Triphenylphosphonium can selectively target various “cargos” to mitochondria based on the high mitochondrial membrane potential of tumor cells.
Triphenylphosphonium can selectively target various “cargos” to mitochondria based on the high mitochondrial membrane potential of tumor cells.
Abstract
Cancer is one of the most important problems that endanger human health. The number of cancer patients is increasing rapidly worldwide. Compared with normal cells, cancer cells exhibit abnormal metabolism (abnormal glycolysis and oxidative phosphorylation, high levels of reactive oxygen species, anti-apoptosis, high mitochondrial membrane potential, and so on), and specific targeting of these metabolic abnormalities would be a promising drug design direction. These physiological characteristics are closely related to tumorigenesis and development, which are mainly regulated by mitochondria. Therefore, mitochondria have become important anticancer drug targets, attracting much attention in recent years. In this review, we systematically summarize various mitochondrial anticancer drugs developed, especially mitocans based on triphenylphosphonium (TPP), and discuss the advantages of TPP in endowing mitochondrial targeting function.
1. Introduction
Cancer is the second leading cause of death after cardiovascular disease. The American Cancer Society released the Global Cancer statistics 2018, citing 36 types of cancer in 185 countries worldwide. According to the report, 18.1 million new cancer cases and 9.6 million cancer-related deaths were registered worldwide in 2018. Asia accounted for nearly half of the new cancer cases and nearly 70% of the cancer-related deaths. Compared with other countries, cancer incidence and mortality rates in China are the highest in the world.1 Based on expected population growth and aging alone, the number of global cancer cases is expected to increase by 60% by 2040.2
Researchers compared the survival rate of cancer patients between the 1975–1977 and 2006–2012 periods and found that the five-year survival rate of the most common cancer increased from 50% to 66%.3 Despite these achievements, the cancer-related health threat and psychological panic are still major problems that must be solved. Non-oncogenic pathways for cancer treatment have recently received widespread attention due to the increased resistance to oncogenic targeted therapy.4,5 For example, the epidermal growth factor receptor (EGFR), the most common oncogenic target for the targeted treatment of non-small lung cancer, is mutated in about 16% of patients with advanced lung cancer.4 One of the most common approaches to target oncogenic pathways is targeting the ubiquitin–proteasome system (UPS).6 The successful treatment of multiple myeloma with protease inhibitors in clinical situations confirmed the correlation between UPS interference and tumor growth.7 However, protease inhibitor resistance and inactivity in solid tumor environments support the need to develop other non-oncogenic pathway inhibitors.
The non-oncogenic pathways are usually based on tumor cell specificity. The most significant difference between healthy and tumor cells is that the former have healthy proliferative and apoptotic pathways, while the latter exhibit unlimited replication and anti-apoptotic potential.8 Studies have shown that the malignant transformation of cells is caused by the irreversible damage of mitochondrial function, breaking the balance between the oxidative phosphorylation and glycolysis energy supply chains.9,10 The inhibition of aerobic glycolysis in some tumor cells can reportedly restore the mitochondrial oxidative phosphorylation function and inhibit tumor cell growth.11 The ROS level in tumor cells is higher than that of healthy cells, which is another feature of tumor cell metabolism.12–14 The relatively higher ROS levels are involved in tumor cell transformation, proliferation, survival, migration, invasion, and metastasis. Mitochondria, the main ROS production sites, are also the main targets of ROS attack.12,14 Considering the importance of mitochondrial function in tumorigenesis and development, mitochondria are becoming important anticancer drug targets that attract increasing attention.
In the past decade, mitocan development went through relevant progress.15,16 “Mitocan” is a fusion abbreviation that stands for “mitochondria” and “cancer” to reflect mitochondria-mediated anticancer effects.15 Mitocans are anticancer agents that specifically target tumor cell mitochondria. Mitochondria are life-and-death organelles and the main ATP sources.17 They are involved in many complicated regulatory processes leading to apoptosis, and their function is also closely related to tumorigenesis and development. An increasing number of anticancer drugs have been found to induce tumor cell death by targeting mitochondria. These drugs are referred to as “mitocans”, which disrupt mitochondrial stability and lead to the release of apoptotic factors in the cells.18 Several recent reviews have highlighted the importance of mitochondria as new anticancer drug targets.19–24 This study mainly focuses on TPP-oriented mitocans.
2. Mitocan types and mechanisms
Mitocans are currently classified into eight categories (Table 1),15 based on the different mitochondrial targets, including hexokinase inhibitors, Bcl-2 family protein-targeting drugs, thiol redox inhibitors, voltage-dependent anion channel/adenine nucleotide translocase (VDAC/ANT)-targeting drugs, electron transport chain targeting drugs, mitochondrial inner membrane-targeted lipophilic cations, tricarboxylic acid (TCA) cycle-targeting drugs, and mtDNA-targeting drugs.15,16,25–27
Table 1. The classification of mitocans.
| Class | Drug function type | Agent |
| I | Hexokinase inhibitors | 2-Deoxyglucose, 3-bromopyruvate, oxamate, mannoheptulose, 5-thioglucose, lonidamine |
| II | Targeting Bcl-2 family proteins | Gossypol, lonidamine, ABT-737, ABT-263, α-tocopheryl succinate, MKT-077, HA14-1, bongkrekic acid, BH31-2, Genasense, obatoclax (GX15-070), chelerythrine, sanguinarine |
| III | Thiol redox inhibitors | Isothiocyanates, arsenites, arsenic trioxide, arsenic |
| IV | VDAC/ANT targeting drugs | GSAO, lonidamine, CD437, betulinic acid, sophoranone, cyclosporin A, F16, arsenic trioxide |
| V | Electron transport chain targeting drugs | Tamoxifen, adaphostin, α-tocopheryl succinate, MitoVES, 3-bromopyruvate, 4-hydroxy retinamide, thenoyltrifluoro-acetone (TTFA), aroglitazone, antimycin A, rotenone, tollinastatin-1, resveratrol |
| VI | Lipophilic cations targeting inner membrane | Rhodamine-123, F16, MKT-077, mastoparan, dequalinium |
| VII | TCA cycle targeting drugs | Dichloroacetic acid, 3-bromopyruvate |
| VII | mtDNA targeting drugs | Menadione, fialuridine, ethidium bromide, ditercalinium, nalidixic acid, ciprofloxacin, etoposide |
Some mitocans have been studied or are currently undergoing clinical trials, indicating that they are potentially clinically relevant drugs to be developed.15,16 However, an important drawback of mitocan use is their poor selectivity in most cases, which could cause serious damage also in healthy cells. This is a worldwide problem that plagues cancer treatment. At present, using the difference in physiological properties between tumor and healthy cells to achieve targeted delivery of anticancer drugs is the most effective way to solve the side effects of anticancer drugs.
3. Mitochondria-targeted lipophilic cations
At present, there are four main drug delivery strategies for mitochondrial-targeted delivery: 1) using lipophilic cations; 2) using cell-penetrating peptides; 3) using nanoparticles as drug carriers; 4) using physical intrusion.28
Among them, lipophilic cations are the most common anticancer drug-carrying tools.28,29 They are generally attached to small bioactive molecules and act as mitochondria-orienting moieties (Fig. 1). Lipophilic cations are capable of localizing mitochondria due to their large hydrophobic surface and positive charges. Such a hydrophobic surface enables high-level compatibility between the molecules and the phospholipid bilayer, facilitating molecular passage through the membrane. The positive charges of the lipophilic cations allow them to enter mitochondria in two steps:30 1) 30–60 mV (negatively-charged inner membrane) of cell membrane potential guides TPP molecules into the cytoplasm and completes the first enrichment step; 2) a more powerful (150–180 mV) mitochondrial membrane potential (negatively-charged inner membrane) drives them to enter into mitochondria and complete the second enrichment step.31 Most importantly, the negative potential in the tumor cell mitochondrial membrane is 60 mV higher than that of normal cells,23,32 allowing selectivity between tumor and healthy cells, which is a very important anticancer drug feature.
Fig. 1. The design of the lipophilic cation-based drug delivery. Lipophilic cations can specifically target mitochondria, similar to “guided missiles”, then acting directly on the target or releasing their carried cargo specifically in the mitochondria.
Although a wide range of lipophilic cations (Table 2) could be used for drug targeting purposes in principle, only TPP has been extensively characterized yet in this context. TPP consists of a positively charged phosphorus atom surrounded by three hydrophobic phenyl groups, providing an extended hydrophobic surface despite the positive charge of the phosphorus atom (Table 2).
Table 2. clog P values of various lipophilic cationic compounds a .
| Compound | Structure | clog P | Compound | Structure | clog P | Compound | Structure | clog P |
| Pyridinium cation |

