhibitors.
The physicochemical properties of compounds IId, IIe and IIId were studied using the online tool SwissADME.43 Predictions for passive human gastrointestinal (GI) absorption were extracted from the readout of the BOILED-Egg model.44 According to this model, IId and IIe would be poorly absorbed at the GI level (Fig. S1†) suggesting that they could exert α-Glc inhibition in the intestinal lumen, without causing undesirable systemic effects. IIId showed similar properties; however its predicted water solubility was very poor (Table S1†).
Target promiscuity evaluation
The two most promising compounds, IIe and IId, did not show PAINS alerts (pan-assay interference compounds alerts) indicating a low likelihood of being anomalous screening actives. Since this evaluation cannot be regarded as a black-and-white issue,45 target promiscuity for both compounds was also tested with different enzymes: two oxidases, xanthine oxidase (XO) and tyrosinase (Tyrase), and hydrolase acetyl cholinesterase (AChE). In each case, IId and IIe were tested at a concentration similar to the IC50 value of a reference inhibitor for the corresponding enzyme. No inhibition was detected for XO or Tyrase, whereas very low inhibition was observed for AChE (2.31% and 7.14%, respectively) (Fig. 3).
Fig. 3. Comparison of the inhibitory potency (% inhibition) of compounds IId and IIevs. the reference inhibitor (RI) for each enzyme tested. a Allopurinol for XO (IC50 2.52 μM), kojic acid for Tyrase (IC50 40.00 μM), eserine for AChE (IC50 1.17 μM), 1-DNJ for β-Glc (IC50 65.18 μM), galacto-DNJ for β-Gal (IC50 90 μM),46 acarbose for α-Amy (IC50 197.4 μM) and α-Glc (IC50 127.70 μM). bCompounds IId and IIe were tested at 3 μM in the XO assay, at 40 μM in the Tyrase assay, 1.5 μM in the AChE assay, 65 μM in the β-Glc assay, 100 μM in the β-Gal assay, 200 μM in the α-Amy assay and 122 μM in the α-Glc assay. *No inhibition was observed between 100 and 2500 μM. Data are mean ± SD of the experiment done in triplicate.
In addition, the effect of IId and IIe on the activity of other glycosidases was also evaluated. Whilst 79.86% or 72.19% α-Glc inhibition had been observed for IId and IIe, respectively, no β-galactosidase (β-Gal) inhibition was detected, low β-Glc inhibition was observed and, perhaps more surprising, α-Amy inhibition exhibited by both compounds was also quite low, indicating good discrimination between the two tested α-glycosidases (α-Glc and α-Amy).
Altogether, the results indicate that type II compounds showed interesting α-glu activities. Within this series, compound IId showed the lowest IC50 value, predicted poor GI absorption, and interesting selectivity for α-Glc.
Enzyme kinetic analysis of IId against α-Glc
To further investigate the interaction of the best inhibitor of the series IId with α-Glc, we initially performed a jump dilution experiment.47 The enzyme activity recovery was 97% (Fig. S2†), which indicates reversible enzyme–inhibitor complex formation.
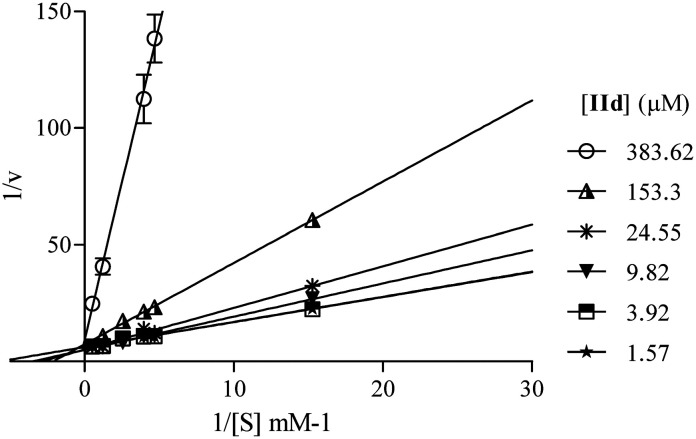
According to the Lineweaver–Burk plot (Fig. 4), IId is a competitive inhibitor. The Ki value is 11.6 μM, almost seven times lower than the value of the reference inhibitor acarbose (Ki = 81 μM), measured under similar conditions.48
Fig. 4. Lineweaver–Burk plot of α-Glc inhibition of p-NPG and compound IId at different concentrations. Data are mean ± SD of the experiment conducted in triplicate.
Docking studies
Molecular docking simulations were performed in order to understand the binding interactions of compound IId with the active site of yeast α-Glc. They show that π-stacking interactions can occur between the aromatic rings of enzyme residues Phe300 and Phe157 and the IId aromatic rings (Fig. 5). In addition, the CF3 groups can interact directly with protein active site residues Asp214 and Glu276. When the IId binding mode is compared with the isomaltose binding mode in the S. cerevisiae protein structure (PDB ID: ; 3axh), it can be observed that the phenyl ring at the Nτ of histamine in IId occupies the place where sugar hydrolysis occurs (Fig. 5 and 6a). The observed interactions may favor a stiffened structure for IId, which can prevent the ligand access to the hydrolysis site, eventually leading to the inhibition of α-Glc. Similar results were obtained for compound IIe (Fig. S3†).
Fig. 5. Superimposed docking results of IId. C atoms from the protein are shown in cyan, C atoms from IId in orange, O atoms in red, N atoms in blue, S atoms in yellow and F atoms in pink.
Fig. 6. Docking results of isomaltose and acarbose ligands. (a) Isomaltose binding mode in the S. cerevisiae protein structure (PDB ID: ; 3axh). (b) Acarbose binding mode. C atoms from the protein are shown in cyan, C atoms from acarbose in orange, O atoms in red, and N atoms in blue.
In previous work, it was shown that apigenin might induce the channel closure to prevent the access of substrates into the active site of α-Glc.49 Compound IId binds in a similar stacking way and, in addition, it may interact directly with hydrolysis residues. When docking studies were performed using acarbose (Fig. 6b), we observed that it establishes multiple polar interactions principally with Asp214 and Glu276 and make stacking with Tyr71 in the bottom of the site, as isomaltose does (Fig. 6a). Furthermore, acarbose make contacts with residues Phe300 and Phe157. If we compare the binding modes of acarbose and IId, the same interactions are clearly observed as described above (Fig. 5 and 6a and b).
Effects of IId on mouse blood glucose levels
Compound IId was able to decrease the postprandial blood glucose level in normal mice. In both control groups, the blood glucose level peak was observed 15 minutes after sucrose administration in untreated mice (Fig. 7A). Since IId was dissolved in 75% ethanolic-physiological solution and acarbose was dissolved in physiological solution, a different control group was used for each compound.50
Fig. 7. A: Glycemia (as % of basal value) at different times after a single sucrose load in mice, together with IId (22.5 mg per kg body weight) or standard drug acarbose (50 mg per kg body weight) and their respective controls. B: Area under the curve (AUC) in the previous image. Data are expressed as mean ± SD (n = 4 mice each). Statistical significance was determined by the one-way analysis of variance followed by Fisher's test. Analysis was performed by using the statistical software GraphPad Prism 5. A p < 0.05 was considered statistically significant.
The glucose blood level peak with IId was lower than that with acarbose. The area under the curve (Fig. 7B) revealed that treatment with 22.5 mg per kg b.w. IId produced a decrease in postprandial glycaemia that was similar to that produced by 50 mg per kg b.w. acarbose. These results give evidence of the potential of IId as a candidate for subsequent studies on T2DM treatment.
No behavioural signs of acute toxicity were detected during the in vivo experiments. The four mouse groups showed similar locomotion and general body positions. In addition, no differences in water and food consumption were observed between groups during 24 h.
Experimental
Chemical reagents and enzymes were purchased from commercial sources and were used without further purification unless noted otherwise. Solvents were of analytical grade or were purified by standard procedures prior to use. Enzymes β-glucosidase (from almonds, EC 3.2.1.21), α-glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20), acetylcholinesterase (from eel, EC 3.1.1.7), xanthine oxidase (from bovine milk, EC 1.17.3.2,) and tyrosinase (from mushroom, EC 1.14.18.1) were acquired from Sigma-Aldrich (St. Louis, MO, USA). The α-amylase enzyme (from pig pancreas, EC 3.2.1.1) was acquired from Roche Diagnostics (Indianapolis, IN, USA), and β-galactosidase (from Kluyveromyces lactis, EC 9031.11.2) was acquired from Laboratorio Dominguez S.A. (Buenos Aires, Argentina).
1H NMR spectra were recorded on a Bruker Avance II at 300 MHz in CDCl3, CD3OD or acetone-d6 in the presence of TMS (0.00 ppm) as the internal standard. 13C NMR spectra were recorded on the same apparatus at 75 MHz in CDCl3, CD3OD or acetone-d6 in the presence of TMS (0.00 ppm) as the internal standard. 19F NMR spectra were recorded on the same apparatus at 282 MHz.
High resolution mass spectra were recorded on a Bruker micrOTOF-Q II spectrometer (Bruker-Daltonics) with an electrospray ionization (ESI) source. Acetonitrile (acquired from Carlo Erba) was used for sample preparation. MS and MS-MS parameters: source type, ESI; ion polarity, positive; set nebulizer, 0.4 bar; set dry heater, 180 °C; set dry gas, 4.0 L min–1; set capillary, 4500 V; set end plate offset, 500 V; set collision cell radio frequency, 150.0 Vpp. ISC collision energy for MS-MS experiments: 30 eV.
The Nα-arylsulfonyl and Nα,Nτ-di-arylsulfonyl histamine derivatives (type I and type II compounds) were prepared as previously desribed.40
N α,Nα,Nτ-Tri-arylsulfonyl histamine analogue synthesis
General procedure
A solution of Nα,Nτ-di-benzenesulfonyl histamine (1.0 mmol), arylsulfonyl chloride (2.0 mmol) and DMAP (2.0 mmol) were dissolved in 25 mL of freshly distilled acetonitrile with stirring. After Et3N (2.85 mmol) was added, the mixture was kept under stirring for 16 hours at 50 °C. The solvent was eliminated under reduced pressure, and the resulting residue was chromatographed on silica gel (hexane : AcOEt gradient) to obtain type III compounds.