|
–4.20 | Quinolinium cation |

|
–2.82 | Quaternary ammonium cation |

|
–3.67 |
| Acridinium cation |

|
–0.33 | Diphenyliodonium cation |

|
2.66 | TPP cation |

|
6.29 |
| Rhodamine cation |

|
4.93 | Janus Green cation |

|
3.31 | Thiopyrylium cation |

|
2.39 |
| Benzimidazolium cation |

|
–0.61 | Benzothiazolium cation |

|
–1.73 | Benzoxazolium cation |

|
–2.62 |
| Indolium cation |

|
4.31 | Berberine cation |

|
–0.75 | Tetraguanidinium cation |

|
4.12 |
aclog P (calculated log P): calculated by ChemDraw 2014.
4. Advantages of TPP in mitochondria-targeted drug delivery
4.1. Easy membrane barrier crossing
Both the cell and the mitochondrial membranes are bilayer polar membranes composed of lipid molecules. They form a continuous barrier around cells or mitochondria, preventing free and limitless diffusion of ions, proteins, and other molecules. Moreover, they are impermeable to most water-soluble (hydrophilic) molecules.33
TPP cations can easily pass through the phospholipid bilayer due to their liposolubility (or hydrophobicity).30,34 Within a certain range, the higher the liposolubility of cations, the easier they pass through the cell membrane. The clog P value is a measure of lipophilicity or hydrophobicity. Table 2 summarizes the clog P values of various lipophilic cations. The high clog P value of the TPP cation partially explains why it can easily pass through the membrane barrier.
Another reason allowing TPP cations to easily cross biological membranes is their low activation energy for movements through the hydrophobic membrane core.30 This is in contrast with other hydrophilic cations (such as Na+), which cannot cross biological membranes unless their transport is facilitated by ionophores or carrier proteins. The impermeability of the biological membranes for hydrophilic cations is mainly due to the excessive activation energy required for transferring them from the aqueous environment to the hydrophobic membrane core.33 Electrostatic interactions and hydrophobic forces also contribute to the activation energy.33 Among them, the main electrostatic energy can be described by the Born energy (WB: the free energy of transfer in moving a charged cation from the aqueous environment to the membrane lipid core).
 |
1 |
In eqn (1), ε0, ε1, and ε2 are universal constants (ε0: vacuum permittivity, ε1: dielectric constant within the membrane core, ε2: the dielectric constant of water), q is the electrical charge per cation mole (the lipophilic cations listed in Table 2 have one positive charge per molecule), and r is the ionic radius. Therefore, the formula can be simplified to WB = Q/r (eqn (1)), so the activation energy (WB) required for driving the cation into the membrane is inversely proportional to the ionic radius (r).30 The TPP cation has a large ionic radius (∼4 Å) due to its three hydrophobic phenyl groups. This, combined with the strong hydrophobicity, makes the activation energy required for TPP to penetrate biological membranes significantly lower than that for other lipophilic cations.
4.2. Efficient mitochondrial uptake and accumulation
All cell and mitochondrial membrane potentials could contribute to the TPP cation accumulation in mitochondria, which also raises concerns about cytotoxicity in normal cells. However, the mitochondrial membrane potential of cancer cells is usually about 60 mV higher than that of healthy cells, which is a major benefit for potential-driven drugs and could enable their selectivity between healthy and cancer cells.23,32
Driven by the high mitochondrial membrane potential, TPP-containing compounds can be rapidly taken up by mitochondria in vivo.28,30 This process can be described by the Nernst equation (eqn (2)). At equilibrium, the ion concentrations at both sides of the charged membrane can be described by the following equation:
 |
2 |
where ΔΨ is the potential variation, R is the universal gas constant, T is the temperature, n is the valence of the charged species, F is Faraday's constant, C[TPP cation]in is the concentration inside the membrane-enclosed compartment, and C[TPP cation]out is the concentration outside the membrane. For a single-charged cationic species accumulating in a membrane-surrounded space with a potential ΔΨ, which is negative inside and at 37 °C, the equation can be simplified as eqn (2).
At 37 °C, each 61.5 mV increase in the membrane potential will increase the cation uptake by 10-fold, leading to a 5-to-10-fold increase in the TPP cation concentration within the cytosol and a 100-to-1000-fold increase within the mitochondria (Fig. 2).28
Fig. 2. Cellular uptake of TPP-based compounds driven by cell membrane potentials and mitochondrial membrane potentials.
The mitochondrial uptake of the TPP-based compounds has been assessed in vivo in detail.35–37 Surprisingly, such compounds could be completely absorbed by mitochondria in the tissues 5 min after their intravenous administration.38 For instance, the mitochondrial accumulation ratio of all AntiOxCINs (a series of mitochondria-targeted antioxidants based on caffeic acid) is almost greater than 2000 in energized rat liver mitochondria.39 At a given membrane potential, the mitochondrial uptake of MitoQ is always ∼5-fold greater than that of TPMP (methyltriphenylphosphonium), even though the Nernst equation predicted identical ratios of MitoQ and TPMP.38 This is because the stronger binding of MitoQ to the proteins on the mitochondrial inner membrane increases its ΔΨm-dependent uptake.38
4.3. TPP-based compound safety
Most lipophilic cations are usually toxic to mitochondria at high concentrations. These compounds share mitochondrial accumulation as a common mechanism.25 However, their mitochondrial toxicity mechanisms vary greatly. For example, the pyridinium compound (dequalinium chloride) and thiacarbocyanines (benzothiazolium) can inhibit NADH–ubiquinone reductase of the respiratory complex I.40,41 Rhodamine 123 (Rh123) and thiopyrylium AA-1 can affect mitochondrial bioenergetic functions by inhibiting F0F1-ATPase.42,43 Additionally, rhodacyanine MKT-077 can cause a general mitochondrial membrane perturbation and lead to the non-specific inhibition of membrane-bound mitochondrial respiratory enzyme activity.44 The newly discovered F16 induces cell apoptosis by opening mitochondrial permeability transition pores.45 This implies that the TPP-based compound-related cytotoxicity mainly originates from the carried cargo and not TPP itself.
Although TPP conjugated to a stearyl residue (STPP) has been confirmed to contribute to mitochondrial membrane permeabilization, and induces instability and lipid bilayer property modifications,46 TPP cations exhibit cytotoxicity only at very high concentrations. TPP-based compounds are relatively less toxic and can be administered at high doses indefinitely without significant damage to organs, such as the heart, liver, and kidneys,36,37 and have been given safely long-term to several rodent models.35,47 Importantly, they have also been administered orally to patients in Phase II studies for up to 1 year with no safety concerns.48,49 At present, MitoQ and SkQ1 have entered clinical trials, and neither compound has shown any sign of systemic toxicity when used at pharmacologically relevant doses.48–50 MitoQ is currently available in health food stores and skincare shops, with more than 10 skincare products available. Additionally, the results obtained so far in humans indicate that MitoQ can be safely administered daily for one year at a dosage (1 mg kg–1) high enough to decrease liver damage in patients with hepatitis C virus.48,49
Regarding the delivery and metabolism of TPP-based compounds in the body, extensive work has shown that they can be delivered to mitochondria via oral administration in drinking water or tablets, intraperitoneal (IP) or intravenous (IV) injections, eye drops and ex vivo organ infusions.35,48,49,51 Studies to date have shown that the metabolism of TPP-based compounds occurs mainly through the response of the “cargo” component, while the TPP function remains unmodified and the compounds are excreted into the bile and urine. For example, the ubiquinol moiety in MitoQ is modified by sulfation or glucuronidation and then excreted through the biliary pathway, or into the urine through the kidneys.52,53 Thus, the metabolism of the TPP-based compounds is highly dependent on the chemical properties of the “cargo” component.
4.4. Advantages of the synthesis
As the crucial intermediates of various Wittig reactions, TPP salts have been extensively studied.54 In most cases, the TPP moiety can be easily introduced into molecules by simply replacing a leaving group with triphenylphosphine at the final step of the synthetic procedure in order to obtain the target products. Certainly, during the synthesis of mitochondrial-targeting drugs, various synthetic strategies have been further studied.
The most common method for synthesizing TPP salts is to remove a leaving group, such as a halide, mesylate, or tosylate, from an appropriate alkyl or benzyl precursor through nucleophilic substitution (Fig. 3A).55–58 In most reports, such a substitution reaction occurs on primary carbon atoms in good to high yields and is performed under reflux in a solvent, such as toluene, acetonitrile, or acetone, for a period of 4–20 h. Moreover, TPP salts can also be synthesized from halogenated benzene or pyridine,59 substituted pyridine or pyrimidine,60 1,3-propyl sultone or 1,4-butane sultone,61,62 substituted ethylene oxide,63 and so on (Fig. 3).
Fig. 3. Five main strategies for TPP synthesis.
5. TPP-based mitocans
TPP-based mitocans mainly target cancer cells with high mitochondrial membrane potential and deliver drugs or bioactive molecules to the cancer cell mitochondria to achieve their purpose of treatment or killing. Most of these compounds target a particular aspect of the cancer cell mitochondrial functions, such as high ROS levels, abnormal oxidative phosphorylation, or other important physiological functions.
5.1. TPP-linked antioxidants as anticancer agents
Since cancer development is closely related to ROS, many TPP-based antioxidants have been used in anti-cancer research. There are two main ROS signaling pathways in tumor cells: ROS-induced tumor progression and ROS-induced tumor apoptosis.64 Cancer cells produce ROS to help stimulate growth, cell survival, and inflammation, established sources of carcinogenesis.65–68 Meanwhile, cancer cells also express enhanced levels of antioxidant proteins to prevent the increased ROS levels from reaching such a cytotoxic concentration that could be incompatible with growth (Fig. 4).66 Therefore, it is imperative for cancer cells to maintain a steady ROS balance. Antioxidants can disrupt the ROS balance by participating in redox reactions, thus breaking ROS-related chain reactions, providing hydrogen atoms, and improving the antioxidant enzyme activity,29 thereby suppressing cancer cells.29,65
Fig. 4. Balancing ROS generation and ROS scavenging allows cancer cells to keep ROS levels in the tumorigenic range.66 Adapted from ref. 67 with permission from BioMed Central Ltd., copyright 2014.
As the natural redox substrate of respiratory chain complexes, mitochondrial targeting of ubiquinone and analogs (Fig. 5) has been widely studied.67 MitoQ, as a chain-breaking antioxidant, can block the formation of peroxides, and allow recycling of MitoQ to its ubiquinol form via reduction by complex II. In contrast, MitoQ cannot be oxidized by complex III, explaining why it does not function as an electron carrier in the mitochondrial respiratory chain.69 MitoQ has been widely used in disease models both in vivo and in vitro. Meanwhile, MitoQ has also been reported to exhibit selective toxicity toward cancer cells. MitoQ treatment led to an irreversible inhibition of breast cancer cell clonogenic growth through a combination of autophagic and apoptotic mechanisms.70 SkQ1, a structurally similar analog of MitoQ, has been shown to suppress spontaneous tumorigenesis in p53–/– mice, as well as HCT116/p53–/– tumor xenograft growth in athymic mice.67 Moreover, both MitoQ and SkQ1 have been tested in vivo and have proven clinical safety, but related anticancer products have not yet been launched.
Fig. 5. Mitochondria-targeted antioxidants for anticancer agents.