N α,Nα,Nτ-Tri-4-fluorobenzenesulfonyl histamine (IIIc)
The general procedure was followed employing 0.082 equiv. of Nα,Nτ-di-4-fluorobenzenesulfonyl histamine to afford 15.0 mg of IIIc (31% final yield). 1H NMR (300 MHz, CDCl3) δ = 8.04 (4H, m, Ar), 7.94 (2H, m, Ar), 7.92 (1H, s, N CH–N), 7.18–7.28 (6H, m, Ar), 6.97 (1H, s, N–CH C), 3.95 (2H, t, J1 = J2 = 7.8 Hz, CH2-imidazole), 2.89 (2H, t, J1 = J2 = 7.7 Hz, CH2–CH2-imidazole). 13C NMR (75 MHz, CDCl3) δ = 166.31 (2CF, d, J = 259.5 Hz, Ar), 165.86 (CF, d, J = 257.8 Hz, Ar), 140.88 (C, N–CH = CN), 136.42 (CH, N CH–N), 135.62 (2C, d, J = 3.4 Hz, Ar), 133.83 (C, d, J = 3.4 Hz, Ar), 131.21 (4 CH, d, J = 9.8 Hz, Ar), 130.38 (2 CH, d, J = 9.8 Hz, Ar), 117.36 (2CH, d, J = 23.1 Hz, Ar), 116.52 (4 CH, d, J = 22.5 Hz, Ar), 114.28 (CH, N–CH C), 48.15 (CH2, CH2-imidazole), 28.75 (CH2, CH2–CH2-imidazole). 19F NMR (282 MHz, CDCl3): δ = –100.37 (m, 1F), –102.29 (m, 2F). HRMS: found m/z = 608.0206, calculated m/z for C23H18F3NaN3O6S3 [M + Na]+ 608.0202 (0.4 mDa error). MS-MS showed the fragment ion corresponding to Nα-4-fluorobenzenesulfonyl histamine at m/z = 253 and to the 4-fluorobenzenesulfonyl radical at m/z = 159.
N α,Nα,Nτ-Tri-4-(trifluoromethyl)benzenesulfonyl histamine (IIId)
The general procedure was followed employing 0.076 equiv. of Nα,Nτ-di-4-(trifluoromethyl)benzenesulfonyl histamine to afford 40.0 mg of IIId (72% final yield). 1H NMR (300 MHz, CDCl3) δ = 8.16 (4H, d, J = 8.3 Hz, Ar), 8.04 (2H, d, J = 8.3 Hz, Ar), 7.94 (1H, s, N CH–N), 7.80–7.86 (6H, m, Ar), 6.98 (1H, s, N–CH C), 4.02 (2H, t, J1 = J2 = 7.6 Hz, CH2-imidazole), 2.92 (2H, t, J1 = J2 = 7.6 Hz, CH2–CH2-imidazole). 13C NMR (75 MHz, CDCl3) δ = 142.82 (C, N–CH CN), 141.25 (C, Ar), 140.91 (2C, Ar), 136.60 (CH, N CH–N), 136.48 (CCF3, m, Ar), 135.78 (2CCF3, m, Ar), 128.86 (4CH, Ar), 127.95 (2CH, Ar), 127.10 (2CH, m, Ar), 126.44 (4CH, m, Ar), 122.96 (2CF3, m), 122.75 (CF3, m), 114.41 (CH, N–CH C), 48.50 (CH2, CH2-imidazole), 28.75 (CH2, CH2–CH2-imidazole). 19F NMR (282 MHz, CDCl3): δ = –63.31 (s, 6F), –63.45 (s, 3F). HRMS: found m/z = 758.0098, calculated m/z for C26H18F9NaN3O6S3 [M + Na]+ 758.0106 (0.8 mDa error). MS-MS showed the fragment ion corresponding to Nα,Nτ-di-4-(trifluoromethyl)benzenesulfonyl histamine at m/z = 549 and to the 4-(trifluoromethyl)benzenesulfonyl radical at m/z = 209.
N α,Nα,Nτ-Tri-2-fluor-5-methylbenzenesulfonyl histamine (IIIe)
The general procedure was followed employing 0.076 equiv. of Nα,Nτ-di-2-fluor-5-methylbenzenesulfonyl histamine to afford 30 mg of IIIe (60% final yield). 1H NMR (300 MHz, CDCl3) δ = 7.99 (1H, s, N CH–N), 7.79 (1H, dd, J1 = J2 = 2.1 Hz and J3 = 6.9 Hz, Ar), 7.62 (1H, dd, J1 = J2 = 2.1 Hz and J3 = 6.9 Hz, Ar), 7.45 (1H, m, Ar), 7.36 (2H, m, Ar), 7.13 (1H, s, N–CH C), 7.10 (1H, dd, J1 = J2 = 8.6 Hz and J3 = 10.1 Hz, Ar), 6.99 (2H, dd, J1 = J2 = 8.3 Hz and J3 = 10.0 Hz Ar), 4.25 (2H, t, J1 = J2 = 7.2 Hz, CH2-imidazole), 3.08 (2H, t, J1 = J2 = 7.2 Hz, CH2–CH2-imidazole), 2.43 (3H, s, CH3–Ar), 2.36 (6H, s, 2CH3–Ar). 13C NMR (75 MHz, CDCl3) δ = 157.32 (CF, d, J = 256.6 Hz, Ar), 157.05 (2CF, d, J = 256.0 Hz, Ar), 140.90 (C, N–CH CN), 137.87 (CH, d, J = 8.4 Hz, Ar), 136.90 (CH, N CH–N), 136.81 (2CH, d, J = 8.4 Hz, Ar), 135.25 (C, d, J = 3.8 Hz, Ar), 134.43 (2C, d, J = 3.8 Hz, Ar), 131.47 (2CH, Ar), 129.90 (CH, Ar), 126.58 (2C, d, J = 13.7 Hz, Ar), 125.44 (C, d, J = 13.1 Hz, Ar), 117.63 (CH, d, J = 21.3 Hz, Ar), 116.90 (2CH, d, J = 21.3 Hz, Ar), 114.35 (CH, N–CH C), 48.58 (CH2, CH2-imidazole), 29.39 (CH2, CH2–CH2-imidazole), 20.64 (ArCH3), 20.56 (2ArCH3). 19F NMR (282 MHz, CDCl3): δ = –112.24 (m, 2F), –112.45 (m, F). HRMS: found m/z = 650.0671, calculated m/z for C26H24F3N3NaO6S3 [M + Na]+ 650.0672 (0.1 mDa error). MS-MS showed the fragment ion corresponding to 2-fluor-5-methylbenzenesulfonyl histamine at m/z = 281 and to the 2-fluor-5-methylbenzenesulfonyl radical at m/z = 173.
Microplate assay protocols
α-Glu
The hydrolysis of p-nitrophenyl α-O-d-glucopyranoside (α-pNPG) was continuously measured in a 96-well microplate using a similar method to that applied by Arnaldos et al.51 Wells were filled in triplicate with α-Glu (yeast) in 0.1 M, pH 7 phosphate buffer (7.10 μU mL–1 end concentration per well), α-cyclodextrin, the same buffer solution (1.22 mM end concentration per well) and 10 μL of test compound in dimethylsulfoxide (DMSO) solution (100 or 122 μM end concentration per well). Wells containing the corresponding volume of DMSO without an inhibitor were used as the references of maximum enzymatic rates. The final volume per well was 270 μL. The enzymatic reaction was initiated by addition of α-pNPG (1.63 mM end concentration per well). The plate was shaken for 2 s and the increase in absorbance at 405 nm was monitored at 37 °C for 10 min. For IC50 determination, ten serial dilutions of the compounds were prepared in DMSO, following equally spaced points on a neperian logarithm scale, starting at 64.7 mM (or 9.6 mM) and finishing at 0.00647 mM (end concentration per well: 2397 (or 355) to 0.2397 μM). IC50 was calculated using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA) applying a non linear regression curve fit for a log[inhibitor] vs. normalized answer model with variable slope. Standard drug acarbose was used as enzyme inhibition control.
β-Glc
The hydrolysis of p-nitrophenyl β-O-d-glucopyranoside (β-pNPG) was continuously measured in a 96-well microplate. According to Arnaldos et al.,51 wells were filled in triplicate with β-Glc (from almonds) in 0.1 M phosphate buffer, pH 7 (7.10 μU mL–1 final concentration per well), α-cyclodextrin, in 0.1 M phosphate buffer, pH 7 (1.22 mM end concentration per well) and 10 μL of test compound in DMSO solution (65 μM end concentration per well). Wells containing the corresponding volume of DMSO without an inhibitor were used as the references of maximum enzymatic rates whilst 1-DNJ was used as an enzyme inhibition control. The final volume per well was 270 μL. The enzymatic reaction was initiated by addition of β-pNPG (1.63 mM end concentration per well). The plate was shaken for 2 s, and the increase in absorbance at 405 nm was monitored at 37 °C for 10 min. Data were processed. For IC50 determination, ten serial dilutions of the compounds were prepared in DMSO, following equally spaced points on a neperian logarithm scale, starting at 600 μM and finishing at 0.06 μM. IC50 was calculated using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
β-Gal
Similar to the method applied in the β-Glc assay, the hydrolysis of o-nitrophenyl-β-O-d-galactopyranoside (β-oNPG) was continuously measured in a 96-well microplate. Wells were filled in triplicate with β-Gal (Kluyveromyces lactis) in 50 mM phosphate buffer, pH 7 (7.20 μU mL–1 end concentration per well) and 10 mL of test compound in DMSO (100 mM end concentration per well). The enzymatic reaction was initiated by addition of β-oNPG (3.23 mM end concentration per well). The final volume per well was 270 mL. The plate was shaken for 2 s and the increase in absorbance at 405 nm was monitored at 37 °C for 10 min. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
α-Amy
The starch-iodine method52 was followed with modifications. Each well was filled in triplicate with 10 μL of test compound in DMSO solution (122 μM end concentration per well), 54 μL of boiled starch solution in 100 mM pH 7 phosphate buffer (500 mg L–1) and 40 μL of α-amylase (porcine pancreatic) in the same buffer (7 U mL–1). After 15 minutes at 37 °C, the reaction was stopped by adding 54 μL of 0.01 N iodine solution in 0.02 N HCl. Finally, 112 μL of water was added to dilute each well. Absorbance at 640 nm was immediately measured. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA). Wells containing the corresponding volume of DMSO without an inhibitor were used as the references of maximum enzymatic activity, whilst similar wells without an enzyme at all were considered as the references of maximum inhibition. Standard drug acarbose was used as an enzyme inhibition control. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
Tyrase
According to the reported method by Atta-ur-Rahman,53 the formation of dopachrome was continuously measured in 96-well microplates. Wells were filled in triplicate with 10 μL test compound in DMSO solution (1.48 μM end concentration per well) and mushroom Tyrase in 0.1 M phosphate buffer, pH 7 (15.55 U mL–1 end concentration per well). Wells containing the corresponding volume of DMSO without an inhibitor were used as references of maximum enzymatic rates, whilst kojic acid was used as an enzyme inhibition control. The enzymatic reaction was initiated by addition of the substrate L-TYR (0.63 mM end concentration per well). The final volume per well was 270 μL. The plate was shaken for 2 s and the increase in absorbance at 475 nm was monitored at 37 °C for 30 min. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
XO
Following the method previously reported by Chu,54 the formation of uric acid was continuously measured in 96-well microplates. Each well was filled in triplicate with 10 μL test compound in DMSO (0.11 μM end concentration per well) and XO (bovine) in 0.2 M phosphate buffer, pH 7.5 (2.81 μU mL–1 end concentration per well). Wells containing the corresponding volume of DMSO without an inhibitor were used as references of maximum enzymatic rates, and standard drug allopurinol was used as an enzyme inhibition control. The enzymatic reaction was initiated by addition of xanthine (0.04 mM end concentration per well). The final volume per well was 270 μL. The plate was shaken for 2 s and the increase in absorbance at 295 nm was monitored at 30 °C for 20 min. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