Mito-CP (Fig. 5) is a mitochondria-targeted nitroxide developed as an antioxidant agent and mitochondria-targeted mimetic of superoxide dismutase.71 It exhibited an inhibitory effect on anchorage-independent growth in HCT-116 colon cancer cells.72 Mito-CP also reportedly suppresses medullary thyroid carcinoma cell survival in vitro and effectively suppresses the growth of xenografts in mice (dosage: 40 mg kg–1) without obvious side effects.73 Its main effector mechanism involves the induction of caspase-dependent apoptosis.74
As for mitochondria-targeted vitamin E, Mito-ChM and its acetate (Mito-ChMAc) were capable of inducing cell death in several lines of breast cancer cells.75 Both Mito-ChM and Mito-ChMAc exhibited anti-proliferative effects and cytotoxicity in several breast cancer cells with different genetic backgrounds. Furthermore, Mito-ChM effectively inhibited tumor growth in breast cancer xenograft mice (60 mg kg–1 five times a week, from Monday to Friday) without causing significant changes in kidney, liver, and heart weights or other major morphological changes.75
5.2. TPP-linked anticancer agents based on natural products
Many early cancer chemopreventive drugs are limited to nutrients, such as vitamin C, vitamin A, calcium, and so on. However, in recent decades, non-nutritive chemical plant constituents have also received increasing attention.
Curcumin ([1E,6E]-1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-dien-3,5-dion), a polyphenolic compound found in turmeric, exhibits anti-inflammatory, antimutagenic, anticancer, and antioxidant activities.76 Curcumin can inhibit the proliferation and survival of almost all types of tumor cells, although it shows limited efficacy due to its low bioavailability in the blood plasma and tissues, especially insufficient intracellular accumulation.77 Mito-curcumin (Fig. 6) exhibited significant cytotoxicity and antiproliferative activity against MCF-7, MDA-MB-231, SKNSH, DU-145, and HeLa cancer cells with a much lower IC50 value compared to curcumin. It exhibited a minimal effect on healthy mammary epithelial cells (MCF-10A).77
Fig. 6. TPP-linked natural products for anticancer agents.
Resveratrol (3,5,4-(trihydroxystilbene)), a triphenolic compound that naturally occurs in grapes and other plants, can scavenge superoxide, peroxynitrite, hydroxyl radicals, and radicals induced by metals. As an inhibitor of protein peroxidation, it can cross the blood–brain barrier and reduce malondialdehyde levels in rat brain.78 Mito-resveratrol (Fig. 6), a mitochondria-targeted product of resveratrol, exhibited powerful antiproliferative activity against colon tumor cells. The postulated mechanism involved in this process was to increase the formation of H2O2, as exogenously added PEG-catalase (rather than PEG-SOD) attenuated the antiproliferative effects of the compound.79 Further studies have clarified that mitochondrial complexes I and III have been identified as potential sources of superoxide following Mito-resveratrol treatment. Interestingly, Mito-resveratrol also behaves as a mitochondrial uncoupler.80
Quercetin belongs to the flavonoid family and is mainly found in most plants, including fruits, vegetables, green tea, and even red wine with antioxidant activities.81 Quercetin has anti-inflammatory, anticancer, and anti-prostate cancer activities, and has good curative effects on high cholesterol, kidney transplantation, asthma, diabetes, viral infections, pulmonary diseases, schizophrenia, and cardiovascular diseases. Quercetin has the ability to scavenge hydroxyl radicals, hydrogen peroxide, and superoxide anions.82 TPP-conjugated quercetin can increase its mitochondrial accumulation and biological activity. Mito-quercetin (Fig. 6) reportedly inhibits ATPase in isolated rat liver mitochondria and colon tumor cell proliferation more efficiently than quercetin itself.83 When used in the range of 5–20 μM, Mito-quercetin acts as an inducer of the mitochondrial permeability transition and has pro-oxidant activity. Furthermore, Mito-quercetin behaves as an uncoupler in isolated mitochondria, causing depolarization and stimulating oxygen consumption.84
Honokiol (HNK), a key bioactive compound present in magnolia bark extracts, exhibits anticancer and anti-metastatic properties in a variety of in vitro and in vivo models. In carcinogen-induced mouse models, high-dose HNK can inhibit the development of squamous cell carcinoma (SCC),85 where HNK can suppress mitochondrial respiration, decrease ATP levels, and increase the generation of ROS. Mitochondria-targeted honokiol (Mito-HNK) (Fig. 6) facilitated mitochondrial HNK accumulation. Such a significant increase in HNK efficacy against high metastatic lung cancer lines in vitro.86 Mito-HNK was >100-fold more potent than conventional HNK in inhibiting cell proliferation, inhibiting mitochondrial complex I, stimulating ROS generation, oxidizing mitochondrial peroxiredoxin-3, and suppressing mitoSTAT3 phosphorylation. For mice with brain metastases from lung cancer, Mito-HNK can induce the production of mediators associated with cell death and decrease the pathways that support invasion and proliferation. In contrast, in non-malignant stroma, Mito-HNK can suppress pathways that support metastatic lesions, including those involved in inflammation and angiogenesis. Moreover, Mito-HNK showed no toxicity in mice over 8 weeks of administration (3.75 μmol kg–1 each mouse) and it can preferentially target the metabolic vulnerabilities of primary and metastatic lung cancers.86
Betulinic acid is a pentacyclic triterpenoid extracted from birch with antitumor, antiviral, and anti-inflammatory activities. Previous studies have found that betulinic acid can induce cancer cell apoptosis, inhibit tumor growth, and fight angiogenesis. It exhibits selective toxicity against cancer cells, demonstrating its potential for cancer therapy.87 A series of mitochondria-targeted analogs of betulinic acid have been synthesized and tested for cytotoxic effects against cancer cells. Among them, Mito-dihydro betulinic acid (Fig. 6) exhibits enhanced antitumor activity in mastocytoma P-815 and Ehrlich carcinoma cell lines.88
Isosteviol (16-oxo-ent-beyeran-19-oic acid) is a derivative of stevioside and is structurally related to diterpenoid acids. Isosteviol can markedly inhibit chemically induced mouse skin carcinogenesis in vivo, suggesting its potential chemopreventive properties.89 However, the in vitro isosteviol biological evaluation failed to reveal its cytotoxic effects on human cancer cell lines. However, TPP-conjugated isosteviols (Fig. 6) have shown excellent antimitotic activity, which suggests their potential application in the anticancer field.90
5.3. TPP-linked anticancer agents based on commercialized drugs
Several studies have shown that the utilization rate and efficacy of certain commercialized drugs with anticancer activity could be improved through their mitochondrial targeting.
Artemisinin (ART) is a specific antimalarial drug that can effectively kill malaria parasites and save millions of lives. Four decades after its discovery, ART-based combination therapies (ACTs) remain the first-line treatment for malaria recommended by the World Health Organization (WHO).91 Interestingly, recent studies reported that ART analogs showed limited efficacy in certain tumor cell lines, which suggested their potential as anticancer agents.92 Remarkably, ART TPP-conjugation (Fig. 7) allowed mitochondrial ART delivery and it was a more potent cancer inhibitor than its parent compound.93
Fig. 7. TPP-linked commercialized drugs for anticancer agents.
Chlorambucil is an anti-cancer drug and immunosuppressive agent.94 It is a member of the nitrogen mustard class of DNA alkylating agents, mainly used for the treatment of chronic lymphocytic leukemia, lymphomas, such as Hodgkin's disease, non-Hodgkin lymphoma, and Waldenstrom's macroglobulinemia, and some solid tumors.95 However, it exhibits limited safety and efficacy. Selective mitochondrial targeting of chlorambucil to cancer cells based on ΔΨm could help in overcoming these limitations.96 Mito-chlorambucil (Fig. 7) localizes into the cancer cell mitochondria, where it affects mtDNA and induces cell cycle arrest and cell death, resulting in an 80-fold-enhanced anticancer effect among such breast and pancreatic cancer cell lines that are insensitive to the parent drug. Significantly, Mito-chlorambucil could also delay tumor progression in a mouse xenograft model of human pancreatic cancer.96
Metformin is a biguanide oral hypoglycemic drug that is currently used in the treatment of type 2 diabetes. It is an inhibitor of mitochondrial electron transfer chain complex I97 and an activator of AMP-activated protein kinase, which might be mediated by mitochondria-derived active nitrogen species.98 Metformin can selectively kill tumor stem cells in breast cancer cell lines both in vitro and in vivo. It is a weak cationic compound that targets the mitochondria to induce cytotoxic effects in tumor cells but is not sufficiently effective.99 However, by attaching TPP to metformin via a 10-carbon aliphatic side chain, Mito-metformin (Fig. 7) is a nearly 1000 times more effective cell proliferation inhibitor in pancreatic ductal adenocarcinoma (PDAC) than metformin. Notably, Mito-metformin potently inhibits mitochondrial complex I in PDAC cells, stimulating superoxide and AMPK activation, and does not affect healthy cells. Moreover, compared with metformin models, Mito-metformin can efficiently trigger G1 cell-cycle phase arrest in PDAC cells, enhance their radiosensitivity, and inhibit PDAC cell growth in preclinical mice.99
Doxorubicin (DOX) is an anticancer drug that can intercalate into DNA and inhibit the progression of topoisomerase II, leading to cell death.100,101 The potency of doxorubicin is limited by the resistance cancer cells could acquire against it. To overcome such resistance, DOX was linked to the TPP cation and tested for its toxicity against MDA-MB-453 breast cancer cells and DOX-resistant strains.102 Interestingly, Mito-doxorubicin did not show increased cytotoxic efficiency compared with free DOX in DOX-sensitive A2780 cells. However, Mito-doxorubicin exhibited greater therapeutic effects in Dox-resistant A2780/ADR cells compared with free DOX. Mito-doxorubicin (Fig. 7) has proven to be not a substrate of the p-glycoprotein (P-gp) efflux pump, while DOX is.102
5.4. TPP-linked anticancer agents based on enzyme inhibitors
The pyruvate dehydrogenase complex (PDC) is a gatekeeper multienzyme complex that links glycolysis with the tricarboxylic acid cycle to regulate pyruvate flux into the mitochondria for OXPHOS.103 The PDC activity can be lost by the reversible phosphorylation of four PDK isoforms (PDK1, 2, 3, and 4), while the dephosphorylation of two pyruvate dehydrogenase phosphatases (PDPs) restores its activity.104 Therefore, the inhibition of PDKs to activate PDC is an attractive therapeutic strategy to reverse the abnormal metabolic pathways and inhibit cancer cell proliferation. Among the four PDK isoforms, PDK1 is most associated with cancer malignancy.105 Thus, PDK1 is an attractive target, and PDK1 inhibition could provide a promising approach to terminate or at least greatly reduce cancer cell growth.106
Dichloroacetophenone (DAP) (Fig. 8) has been identified as a PDK inhibitor in a screening campaign based on pyruvate dehydrogenase activity. Compound 1a (Fig. 8) was a more efficient PDK1 inhibitor than DAP. When incorporated with the TPP moiety,106 all TPP-conjugated compounds exhibited significant activities in vitro, especially compound 1f (Fig. 8), which inhibited PDK1 with an EC50 value of 0.12 μM and reduced NCI-H1650 cell proliferation with an IC50 value of 0.21 μM.106 In addition, 1f decreased the extracellular acidification rate and lactate formation, increased ROS production, and depolarized the mitochondrial membrane potential in NCI-H1650 cells. 1f could also serve as a potential modulator of mitochondrial oxidative phosphorylation and reprogram the glucose metabolic pathways in cancer cells.106
Fig. 8. Chemical structures of DAP, 1a, DCA, 1f, and Mito-DCA.