AChE
AChE activity measurement was carried out based on Ellman's method. Wells were filled in triplicate with AChE (electric eel) in 0.1 M phosphate buffer, pH 7.5 (13.7 μU mL–1 end concentration per well), Ellman's reagent (DTNB, 5,5-dithio-bis-(2-nitrobenzoic acid)), the same buffer solution (0.31 mM end concentration per well) and 10 μL of test compound in DMSO solution (0.05 μM end concentration per well). Wells containing the corresponding volume of DMSO without an inhibitor were used as references of maximum enzymatic rates, and standard drug eserine was used as the control for enzyme inhibition. The enzymatic reaction was initiated by addition of acetylthiocholine iodide (ATCI) (0.46 mM end concentration per well). The final volume per well was 270 μL. The plate was shaken for 2 s and the increase in absorbance at 405 nm was monitored at 37 °C for 15 min. Data were processed using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
Jump dilution assay
A DMSO solution of compound IId was incubated at 10-fold its IC50, with the enzyme solution at over 100-fold the concentration used in the microplate assay. After 30 minutes of incubation, the mixture was diluted 100-fold and mixed with the substrate and α-CD to start the reaction. The progress curve of this sample was measured and compared to an enzyme sample incubated and diluted in the absence of an inhibitor. Each incubation and dilution were prepared and measured in triplicate. The percentage of activity recovery was obtained using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
In silico ADME property predictions
ADME properties were predicted in silico using SwissADME (; http://www.swissadme.ch/). Results for each tested analogue are tabulated and listed in Table S1 in the ESI.†
Kinetic experiments
Microplate assay was carried out at varying substrate concentrations between 0.022 and 7.2 mM and at inhibitor concentrations between 0.239 and 0.0061 mM. Initial velocities vs. [S] curves were obtained with Michaelis–Menten equation adjustment. After that, the Lineweaver–Burk linearization plot and the secondary linearization of slopes vs. [I] revealed the inhibition mode and the Ki value, respectively. All the data acquired were treated using Prism V5.01 (GraphPad Software Inc., La Jolla, CA, USA).
Docking studies
Homology model
Since the Saccharomyces cerevisiae α-Glu enzyme has not been crystallized, we resorted to homology modeling. The sequence in the FASTA format of α-Glu was obtained from UniProt (access code P53341). Template search was conducted using the HHpred tool of the Max Planck Institute Bioinformatics Toolkit.55 We used as a template the crystal structure of S. cerevisiae isomaltase which has been resolved at 1.3 Å resolution (PDB entry: ; 3AJ7). The comparison of both sequences results in 72% identity. Homology models were generated using the Modeller v.9.9 software.56
Molecular docking studies
The solvent site biased docking method (SSBDM) was carried out using Autodock 4.2.6 software.57 The SSBDM is based on the method developed and thoroughly tested in previous work.58–60 This strategy take advantage of using information of crystal solvent structures or calculated water sites from molecular dynamics simulations to modify the Autodock4 scoring function, adding an additional energy term for polar or nonpolar atoms to the original function. In order to dock the acarbose ligand, the SSBDM was performed by using crystallographic water molecules from a template protein structure (PDB entry: ; 3AJ7) while for docking of IId and IIe compounds, calculated molecular dynamics ethanol sites were used.
The model obtained by homology modeling was used as the receptor protein. We prepared the receptor by adding hydrogen atoms with AutoDockTools4.57 The structures of compounds IId, IIe and acarbose were optimized in a vacuum at PBE/6-31G* level using Gaussian 09,61 then the optimized structures of the ligands were prepared in a pdbqt format using AutoDockTools4.57
The grid map was set to 90 × 90 × 90 points with a grid spacing of 0.375 Å centered on catalytic sites (Asp 214 and Glu 276). For each calculation, 100 different docking runs were performed, and the resulting 100 poses were clustering according to a ligand RMSD cut-off of 2 Å. To analyze the performance of the docking runs, we used the Autodock capacity to discriminate the correct ligand pose from wrong predictions by using the predicted binding free energy score (ΔG) and the population, which is the number of individual docking results that predicted the same ligand pose in the whole docking experiment (100 runs). The poses selected as correct were those with high population and a negative binding free energy score.
Molecular dynamics simulation
The homology model structure was refined using molecular dynamics simulation as described previously by Blanco et al.,60 and solvent sites were calculated with explicit solvent mixture molecular dynamics simulation using a clustering algorithm.58,62
In vivo experiments
Male C57BL/6 mice (6–8 weeks old) were provided by the School of Medicine, National University of Rosario and were maintained at the animal facilities of the School of Biochemistry of the National University of Rosario. All animals were housed individually in light/dark cycles (12 h on/12 h off) and a temperature-controlled room at 22 °C with pelleted food and tap water available ad libitum.
Oral sucrose load test
Mice were divided into 4 groups (n = 4 mice each). Fasted animals were deprived of food for at least 12 h, but allowed free access to water. After collection of an unchallenged blood sample (time 0), a solution of sucrose (3.0 g per kg body weight) together with IId (22.5 mg per kg body weight) or standard drug acarbose as the positive control (50 mg per kg body weight) was administered to the mice by gavage. Control groups were treated under the same conditions, only that acarbose and IId solutions were replaced with physiological solution or 75% ethanolic-physiological solution, respectively. Blood samples were collected from the tail after 15, 30, 60, and 90 min for determination of glucose concentrations. Glucose levels were measured with an Accu-Check glucometer (Roche). Data are expressed as the mean (mg dL–1) ± standard error of the mean (n = 4). All the experimental protocols were performed according to the Regulation for the Care and Use of Laboratory Animals (Expedient 6109/012 E.C. Resolution 267/02) and approved by the Institutional Animal Use Committee of the National University of Rosario, Argentina. Animals received human care according to criteria outlined in the ARRIVE guidelines which were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Conclusions
The in vitro evaluation of α-Glc inhibition by a series of Nα arylbenzenesulfonyl (type I), Nα,Nτ-di-arylbenzenesulfonyl (type II), and Nα,Nα,Nτ-tri-arylbenzenesulfonyl histamine derivatives (type III) clearly showed that the di-arylbenzenesulfonyl compounds of type II displayed the most promising properties.
Two library members, compounds IId and IIe, exhibited an α-Glc inhibitory potency significantly higher than acarbose. Both compounds possess at least one fluorine atom and methyl substituent type (CF3 and CH3).
Upon prediction using SwissADME, IIe and IId express several aspects of a promising drug-like lead compound, including poor absorption in the GI tract.
The best inhibitor (IId) is a reversible competitive inhibitor with a Ki value almost seven times lower than that of acarbose. In addition, it showed interesting in vitro enzyme selectivity and an in vivo hypoglycemic effect in mice, causing an effect comparable to that produced by acarbose with a two-fold higher dose.
To the best of our knowledge, in spite of its simplicity, compound IId is the first non-sugar-related inhibitor that displays these interesting properties.
Conflicts of interest
The authors have declared no conflicts of interest.
Supplementary Material
Acknowledgments
Financial support for this work was provided by FONCYT (PICT2015-3574), and Universidad Nacional de Rosario (80020180300114UR) to RLEF. We thank the Instituto de Química de Rosario (IQUIR, CONICET-UNR) and the Instituto de Biología Molecular y Celular de Rosario (IBR, CONICET-UNR) for providing access to equipment. MIO, FL and MDG thank CONICET for their fellowships. MOS, DMM, DEF and RLEF are CONICET researchers. We thank Dr. Diego Gomez Casati for his generous loan of the enzyme.
Footnotes
†Electronic supplementary information (ESI) available: In vitro studies (Fig. S1 and Table S1), kinetic analysis (Fig. S2), docking results (Fig. S3) and mass spectra and NMR spectra (H, C and F) of type III compounds. See DOI: 10.1039/c9md00559e
References
- Shi Y., Hu F. B. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- Classification of diabetes mellitus, World Health Organization, Geneva, 2019, Licence: CC BY-NC-SA 3.0 IGO.
- Narender T., Madhur G., Jaiswal N., Agrawal M., Maurya C. K., Rahuja N., Srivastava A. K., Tamrakar A. K. Eur. J. Med. Chem. 2013;63:162–169. doi: 10.1016/j.ejmech.2013.01.053. [DOI] [PubMed] [Google Scholar]
- Nguyen D. V., Shawand L. C., Grant M. B. Front. Endocrinol. 2012;3:1–7. doi: 10.3389/fendo.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanangkool E., Kittikhunnatham P., Damsud T., Wacharasindhu S., Phuwapraisirisan P. Eur. J. Med. Chem. 2013;66:296–304. doi: 10.1016/j.ejmech.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Lebovitz H. E. Endocrinol. Metab. Clin. North Am. 1997;26:539–551. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- Ali H., Houghton P. J., Soumyanath A. J. Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Ghani U. Eur. J. Med. Chem. 2015;103:133–162. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- van de Laar F. A. Vasc. Health Risk Manage. 2008;4:1189–1195. doi: 10.2147/vhrm.s3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar F. A., Lucassen P. L., Akkermans R. P., van de Lisdonk E. H., Rutten G. E., van Weel C. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- Kumar R. V., Sinha V. R. Expert Opin. Drug Delivery. 2012;9:4. doi: 10.1517/17425247.2012.663080. [DOI] [PubMed] [Google Scholar]