Dichloroacetate (DCA) is also considered as a PDK1 inhibitor, causing an increased glycolytic carbon entry into the citric acid cycle as acetyl-CoA.107,108 As a mitochondrial kinase inhibitor, DCA shows poor uptake, weak bioavailability, and limited ability to reach its target.109 To improve DCA bioavailability and increase its mitochondrial accumulation, it was linked to a TPP moiety. Mito-DCA (Fig. 8) was a 100–1000-fold more potent inhibitor of several prostate cancer cell lines than the parent DCA compound. Surprisingly, it did not show any significant metabolic effects on healthy cells, but affected tumor cell mitochondrial dysfunction, inhibiting abnormal glycolytic pathways and subsequent apoptotic cell death.109
MET kinase is a hepatocyte growth factor (HGF) receptor. The HGF-MET pathway is frequently activated during tumor development, growth, and metastasis.110,111 Therefore, Met kinase is an important cancer therapeutic target. MET kinase overexpression in cancer cells is also associated with a primarily mitochondrial Met localization.112 The presence of MET kinase in mitochondria converts cellular metabolism into a state of heightened glycolysis, increases tricarboxylic acid (TCA) cycle activity, and facilitates oxidative phosphorylation. Such metabolic remodeling can conceivably enhance the viability of cancer cells by increasing ATP production and providing abundant TCA cycle intermediates for biomass production.112 PHA-665752 is a selective MET kinase inhibitor. Its TPP-conjugation (TM608) (Fig. 9) results in a rapid mitochondrial localization in MET-overexpressing erlotinib-resistant HCC827 cells and the TM608-mediated inhibition of MET activation is time-dependent. TM608 is reportedly as potent as PHA-665752 in inducing apoptosis and abrogating NSCLC cell viability.113
Fig. 9. Chemical structures of PHA-665752 and TM608.
Hsp90 (heat shock protein 90) is an ATP-dependent molecular chaperon. The mitochondrial Hsp90 pool suppresses cell death and reprograms energy metabolism in cancer cells.114,115 Hsp90 expression and activity are increased in most cancer cells, playing important roles in malignant cell survival, which are often exposed to stress conditions. Since cancer cells rely on the pro-survival function of Hsp90, many chemical Hsp90 inhibitors have been developed to date, and some already entered clinical trials.116 Geldanamycin is a natural antibiotic targeting Hsp90. The TPP-linked derivative of geldanamycin (G-TPP) (Fig. 10) has shown stronger antitumor effects than geldanamycin in xenograft models in vivo.117 In addition, mitochondrial tumor necrosis factor receptor-associated protein 1 (Trap1) is one of the members of the Hsp90 family. In order to efficiently and selectively inhibit Trap-1 in mitochondria, the Hsp90 inhibitors PU-H71 and BIIB (Fig. 10) were linked to TPP (SMTIN-P01 and TPP-BIIB, respectively).116 The results showed that TPP-BIIB had no obvious inhibitory effect on different tumor cell lines. However, SMTIN-P01 was able to induce mitochondrial membrane depolarization and showed stronger cytotoxicity toward cancer cells than its parent inhibitor.116
Fig. 10. Chemical structures of geldanamycin, BIIB-021, PU-H71, and their TPP-conjugated products G-TPP, TPP-BIIB, and SMTIN-P01.
Hexokinase is the first enzyme in the glycolysis pathway, and it is also the rate-limiting enzyme for glycolysis in tumor cells. Its increased expression and activity allow tumor cells to ensure sufficient energy supply under hypoxic conditions. Lonidamine (LND) is a proven hexokinase inhibitor that selectively inhibits aerobic glycolysis and energy metabolism in tumor cells.118,119 LND can also inhibit the activity of succinate–ubiquinone reductase in respiratory complex II, leading to enhanced ROS formation.120 LND was previously evaluated in phase II and phase III trials of lung cancer and was proven safe but with limited efficacy.121 Mito-lonidamine (Mito-LND) (Fig. 11) is a 100-fold more potent tumor-selective inhibitor of oxidative phosphorylation than LND.122 It can effectively inhibit lung cancer cell mitochondrial bioenergetics and reduce lung cancer cell viability, growth, progression, and metastasis of lung cancer xenografts in mice. Mito-LND has been proven unlikely to directly inhibit hexokinase activity, but it significantly inhibits mitochondrial complexes I and II, thereby blocking mitochondrial bioenergetics, stimulating ROS formation, oxidizing mitochondrial peroxiredoxin, inactivating AKT/mTOR/p70S6K signaling, and inducing autophagic cell death in lung cancer cells.122
Fig. 11. Chemical structures of lonidamine and Mito-lonidamine.
5.5. TPP-linked anticancer agents based on photosensitizers
Photosensitizers are central to photodynamic therapy (PDT), a treatment method for certain cancers.123 Photosensitizers can absorb light at specific wavelengths and convert them into useful energy,124 which can be applied for the inactivation of selected proteins or light-induced cell ablation. As an emerging non-invasive treatment modality, PDT involves the application of photosensitizers, light, and endogenous molecular oxygen to eliminate cancer cells.123 Separately, each component is non-toxic, but when a photosensitizer is illuminated with a specific wavelength, the photochemical reaction results in the formation of highly reactive singlet oxygen species, which are responsible for cytotoxicity and cell death.123 One of the key factors in determining the therapeutic function is the photosensitizer action site. The cancer-specific cytotoxicity of the photosensitizers could be precisely regulated by conjugating them to TPP, based on the difference in mitochondrial membrane potential between tumor and healthy cells. Therefore, mitochondria-targeted photosensitizers have been extensively studied recently.125–128
MitoPhotoDNP (Fig. 12A) has three constituent parts: a caged dinitrophenol (DNP), a photocleavable linker, and a mitochondria-targeting unit.128 DNP is a classic mitochondrial uncoupler, the o-nitrobenzyl group is a well-established photoactivatable linker, and TPP is a targeting unit. Upon light irradiation, DNP is released from MitoPhotoDNP, causing mitochondrial membrane depolarization in smooth muscle cells. As a classical photosensitizer, MitoPhotoDNP can release a protonophore within mitochondria, thereby preventing ATP production and calcium ion absorption.128 Potentially, this approach could also be used for mitochondrial photoactivated therapeutic drug delivery.
Fig. 12. A) Chemical structures of DNP and MitoPhotoDNP and the three constituent parts of MitoPhotoDNP.117 B) Chemical structures of G-Mito-Pc and the three constituent parts of G-Mito-Pc.
G-Mito-Pc (Fig. 12B) is a novel photosensitizer with dual targeting of tumors and mitochondria.125 On the one hand, it employs silicon(ii) Pc as a photosensitizing component in combination with an anticancer drug (gefitinib). On the other hand, in the axial direction of silicon(ii) Pc, TPP is linked to G-Pc through an alkyl chain forming G-Mito-Pc (Fig. 12B). For the photosensitizer G-Mito-Pc, the introduction of the TPP moiety can increase the likelihood of the photosensitizer targeting tumor cells and it can further enter the cancer cell mitochondria, directly induce in situ ROS generation, decrease MMP, then subsequently cause apoptosis and necrosis.125 The G-Mito-Pc IC50 value is about a dozen fold lower than that of G-Pc under LED light toward EGFR-overexpressing tumor cells. In addition, G-Mito-Pc did not show remarkable cytotoxicity against healthy cells under the same conditions, indicating its excellent selectivity. G-Mito-Pc exhibits a more precise and effective photodynamic action site, and shows ultra-efficient photodynamic cancer therapeutic effects and excellent targeting ability.125
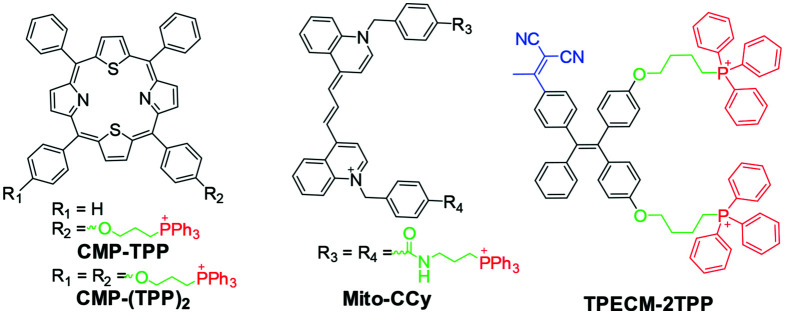
CMP-TPP and CMP-(TPP)2 (Fig. 13) are dithiaporphyrin-based photosensitizers.127 Core-modified porphyrins have been extensively studied as second-generation photosensitizers. The conjugation of CMP to TPP significantly enhanced its intracellular uptake and photodynamic activity in vitro. Under dark conditions, no significant toxicity was observed in cells treated with CMP-TPP and CMP-(TPP)2. This could be due to their inability to generate singlet oxygen in the dark. However, CMP-TPP showed the highest phototoxicity to colon-26 cells after irradiation with a 690 nm diode laser with an IC50 of 0.9 μM, followed by CMP-(TPP)2 with an IC50 of 1.67 μM.127
Fig. 13. Chemical structures of CMP-TPP, CMP-(TPP)2, Mito-CCY, and TPECM-2TPP.
TPECM-2TPP (Fig. 13) was constructed by decorating a typical aggregation-induced emission (AIE) photosensitizer with two positive TPP arms.126 It was designed to target cancer cell mitochondria, and undergo combined chemotherapy and PDT treatment. The TPP moiety contributes to the entry of TPECM-2TPP into HeLa cells and illuminates the mitochondria. Due to the accumulation of positively charged molecules, the mitochondrial membrane potential is lowered, and intracellular oxidative stress increases, leading to the dark toxicity of TPECM-2TPP in HeLa cells.129,130 TPECM-2TPP can generate singlet oxygen upon light irradiation to undergo PDT. Thus, it exhibited more significant toxicity under light irradiation in HeLa cells. Additionally, strong fluorescent signals from TPECM-2TPP in mitochondria have been used to monitor mitochondrial morphology changes during the elimination process.129,130
Mito-CCy (Fig. 13) is a mitochondria-targeted cryptocyanine (CCy)-based photothermogenic photosensitizer.131 CCy-based probes exhibit highly efficient photothermal conversion and represent a new class of photothermal agents in photothermal therapy (PTT). Considering mitochondrial thermosensitivity, Mito-CCy is based on a mitochondria-targeted TPP moiety and cryptocyanine.131 When exposed to 730 nm photoirradiation, Mito-CCy causes a 10 °C temperature rise in the tested system compared to the CCy treatment. It also displayed excellent cytotoxicity toward HeLa cells by inducing endogenous ROS generation. Therefore, it is suggested that Mito-CCy uses heat-induced mitochondria-based ROS to mediate cell elimination, and resends promising PTT photosensitizers.131
5.6. TPP-linked anticancer agents based on thermo-sensitive agents
TPPV is a mitochondria-targeted, thermo-sensitive radical initiator in anticancer therapy. It has two constituent parts, a thermal-sensitive radical initiator (V044) and a mitochondria-targeted TPP moiety132 (Fig. 14). V044 (2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride), a thermo-sensitive water-soluble initiator, was applied as a radical source. According to previous reports, mitochondria maintains physiologically a temperature close to 50 °C, which is approximately 10 °C warmer than the constant body temperature.133 Furthermore, cancer cells are more susceptible to free radicals than normal cells because of their high proliferation rate. Therefore, targeting thermo-sensitive agents to mitochondria is a potential biomedical application for anticancer therapy and can make up for the lack of biomedical applications of cell temperature diversity.132
Fig. 14. TPPV-generated free radicals in the mitochondrial area. Non-targeting V044 generated free radicals at a low rate. However, mitochondria-targeted TPPV was initiated at a higher rate with the mitochondrial heat, leading to a more efficient anticancer effect.
TPPV can effectively accelerate the production of free radicals due to the endogenous mitochondrial heat. Simultaneously, the mitochondria-localized generation of free radicals causes focused damage to mitochondria, especially in cancer cells, inducing cancer cell apoptosis more effectively.132 In addition, it was confirmed that the effective TPPV dose for mice is 30 mg kg–1, which can significantly inhibit tumor growth, and all mice were alive after 30 days, in stark contrast to those without TPPV treatment, which all died within 30 days.132
This therapeutic strategy based on endogenous mitochondrial heat could overcome the restrictions of the tissue penetration depth and the metabolic concerns of nanoparticles, which could be further applied without exogenous limitations.132 More importantly, it was predictable that mitochondrial heat would be a target for mitochondria-targeting therapeutic strategies.
5.7. TPP-linked anticancer agents based on small cytotoxic molecules
In order to kill cancer cells, some cytotoxic small molecules have been used for targeting design. Cytotoxic small molecules, such as alkylating agents and uncouplers, could interact specifically with mitochondrial proteins or induce the production of endogenous toxic substances.