- Hogan S., Zhang L., Li J., Sun S., Canning C., Zhou K. Nutr. Metab. 2010;7:71–80. doi: 10.1186/1743-7075-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerru N., Singh-Pillay A., Awolade P., Singh P. Eur. J. Med. Chem. 2018;152:436–488. doi: 10.1016/j.ejmech.2018.04.061. [DOI] [PubMed] [Google Scholar]
- Worawalai W., Wacharasindhu S., Phuwapraisirisan P. Med. Chem. Commun. 2012;3:1466–1470. [Google Scholar]
- Wang J.-T., Lin T.-C., Chen Y.-H., Lin C.-H., Fang J.-M. Med. Chem. Commun. 2013;4:387–393. [Google Scholar]
- Tanabe G., Xie W., Balakishan G., Amer M. F. A., Tsutsui N., Takemura H., Nakamura S., Akaki J., Ninomiya K., Morikawa T., Nakanishi I., Muraoka O. Bioorg. Med. Chem. 2016;24:3705–3715. doi: 10.1016/j.bmc.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Jenkinson S. F., Best D., Saville W., Mui J., Martínez R. F., Nakagawa S., Kunimatsu T., Alonzi D. S., Butters T. D., Norez C., Becq F., Blériot Y., Wilson F. X., Weymouth-Wilson A. C., Kato A., Fleet G. W. J. J. Org. Chem. 2013;78:7380–7397. doi: 10.1021/jo4005487. [DOI] [PubMed] [Google Scholar]
- Kato A., Hayashi E., Miyauchi S., Adachi I., Imahori T., Natori Y., Yoshimura Y., Nash R. J., Shimaoka H., Nakagome I., Koseki J., Hirono S., Takahata H. J. Med. Chem. 2012;55:10347–10362. doi: 10.1021/jm301304e. [DOI] [PubMed] [Google Scholar]
- Wang K., Bao L., Ma K., Zhang J., Chen B., Han J., Ren J., Luo H., Liu H. Eur. J. Med. Chem. 2017;127:1035–1046. doi: 10.1016/j.ejmech.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Taha M., Ismail N. H., Imran S., Wadood A., Ali M., Ur Rehman A. Med. Chem. Commun. 2015;6:1826–1836. [Google Scholar]
- Wang G.-C., Peng Y.-P., Xie Z.-Z., Wang J., Chen M. Med. Chem. Commun. 2017;8:1477–1484. doi: 10.1039/c7md00173h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhameja M., Gupta P. Eur. J. Med. Chem. 2019;176:343–377. doi: 10.1016/j.ejmech.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Özil M., Emirik M., Beldüz A., Ülker S. Bioorg. Med. Chem. 2016;24:5103–5114. doi: 10.1016/j.bmc.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Yar M., Bajda M., Shahzad S., Ullah N., Gilani M. A., Ashraf M., Rauf A., Shaukat A. Bioorg. Chem. 2015;58:65–71. doi: 10.1016/j.bioorg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Zawawi N. K. N. A., Taha M., Ahmat N., Wadood A., Ismail N. H., Wadood A., Rahim F. Bioorg. Chem. 2017;70:184–191. doi: 10.1016/j.bioorg.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Chaudhry F., Naureen S., Huma R., Shaukat A., Al-Rashida M., Asif N., Ashraf M., Munawar M. A., Khan M. A. Bioorg. Chem. 2017;71:102–109. doi: 10.1016/j.bioorg.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Hameed S., Kanwal, Seraj F., Rafique R., Chigurupati S., Wadood A., Ur Rehman A., Venugopal V., Salar U., Taha M., Khan K. M. Eur. J. Med. Chem. 2019;183:111677. doi: 10.1016/j.ejmech.2019.111677. [DOI] [PubMed] [Google Scholar]
- Singh G., Singh A., Verma R. K., Mall R., Azeem U. Comput. Biol. Chem. 2018;72:45–52. doi: 10.1016/j.compbiolchem.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Adib M., Peytam F., Shourgeshty R., Mohammadi-Khanaposhtani M., Jahani M., Imanparast S., Faramarzi M. A., Larijani B., Moghadamnia A. A., Esfahani E. N., Bandarian F., Mahdavi M. Bioorg. Med. Chem. Lett. 2019;29:713–718. doi: 10.1016/j.bmcl.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Kasturi S., Surarapu S., Uppalanchi S., Anireddy J. S., Dwivedi S., Anantaraju H. S., Perumal Y., Sigalapalli D. K., Babu B. N., Ethiraj K. S. Bioorg. Med. Chem. Lett. 2017;27:2818–2823. doi: 10.1016/j.bmcl.2017.04.078. [DOI] [PubMed] [Google Scholar]
- Kasturi S. P., Surarapu S., Uppalanchi S., Dwivedi S., Yogeeswari P., Sigalapalli D. K., Bathini N. B., Ethiraj K. S., Anireddy J. S. Eur. J. Med. Chem. 2017;150:39–52. doi: 10.1016/j.ejmech.2018.02.072. [DOI] [PubMed] [Google Scholar]
- Riaz S., Khan I. U., Bajda M., Ashraf M., Qurat-ul-Ain, Shaukat A., Rehman T. Ur, Mutahir S., Hussain S., Mustafa G., Yar M. Bioorg. Chem. 2015;63:64–71. doi: 10.1016/j.bioorg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Seo W. D., Kim J. H., Kang J. E., Ryu H. W., Curtis-Long M. J., Lee H. S., Yanga M. S., Park K. H. Bioorg. Med. Chem. Lett. 2005;15:5514–5516. doi: 10.1016/j.bmcl.2005.08.087. [DOI] [PubMed] [Google Scholar]
- Wang S., Yan J., Wang X., Yang Z., Lin F., Zhang T. Eur. J. Med. Chem. 2010;45:1250–1255. doi: 10.1016/j.ejmech.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Kasturi S. P., Surarapu S., Uppalanchi S., Dwivedi S., Yogeeswari P., Sigalapalli D. K., Bathini N. B., Ethiraj K. S., Anireddy J. S. Eur. J. Med. Chem. 2018;150:39–52. doi: 10.1016/j.ejmech.2018.02.072. [DOI] [PubMed] [Google Scholar]