APPCL and APPI (Fig. 15A) were designed by linking tri-(dimethylaminophenyl) phosphonium to chloropropane or iodopropane, respectively,134 while IBTP by linking TPP to iodobutane. Because of the presence of the haloalkyl moiety, APPCL, APPI, and IBTP were assumed to possess mitochondria-targeting protein alkylating capabilities. APPCL and APPI have been reported to exert anticancer effects against ovarian cancer cells in vitro at submicromolar concentrations.134 They could damage the mitochondrial membrane and decrease the number of mitochondria per cell. The disease-free survival of mice with in vivo ovarian cancer xenografts reached 12.5% and 37.5% after 180 days of their treatment with APPI or APPCL, respectively, while all untreated mice died within the first 50 days. The terminal iodine of IBTP makes it susceptible to mitochondrial protein thiol attack to form butylated products.135 It (Fig. 15A) is toxic to MDA-MB-231 breast cancer cells but not to non-tumorigenic MCF-10A epithelial cells. IBTP can also inhibit cell migration and mitochondrial respiration.
Fig. 15. A) Chemical structures of APPCL, APPI, and IBTP. B) Chemical structures of MitoDNP-SUM and the four constituent parts of MitoDNP-SUM.
MitoDNP-SUM (Fig. 15B) is a DNP uncoupler-based mitochondria-targeted uncoupler.136 MitoDNP-SUM has four important functional features: a TPP group to target the pro-drug into the mitochondrial matrix, an arylboronate group to act as an H2O2-sensitive trigger, a 2,4-dinitrophenoxyl group that is a caged form of the classic uncoupler, 2,4-dinitrophenol (DNP), and a linker to link the targeting group and the H2O2-sensitive trigger.136 MitoDNP-SUM has shown to release “free” DNP in mitochondria under the conditions of H2O2 production. Therefore, MitoDNP-SUM could reduce the high membrane potential responsible for ROS production, and it is considered as a potential anti-proliferative drug.
Mito-15d-PGJ2, MitoPQ, and MitoK3 (Fig. 16) all exhibited excellent anticancer activities. 15d-PGJ2 (Fig. 16) is a cyclopentenone-like eicosanoid electrophile. It reportedly localizes into the mitochondria and is responsible for inducing ROS formation in endothelial cells.137 However, research has shown that the target proteins of 15d-PGJ2 could be also found in the cytosol.138 Mito-15d-PGJ2 can make 15d-PGJ2 less effective in targeting cytosolic pathways. It profoundly affected mitochondrial bioenergetics and membrane potential, and increased apoptosis in breast cancer cells, in contrast to 15d-PGJ2.139
Fig. 16. Chemical structures of 15d-PGJ2, paraquat, menadione, and their TPP-conjugated products.
Paraquat (PQ) (Fig. 16) is a sterilant herbicide, regarded as an electron acceptor that acts on the cellular redox system, resulting in excessive superoxide production, which causes cell membrane lipid peroxidation.140 MitoPQ showed a strong inhibitory effect on the HCT-116 human colon cancer cell viability with a cell death rate greater than 50% at 10 μmol L.141 Moreover, MitoPQ could accumulate rapidly within the mitochondria and increase mitochondrial superoxide, showing that it is 1000-fold more potent than untargeted paraquat.141
Menadione (Fig. 16), also known as the precursor of vitamin K3, is a potent cytotoxic compound mainly due to its quinone redox-cycling with ROS production.142 Menadione could also cause hemolytic anemia, hyperbilirubinemia, and nuclear jaundice in infants due to its reaction with thiol groups.143 MitoK3 has changed the redox properties of naphthoquinone due to the chemical modification of its structure.144 It was confirmed that the MitoK3-induced toxicity was due to its capacity to induce mitochondrial dysfunction. In this way, MitoK3 can render cancer cell mitochondria more sensitive to cancer drugs, such as DOX.144
6. TPP-based compound optimization and implications
The development of TPP-based anticancer drugs has been in full swing, and the optimization and improvement of these drugs are also ongoing. Some studies inspired the design of new TPP-based compounds.
6.1. In the TPP moiety
The TPP component could be modified to improve the mitochondrial uptake and cell activity by introducing methyl, alkoxyl, halogen, trifluoromethyl, and other substituents.
In the methylated modified TPP series (1–7) (Fig. 17A), TPP cation 7 (trimethyl-substituted TPP) had the highest log P value followed by cations with dimethyl-substitution (5 and 6) and monomethyl-substitution (2–4).145 As for the mitochondrial uptake, TPP cation 7 has relatively high mitochondrial uptake followed by 5 > 6 > 2 > 3 > 4 and 1. In particular, polymethyl-substituted TPP cations (5, 6, and 7) have a much higher uptake rate than monomethyl-substituted TPP cations (2, 3, and 4).145 In methoxylated modified TPP series (8 and 9), TPP cation 9 has the highest mitochondrial uptake followed by the unmodified TPP cation (1) and 4-methoxy-substituted 8.146 Due to insufficient data, only a few modified TPP derivatives were analyzed in this study. The order of decreasing activity against African trypanosomes was 4-CH3 (2) > 3-CH3 (3) > 4-OCH3 (8) ∼ H (1) > 4-Cl (11) and 4-F (10) > 4-CF3 (12).147 The dimethyl-substituted 5 exhibited higher biological activities against HeLa and FU97 cells than the 4-methyl substituted 2.145,148
Fig. 17. A) Chemical structures of TPP (1), methylated modified TPP series (1–7), methoxylated modified TPP series (8 and 9), halogenated TPP series (10 and 11), and a trifluoromethyl substituted TPP derivative (12). B) Strategies for conjugating the TPP with the cargo. C) Different types of linkers.
In general, the presence of methyl or methoxy groups at key positions on the phenyl ring of TPP results in increased hydrophobicity and mitochondrial uptake. Particularly, polymethyl-substituted TPP cations have much higher uptake rates and their TPP derivatives have better biological activities than monomethyl-substituted TPP derivatives. This could provide a drug design platform for exploring alternative TPP derivatives that can act as optimal drug transporters for enhanced mitochondria-targeted therapies.
6.2. In the linker
The type and length of linkers reportedly affect drug conjugate stability and toxicity.149 As for TPP-based compounds, the linker has two main functions. One is to connect the TPP moiety and the cargo. The other is to adjust the hydrophilicity and hydrophobicity of the compound to further change mitochondrial uptake.
The use of linkers is very flexible. They can be linked with the cargo and then coupled with triphenylphosphine through the above-described methods and vice versa (Fig. 17B). In many cases, it is more practical to first synthesize the TPP-based linker bearing a functional group and subsequently couple it to the drug or bioactive molecule of interest via this functional group. Examples include the formation of ester, amide, ether azide, ether, thioether, or secondary amine bonds (Fig. 17C). The length of the linker is typically 2–10 atoms for TPP-based compounds. In most cases, conjugation to TPP using a long-chain hydrophobic linker is an efficient strategy for mitochondrial uptake and delivery. However, a longer hydrophobic linker does not mean a better activity. Representative examples are MitoQ3, MitoQ5, MitoQ10 (MitoQ), and MitoQ15. MitoQ15 shows the best mitochondrial uptake coupled with a less-efficient activity.34
Therefore, we can choose the linker based on the properties of the compound and the target protein. However, in general, hydrophobic alkyl chains with a 2–10 carbon atom length are the most popular linkers.
6.3. In the cargo
As for the cargo, we have reviewed many types of compounds for a bioactive molecule targeted design. In many cases, TPP-based compounds have ionizable groups, such as Mito-VES, Mito-metformin, and TPP-BIIB, typically bearing carboxylic acid or amine functions.
Here, we summarize the effect of the protonation equilibria of weak acids and bases on their mitochondrial accumulation. For these compounds, the mitochondrial uptake is controlled not only by the mitochondrial membrane potential but also by the cytosolic and mitochondrial pH values (cytosolic pH ≈ 7.2; mitochondrial pH ≈ 8.0). In the case of TPP-based carboxylic acid (pKa ≈ 5) and TPP-based aliphatic amine (pKa ≈ 10), only the uncharged forms cross the membrane and thereby equilibrate with the ΔΨm, as described by the Nernst equation (Fig. 18). Then, the TPP-based carboxylic acid generates a zwitterion by deprotonation, and the TPP-based aliphatic amine generates a dication by protonation. Neither of the zwitterions and the dication is membrane-permeant. However, due to the existence of a pH gradient across the mitochondrial inner membrane (ΔpH ≈ 0.8, basic inside), it has different effects on the TPP-based carboxylic acid and TPP-based aliphatic amine. ΔpH could lead to a greater TPP-based carboxylic acid uptake into the matrix compared to a simple TPP cation, while the TPP-based aliphatic amines are excluded by the ΔpH.28,150
Fig. 18. Scheme of the transport of TPP-based weak acids and TPP-based weak bases.
Finally, we summarize the mitochondrial uptake of TPP-based weak acids and bases. For TPP-based compounds with acidic functions, the mitochondrial uptake increases compared to that of a simple TPP cation as the pKa drops below 8. In contrast, the mitochondrial uptake of the TPP-based compounds with basic functions decreases relative to that of a simple TPP cation as the pKa increases. This is due to the extra accumulation or exclusion, driven by ΔpH, in addition to the accumulation by ΔΨm. This could open new perspectives for rational TPP-based drug design.
7. Conclusion and perspectives
In this study, we comprehensively summarized various types of mitocans and the advantages of the TPP delivery system. TPP cations can easily cross the membrane barrier and be efficiently taken up and accumulated by mitochondria. Several TPP-based mitocans have confirmed clinical efficiency and metabolic safety. Moreover, many important existing bioactive molecules can be transplanted into new mitochondrial targeting functional interferents through simple TPP conjugation, which is of great significance for the development of new mitochondrial drugs, especially mitocans.
Nevertheless, there are many points to pay attention to concerning the TPP-based mitocans. One of them is to improve the TPP molecules. In current drug research, TPP without substituents is widely used. Few studies have been conducted on the effect of the substituents on TPP.145,151 Methyl-functionalized TPP exhibited significant cellular accumulation enhancement of both the cation and its conjugated cargo.146,148 Moreover, TPP species comprising multiple charges, bis-TPP or multi-TPP derivatives, are predicted to be superior mitochondria-targeted vectors and their mitochondrial accumulation is expected to be 1000-fold greater than that of TPP.151 For example, in a series of mitochondria-targeted curcumin derivatives, the accumulation ratio of Mitocur-1 (two TPPs are linked to both hydroxyl groups of the curcumin molecule) in MCF-7 cells was 1010-fold higher than that of Mitocur-2 (Mito-curcumin, shown in Fig. 6, one TPP is linked to one hydroxyl group of the curcumin molecule).77 Compared with Mitocur-2, Mitocur-1 exhibited higher tumor cell toxicity, such as in MCF-7 (IC50: 2.31 μM vs. 5.31 μM), MDA-MB-231 (IC50: 5.10 μM vs. 8.33 μM), DU-145 (IC50: 5.10 μM vs. 8.62 μM), HeLa (IC50: 4.46 μM vs. 7.69 μM) and SKNSH (IC50: 5.12 μM vs. 8.19 μM) cells.77 Certainly, the characteristics and toxicology of these compounds need further study.
In addition, the application of TPP-based mitocans to address anticancer drug-resistance problems is a new development direction. Drug resistance is an important matter affecting all drugs and it seriously threatens their service life. Studies have reported that TPP conjugation could restore the activity of compounds (efflux pump-mediated resistance to these compounds) and showed a low tendency to induce drug resistance.152 Combining TPP with resistance-affected drugs might be a promising approach for drug development.
Finally, the importance of TPP in mitochondria-targeted anticancer drugs has already been known, which provides an opportunity to design new mitochondria-targeted anticancer drugs. Furthermore, the establishment of a more effective drug delivery platform and a TPP-based new application perspective are of great significance for mitocan research and development.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No. 21877125).
Biographies