- Ramallo I. A., Salazar M. O., Mendez L., Furlan R. L. E. Acc. Chem. Res. 2011;44:241–250. doi: 10.1021/ar100106n. [DOI] [PubMed] [Google Scholar]
- Lopez S. N., Ramallo I. A., Sierra M. G., Zacchino S. A., Furlan R. L. Proc. Natl. Acad. Sci. U. S. A. 2007;104:441–444. doi: 10.1073/pnas.0608438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramallo I. A., Salazar M. O., García P. and Furlan R. L. E., Chemical Diversification of Natural Product Extracts, in Studies in Natural Products Chemistry, ed. Atta-ur-Rahman, Elsevier, Amsterdam, 2018, ch. 10, vol. 60. [Google Scholar]
- Salazar M. O., Micheloni O., Escalante A. M., Furlan R. L. E. Mol. Diversity. 2011;15:713–719. doi: 10.1007/s11030-010-9301-2. [DOI] [PubMed] [Google Scholar]
- Salazar M. O., Osella M. I., Ramallo I. A., Furlan R. L. E. RSC Adv. 2018;8:36209–36218. doi: 10.1039/c8ra06625f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Tang B., Liang S. H., Jiang X. Curr. Top. Med. Chem. 2016;16:1200–1216. doi: 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popović-Djordjević J. B., Jevtić I. I., Dj Grozdanić N., Šegan S. B., Zlatović M. V., Ivanović M. D., Stanojković T. P. J. Enzyme Inhib. Med. Chem. 2017;32:298–303. doi: 10.1080/14756366.2016.1250754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Michielin O., Zoete V. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Zoete V. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J. B., Nissink J. W. M. ACS Chem. Biol. 2018;13:36–44. doi: 10.1021/acschembio.7b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Kato N., Kano E., Adachi I., Ikeda K., Yu L., Okamoto T., Banba Y., Ouchi H., Takahata H., Asano N. J. Med. Chem. 2004;48:2036–2044. doi: 10.1021/jm0495881. [DOI] [PubMed] [Google Scholar]
- Copeland R. A., Basavapathruni A., Moyer M., Scott M. P. Anal. Biochem. 2011;416:206–210. doi: 10.1016/j.ab.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Dong W., Jespersen T., Bols M., Skrydstrup T., Sierks M. R. Biochemistry. 1996;35:2788–2795. doi: 10.1021/bi9522514. [DOI] [PubMed] [Google Scholar]
- Zeng L., Zhang G., Lin S., Gong D. J. Agric. Food Chem. 2016;64:6939–6949. doi: 10.1021/acs.jafc.6b02314. [DOI] [PubMed] [Google Scholar]
- Although the corresponding control group was used for each calculation, it is worth mentioning that the areas under the blood glucose curves of the two vehicle-treated control groups (IId Ctrl versus Acarb Ctrl, Fig. 7B) did not show significant differences between them (p > 0.05)
- Arnaldos T. L., Serrano M. L., Calderon A. A., Muñoz R. Phytochem. Anal. 1999;10:171–174. [Google Scholar]
- Xiao Z., Storms R., Tsang A. Anal. Biochem. 2006;351:146–148. doi: 10.1016/j.ab.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Atta-ur-Rahman, Choudhary W. J. and Thomsen M. I., Bioassay Techniques for Drug Development, Amsterdam, 2001. [Google Scholar]
- Chu Y.-H., Chen C.-J., Wu S.-H., Hsieh J.-F. J. Agric. Food Chem. 2014;62:3742–3749. doi: 10.1021/jf5004094. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Stephens A., Nam S.-Z., Rau D., Kübler J., Lozajic M., Gabler F., Söding J., Lupas A. N., Alva V. J. Mol. Biol. 2018;430:2237–2243. doi: 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Webb B., Sali A., Webb B. and Sali A., Comparative Protein Structure Modeling Using MODELLER, in Current Protocols in Bioinformatics, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2014, pp. 5.6.1–5.6.32. [DOI] [PubMed] [Google Scholar]
- Morris G. M., Huey R., Lindstrom W., Sanner M. F., Belew R. K., Goodsell D. S., Olson A. J. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcon J. P., Defelipe L. A., Modenutti C. P., López E. D., Alvarez-Garcia D., Barril X., Turjanski A. G., Martí M. A. J. Chem. Inf. Model. 2017;57:846–863. doi: 10.1021/acs.jcim.6b00678. [DOI] [PubMed] [Google Scholar]
- Modenutti C., Gauto D., Radusky L., Blanco J., Turjanski A., Hajos S., Marti M. A. Glycobiology. 2015;25:181–196. doi: 10.1093/glycob/cwu102. [DOI] [PubMed] [Google Scholar]
- Blanco Capurro J. I., Di Paola M., Gamarra M. D., Martí M. A., Modenutti C. P. Glycobiology. 2018;29:124–136. doi: 10.1093/glycob/cwy102. [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Had M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery, Jr. J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J. and Fox D. J., Gaussian 09, Gaussian, Inc., Wallingford CT, 2009.
- Arcon J. P., Modenutti C. P., Avendano D., Lopez E. D., Defelipe L. A., Ambrosio A. F., Turjanski A. G., Forli S., Marti M. A. Bioinformatics. 2019;35:3836–3838. doi: 10.1093/bioinformatics/btz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.