Jiayao Wang
Jiayao Wang is a doctoral student studying in China Agricultural University, and his tutor is Professor Zhaohai Qin. His main research direction is the design, synthesis and biological research of novel mitochondria-targeted drugs.

Jiaqi Li
Jiaqi Li studied N-heterocyclic carbene chemistry at Beijing Normal University where he received his BSc in 2005 and MSc in 2008 under the supervision of Prof. Ying Cheng. He obtained his Ph. D at Uppsala University with Prof. Pher G. Andersson in 2012. After a postdoctoral stay at Stockholm University, he joined the China Agricultural University as an Assistant Professor in 2013 and became Associate Professor in 2015. His research interests involve stereoselective synthesis and asymmetric catalysis.

Yumei Xiao
Yumei Xiao received her Ph.D. degree in 2003 from China Agricultural University and is currently a professor of chemistry at that institution. In 2013, she worked as a visiting scholar at the National Institutes of Health, Bethesda, Maryland. Her current research interests include development of novel pesticides, organic synthesis, and bioorganic chemistry.

Bin Fu
Bin Fu received his Ph.D. degree from the College of Chemistry, Peking University, in 2000. After postdoctoral research in Peking University and Korea Advanced Institute of Technology in 2003, he then joined the faculty of the College of Science, China Agricultural University. He was promoted to Professor in 2011. His research interests focus on the development of new synthetic methodologies and innovation of new agrochemicals.

Zhaohai Qin
Professor Zhaohai Qin received his Ph.D degree from Shanghai Institute of Materia Medica, Chinese Academy of Sciences in 1992 and was employed as a professor at China Agricultural University in 2001. He is the host of a number of national scientific research projects. His main research interests include total synthesis and structural modification of natural products, design and synthesis of novel bioactive molecules, and chemical biology.
References
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Ca-Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Jemal A., Torre L., Soerjomataram I. and Bray F., The Cancer Atlas, American Cancer Society, Atlanta, GA, 3rd edn, 2019. [Google Scholar]
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C. J., Esteve J., Ogunbiyi O. J., Silva G. A. E., Chen W. Q., Eser S., Engholm G., Stiller C. A., Monnereau A., Woods R. R., Visser O., Lim G. H., Aitken J., Weir H. K., Coleman M. P., Grp C. W. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeten M. S. F., Cloos J., Jansen G. Cancer Chemother. Pharmacol. 2018;81(2):227–243. doi: 10.1007/s00280-017-3489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotow J., Bivona T. G. Nat. Rev. Cancer. 2017;17(11):637–658. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- Yang Y. L., Kitagaki J., Wang H., Hou D. X., Perantoni A. O. Cancer Sci. 2009;100(1):24–28. doi: 10.1111/j.1349-7006.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng E. A., Carlson L. M., Gutman D. M., Harrington W. J., Lee K. P., Boise L. H. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Heiden M. G. V., Cantley L. C., Thompson C. B. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. Science. 1956;124(3215):267–269. doi: 10.1126/science.124.3215.267. [DOI] [PubMed] [Google Scholar]
- Ashton T. M., McKenna W. G., Kunz-Schughart L. A., Higgins G. S. Clin. Cancer Res. 2018;24(11):2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- Gupta S. C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B. B. Antioxid. Redox Signaling. 2012;16(11):1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A., Agnihotri S., Guha A. Oncotarget. 2010;1(7):552–562. doi: 10.18632/oncotarget.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Alexandre J., Huang P. Nat. Rev. Drug Discovery. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Boukalova S., Rohlenova K., Rohlena J. and Neuzil J., in Mitochondrial Biology and Experimental Therapeutics, ed. P. J. Oliveira, Springer International Publishing, Cham, 2018, ch. Mitocans: Mitochondrially Targeted Anti-cancer Drugs, pp. 613–635. [Google Scholar]
- Panda V., Khambat P., Patil S. Int. J. Clin. Med. 2011;2(4):515–529. [Google Scholar]
- Newmeyer D. D., Ferguson-Miller S. Cell. 2003;112(4):481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Green D. R., Kroemer G. Science. 2004;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Dijk S. N., Protasoni M., Elpidorou M., Kroon A. M., Taanman J.-W. Sci. Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-61381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmes C. Trends Mol. Med. 2020;26(1):1–2. doi: 10.1016/j.molmed.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Roth K. G., Mambetsariev I., Kulkarni P., Salgia R. Trends Mol. Med. 2019;26(1):119–134. doi: 10.1016/j.molmed.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P., Hartley R. C. Nat. Rev. Drug Discovery. 2018;17(12):865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- Wang Huanan X. W. Liaoning Zhongyiyao Daxue Xuebao. 2017;19(8):158–162. [Google Scholar]
- Milane L., Trivedi M., Singh A., Talekar M., Amiji M. J. Controlled Release. 2015;207:40–58. doi: 10.1016/j.jconrel.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Rohlena J., Dong L. F., Ralph S. J., Neuzil J. Antioxid. Redox Signaling. 2011;15(12):2951–2974. doi: 10.1089/ars.2011.3990. [DOI] [PubMed] [Google Scholar]
- Dakubo G. D., Mitochondrial Genetics and Cancer, Springer, Berlin, Heidelberg, 2010, ch. 14, pp. 321–344. [Google Scholar]
- Elimadi A., Jullien V., Tillement J. P., Morin D. Eur. J. Pharmacol. 2003;468(2):93–101. doi: 10.1016/s0014-2999(03)01671-6. [DOI] [PubMed] [Google Scholar]
- Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., Cheng G., Lopez M., Kalyanaraman B. Chem. Rev. 2017;117(15):10043–10120. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Li J. Q., Xiao Y. M., Fu B., Qin Z. H. ChemMedChem. 2020;15(5):404–410. doi: 10.1002/cmdc.201900695. [DOI] [PubMed] [Google Scholar]
- Ross M. F., Kelso G. F., Blaikie F. H., James A. M., Cocheme H. M., Filipovska A., Da Ros T., Hurd T. R., Smith R. A. J., Murphy M. P. Biochemistry. 2005;70(2):222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- Yousif L. F., Stewart K. M., Kelley S. O. ChemBioChem. 2009;10(12):1939–1950. doi: 10.1002/cbic.200900185. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Singh K. K. Mitochondrion. 2004;4(5–6):755–762. doi: 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Vitkova V., Petrov A. G. Adv. Planar Lipid Bilayers Liposomes. 2013;17:89–138. [Google Scholar]
- James A. M., Cocheme H. M., Smith R. A. J., Murphy M. P. J. Biol. Chem. 2005;280(22):21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S., Cocheme H. M., Logan A., Abakumova I., Prime T. A., Rose C., Vidal-Puig A., Smith A. C., Rubinsztein D. C., Fearnley I. M., Jones B. A., Pope S., Heales S. J. R., Lam B. Y. H., Neogi S. G., McFarlane I., James A. M., Smith R. A. J., Murphy M. P. Free Radical Biol. Med. 2010;48(1):161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Porteous C. M., Logan A., Evans C., Ledgerwood E. C., Menon D. K., Aigbirhio F., Smith R. A. J., Murphy M. P. Biochim. Biophys. Acta, Gen. Subj. 2010;1800(9):1009–1017. doi: 10.1016/j.bbagen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Smith R. A. J., Porteous C. M., Gane A. M., Murphy M. P. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. F., Prime T. A., Abakumova I., James A. M., Porteous C. M., Smith R. A. J., Murphy M. P. Biochem. J. 2008;411:633–645. doi: 10.1042/BJ20080063. [DOI] [PubMed] [Google Scholar]
- Teixeira J., Cagide F., Benfeito S., Soares P., Garrido J., Baldeiras I., Ribeiro J. A., Pereira C. M., Silva A. F., Andrade P. B., Oliveira P. J., Borges F. J. Med. Chem. 2017;60(16):7084–7098. doi: 10.1021/acs.jmedchem.7b00741. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Watanabe A., Shibuya H., Toru M. Pharmacol., Biochem. Behav. 1993;45(3):691–695. doi: 10.1016/0091-3057(93)90526-y. [DOI] [PubMed] [Google Scholar]
- Anderson W. M., Patheja H. S., Delinck D. L., Baldwin W. W., Smiley S. T., Chen L. B. Biochem. Int. 1989;19(4):673–685. [PubMed] [Google Scholar]
- Modicanapolitano J. S., Aprille J. R. Cancer Res. 1987;47(16):4361–4365. [PubMed] [Google Scholar]
- Modicanapolitano J. S., Weiss M. J., Chen L. B., Aprille J. R. Biochem. Biophys. Res. Commun. 1984;118(3):717–723. doi: 10.1016/0006-291x(84)91453-0. [DOI] [PubMed] [Google Scholar]
- ModicaNapolitano J. S., Koya K., Weisberg E., Brunelli B. T., Li Y., Chen L. B. Cancer Res. 1996;56(3):544–550. [PubMed] [Google Scholar]
- Fantin V. R., Berardi M. J., Scorrano L., Korsmeyer S. J., Leder P. Cancer Cell. 2002;2(1):29–42. doi: 10.1016/s1535-6108(02)00082-x. [DOI] [PubMed] [Google Scholar]
- Fuller N., Rand R. P. Biophys. J. 2001;81(1):243–254. doi: 10.1016/S0006-3495(01)75695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus M. J., Murphy M. P., Franklin J. L. J. Neurosci. 2011;31(44):15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow B. J., Rolfe F. L., Lockhart M. M., Frampton C. M., O'Sullivan J. D., Fung V., Smith R. A. J., Murphy M. P., Taylor K. M., Grp P. S. Mov. Disord. 2010;25(11):1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- Gane E. J., Weilert F., Orr D. W., Keogh G. F., Gibson M., Lockhart M. M., Frampton C. M., Taylor K. M., Smith R. A. J., Murphy M. P. Liver Int. 2010;30(7):1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- Brzheskiy V. V., Efimova E. L., Vorontsova T. N., Alekseev V. N., Gusarevich O. G., Shaidurova K. N., Ryabtseva A. A., Andryukhina O. M., Kamenskikh T. G., Sumarokova E. S., Miljudin E. S., Egorov E. A., Lebedev O. I., Surov A. V., Korol A. R., Nasinnyk I. O., Bezditko P. A., Muzhychuk O. P., Vygodin V. A., Yani E. V., Savchenko A. Y., Karger E. M., Fedorkin O. N., Mironov A. N., Ostapenko V., Popeko N. A., Skulachev V. P., Skulachev M. V. Adv. Ther. 2015;32(12):1263–1279. doi: 10.1007/s12325-015-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime T. A., Blaikie F. H., Evans C., Nadtochiy S. M., James A. M., Dahm C. C., Vitturi D. A., Patel R. P., Hiley C. R., Abakumova I., Requejo R., Chouchani E. T., Hurd T. R., Garvey J. F., Taylor C. T., Brookes P. S., Smith R. A. J., Murphy M. P. Proc. Natl. Acad. Sci. U. S. A. 2009;106(26):10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Fawcett J. P., Tucker I. G. Asian J. Pharm. Sci. 2010;5(3):106–113. [Google Scholar]
- Li Y., Zhang H., Fawcett J. P., Tucker I. G. Rapid Commun. Mass Spectrom. 2007;21(13):1958–1964. doi: 10.1002/rcm.3048. [DOI] [PubMed] [Google Scholar]
- McMurry J. I., Organic Chemistry, Thomson Brooks/Cole, 7th edn, 2008. [Google Scholar]
- Zhang X. L., Guo R. Z., Zhao X. D. Org. Chem. Front. 2015;2(10):1334–1337. [Google Scholar]
- Bonnet L. G., Kariuki B. M. Eur. J. Inorg. Chem. 2006;2006(2):437–446. [Google Scholar]
- Kelso G. F., Porteous C. M., Coulter C. V., Hughes G., Porteous W. K., Ledgerwood E. C., Smith R. A. J., Murphy M. P. J. Biol. Chem. 2001;276(7):4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- Burns R. J., Smith R. A. J., Murphy M. P. Arch. Biochem. Biophys. 1995;322(1):60–68. doi: 10.1006/abbi.1995.1436. [DOI] [PubMed] [Google Scholar]
- Toda Y., Komiyama Y., Kikuchi A., Suga H. ACS Catal. 2016;6(10):6906–6910. [Google Scholar]
- Dolewski R. D., Fricke P. J., McNally A. J. Am. Chem. Soc. 2018;140(25):8020–8026. doi: 10.1021/jacs.8b04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S. M., Zolfigol M. A., Baghery S. Appl. Organomet. Chem. 2016;30(5):311–317. [Google Scholar]
- Song H., Jin F., Jin R., Kang M., Li Z., Chen J. Catal. Lett. 2016;146(7):1264–1272. [Google Scholar]
- Wada M., Tsuboi A. J. Chem. Soc., Perkin Trans. 1. 1987;1(1):151–154. [Google Scholar]
- Liou G. Y., Storz P. Free Radical Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athreya K., Xavier M. F. Nutr. Cancer. 2017;69(8):1099–1104. doi: 10.1080/01635581.2017.1362445. [DOI] [PubMed] [Google Scholar]
- Sullivan L. B. C., Navdeep S. Cancer Metab. 2014;2(1):17–28. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapova L. S., Chernyak B. V., Domnina L. V., Dugina V. B., Efimenko A. Y., Fetisova E. K., Ivanova O. Y., Kalinina N. I., Khromova N. V., Kopnin B. P., Kopnin P. B., Korotetskaya M. V., Lichinitser M. R., Lukashev A. L., Pletjushkina O. Y., Popova E. N., Skulachev M. V., Shagieva G. S., Stepanova E. V., Titova E. V., Tkachuk V. A., Vasiliev J. M., Skulachev V. P. Biochemistry. 2008;73(12):1300–1316. doi: 10.1134/s0006297908120031. [DOI] [PubMed] [Google Scholar]
- Dreher D., Junod A. F. Eur. J. Cancer. 1996;32a(1):30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- James A. M., Sharpley M. S., Manas A.-R. B., Frerman F. E., Hirst J., Smith R. A., Murphy M. P. J. Biol. Chem. 2007;282(20):14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- Rao V. A., Klein S. R., Bonar S. J., Zielonka J., Mizuno N., Dickey J. S., Keller P. W., Joseph J., Kalyanaraman B., Shacter E. J. Biol. Chem. 2010;285(45):34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran A., Kotamraju S., Karunakaran C., Kalivendi S. V., Thomas S., Joseph J., Kalyanaraman B. Free Radical Biol. Med. 2005;39(5):567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Weinberg F., Hamanaka R., Wheaton W. W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G. M., Budinger G. R. S., Chandel N. S. Proc. Natl. Acad. Sci. U. S. A. 2010;107(19):8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starenki D., Park J.-I. J. Clin. Endocrinol. Metab. 2013;98(4):1529–1540. doi: 10.1210/jc.2012-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Zielonka J., McAllister D., Hardy M., Ouari O., Joseph J., Dwinell M. B., Kalyanaraman B. Cancer Lett. 2015;365(1):96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Zielonka J., McAllister D. M., Mackinnon A. C., Joseph J., Dwinell M. B., Kalyanaraman B. BMC Cancer. 2013;13(1):285–308. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken R., Veena M. S., Wang M. B., Srivatsan E. S. Mol. Cancer. 2011;10(1):12–30. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Somepalli V., Golakoti T., Kanugula A. K., Karnewar S., Rajendiran K., Vasagiri N., Prabhakar S., Kuppusamy P., Kotamraju S., Kutala V. K. PLoS One. 2014;9(3):e89351. doi: 10.1371/journal.pone.0089351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S., Holme A. L. Antioxid. Redox Signaling. 2009;11(11):2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- Sassi N., Mattarei A., Azzolini M., Bernardi P., Szabo I., Paradisi C., Zoratti M., Biasutto L. Curr. Pharm. Des. 2014;20(2):172–179. doi: 10.2174/13816128113199990034. [DOI] [PubMed] [Google Scholar]
- Sassi N., Mattarei A., Azzolini M., Szabò I., Paradisi C., Zoratti M., Biasutto L. Biochim. Biophys. Acta, Bioenerg. 2014;1837(10):1781–1789. doi: 10.1016/j.bbabio.2014.06.010. [DOI] [PubMed] [Google Scholar]
- D'Andrea G. Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Murakami A., Ashida H., Terao J. Cancer Lett. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Mattarei A., Biasutto L., Marotta E., De Marchi U., Sassi N., Garbisa S., Zoratti M., Paradisi C. ChemBioChem. 2008;9(16):2633–2642. doi: 10.1002/cbic.200800162. [DOI] [PubMed] [Google Scholar]
- Biasutto L., Sassi N., Mattarei A., Marotta E., Cattelan P., Toninello A., Garbisa S., Zoratti M., Paradisi C. Biochim. Biophys. Acta, Bioenerg. 2010;1797(2):189–196. doi: 10.1016/j.bbabio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Pan J., Zhang Q., Liu Q., Komas S. M., Kalyanaraman B., Lubet R. A., Wang Y., You M. Cancer Prev. Res. 2014;7(11):1149–1159. doi: 10.1158/1940-6207.CAPR-14-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Lee Y., Cheng G., Zielonka J., Zhang Q., Bajzikova M., Xiong D., Tsaih S. W., Hardy M., Flister M., Olsen C. M., Wang Y., Vang O., Neuzil J., Myers C. R., Kalyanaraman B., You M. iScience. 2018;3:192–207. doi: 10.1016/j.isci.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S. Int. J. Mol. Sci. 2008;9(6):1096–1107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak A. Y., Nedopekina D. A., Shakurova E. R., Khalitova R. R., Gubaidullin R. R., Odinokov V. N., Dzhemilev U. M., Bel'skii Y. P., Bel'skaya N. V., Stankevich S. A., Korotkaya E. V., Khazanov V. A. Russ. Chem. Bull. 2013;62(1):188–198. [Google Scholar]
- Takasaki M., Konoshima T., Kozuka M., Tokuda H., Takayasu J., Nishino H., Miyakoshi M., Mizutani K., Lee K. H. Bioorg. Med. Chem. 2009;17(2):600–605. doi: 10.1016/j.bmc.2008.11.077. [DOI] [PubMed] [Google Scholar]
- Strobykina I. Y., Belenok M. G., Semenova M. N., Semenov V. V., Babaev V. M., Rizvanov I. K., Mironov V. F., Kataev V. E. J. Nat. Prod. 2015;78(6):1300–1308. doi: 10.1021/acs.jnatprod.5b00124. [DOI] [PubMed] [Google Scholar]
- Greenwood B. N. Engl. J. Med. 2014;371(5):474–475. doi: 10.1056/NEJMe1407026. [DOI] [PubMed] [Google Scholar]
- Ho W. E., Peh H. Y., Chan T. K., Wong W. S. F. Pharmacol. Ther. 2014;142(1):126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang C. J., Wang J. G., Zhang J. B., Lee Y. M., Feng G. X., Lim T. K., Shen H. M., Lin Q. S., Liu B. Angew. Chem., Int. Ed. 2016;55(44):13770–13774. doi: 10.1002/anie.201607303. [DOI] [PubMed] [Google Scholar]
- Parker L. J., Ciccone S., Italiano L. C., Primavera A., Oakley A. J., Morton C. J., Hancock N. C., Lo Bello M., Parker M. W. J. Mol. Biol. 2008;380(1):131–144. doi: 10.1016/j.jmb.2008.04.066. [DOI] [PubMed] [Google Scholar]
- Rai K. R., Peterson B. L., Appelbaum F. R., Kolitz J., Elias L., Shepherd L., Hines J., Threatte G. A., Larson R. A., Cheson B. D., Schiffer C. A. N. Engl. J. Med. 2000;343(24):1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- Millard M., Gallagher J. D., Olenyuk B. Z., Neamati N. J. Med. Chem. 2013;56(22):9170–9179. doi: 10.1021/jm4012438. [DOI] [PubMed] [Google Scholar]
- Wheaton W. W., Weinberg S. E., Hamanaka R. B., Soberanes S., Sullivan L. B., Anso E., Glasauer A., Dufour E., Mutlu G. M., Budigner G. R. S., Chandel N. S. Elife. 2014;3:1–18s. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. J., Romero I. L., Litchfield L. M., Lengyel E., Locasale J. W. Cell Metab. 2016;24(5):728–739. doi: 10.1016/j.cmet.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Zielonka J., Ouari O., Lopez M., McAllister D., Boyle K., Barrios C. S., Weber J. J., Johnson B. D., Hardy M., Dwinell M. B., Kalyanaraman B. Cancer Res. 2016;76(13):3904–3915. doi: 10.1158/0008-5472.CAN-15-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigsloot M., Tijdens I. B., Mulder G. J., de Water B. V. J. Biol. Chem. 2002;277(39):35869–35879. doi: 10.1074/jbc.M200378200. [DOI] [PubMed] [Google Scholar]
- Singh K. K., Russell J., Sigala B., Zhang Y. G., Williams J., Keshav K. F. Oncogene. 1999;18(48):6641–6646. doi: 10.1038/sj.onc.1203056. [DOI] [PubMed] [Google Scholar]
- Han M., Vakili M. R., Abyaneh H. S., Molavi O., Lai R., Lavasanifar A. Mol. Pharmaceutics. 2014;11(8):2640–2649. doi: 10.1021/mp500038g. [DOI] [PubMed] [Google Scholar]
- Patel M. S., Korotchkina L. G. Biochem. Soc. Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- Zhang S. L., Hu X., Zhang W., Yao H., Tam K. Y. Drug Discovery Today. 2015;20(9):1112–1119. doi: 10.1016/j.drudis.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang S. L., Hu X. H., Tam K. Y. Int. J. Biol. Sci. 2015;11(12):1390–1400. doi: 10.7150/ijbs.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Yu Z. M., Xiang S. C., Li Y. S., Zhang S. L., He Y. Eur. J. Med. Chem. 2018;155:275–284. doi: 10.1016/j.ejmech.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Sun R. C., Fadia M., Dahlstrom J. E., Parish C. R., Board P. G., Blackburn A. C. Breast Cancer Res. Treat. 2010;120(1):253–260. doi: 10.1007/s10549-009-0435-9. [DOI] [PubMed] [Google Scholar]
- Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C. Nature. 2008;452(7184):230–U274. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Pathak R. K., Marrache S., Harn D. A., Dhar S. ACS Chem. Biol. 2014;9(5):1178–1187. doi: 10.1021/cb400944y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso S., Giordano S. Cancer Discovery. 2013;3(9):978–992. doi: 10.1158/2159-8290.CD-13-0040. [DOI] [PubMed] [Google Scholar]
- Wilson T. R., Fridlyand J., Yan Y. B., Penuel E., Burton L., Chan E., Peng J., Lin E., Wang Y. L., Sosman J., Ribas A., Li J., Moffat J., Sutherlin D. P., Koeppen H., Merchant M., Neve R., Settleman J. Nature. 2012;487(7408):505–U1652. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T. N., Zhu Y., Gan C. S., Lee S. S., Zhu J. A., Wang H. X., Li X., Christensen J., Huang S. A., Kon O. L., Sze S. K. Mol. Cell. Proteomics. 2010;9(12):2629–2641. doi: 10.1074/mcp.M110.001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. M., Ng W. H., Chen H., Chomchopbun K., Huynh T. H., Go M. L., Kon O. L. ACS Med. Chem. Lett. 2016;7(8):807–812. doi: 10.1021/acsmedchemlett.6b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caino M. C., Chae Y. C., Vaira V., Ferrero S., Nosotti M., Martin N. M., Weeraratna A., O'Connell M., Jernigan D., Fatatis A., Languino L. R., Bosari S., Altieri D. C. J. Clin. Invest. 2013;123(7):2907–2920. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. H., Plescia J., Dohi T., Rosa J., Doxsey S. J., Altieri D. C. Cell. 2007;131(2):257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Park H. K., Jeong H., Lim J., Lee A. J., Cheon K. Y., Kim C. S., Thomas A. P., Bae B., Kim N. D., Kim S. H., Suh P. G., Ryu J. H., Kang B. H. J. Am. Chem. Soc. 2015;137(13):4358–4367. doi: 10.1021/ja511893n. [DOI] [PubMed] [Google Scholar]
- Kang B. H., Plescia J., Song H. Y., Meli M., Colombo G., Beebe K., Scroggins B., Neckers L., Altieri D. C. J. Clin. Invest. 2009;119(3):454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floridi A., Bruno T., Miccadei S., Fanciulli M., Federico A., Paggi M. G. Biochem. Pharmacol. 1998;56(7):841–849. doi: 10.1016/s0006-2952(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Lawson T. A., Gingell R., Nagel D., Hines L. A., Ross A. Cancer Lett. 1981;11(3):251–255. doi: 10.1016/0304-3835(81)90116-6. [DOI] [PubMed] [Google Scholar]
- Nath K., Guo L. L., Nancolas B., Nelson D. S., Shestov A. A., Lee S. C., Roman J., Zhou R., Leeper D. B., Halestrap A. P., Blair I. A., Glickson J. D. Biochim. Biophys. Acta, Rev. Cancer. 2016;1866(2):151–162. doi: 10.1016/j.bbcan.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Madrid D., Romero Y., Duenas-Gonzalez A. BioMed Res. Int. 2015:1–13. doi: 10.1155/2015/690492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Zhang Q., Pan J., Lee Y., Ouari O., Hardy M., Zielonka M., Myers C. R., Zielonka J., Weh K., Chang A. C., Chen G. A., Kresty L., Kalyanaraman B., You M. Nat. Commun. 2019;10(1):1–14. doi: 10.1038/s41467-019-10042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Xu S. D., Liu B. Adv. Mater. 2018;30(45):e1801350. doi: 10.1002/adma.201801350. [DOI] [PubMed] [Google Scholar]
- Yoo J. O., Ha K. S. Int. Rev. Cell Mol. Biol. 2012;295:139–174. doi: 10.1016/B978-0-12-394306-4.00010-1. [DOI] [PubMed] [Google Scholar]
- Zhao X., Huang Y. C., Yuan G. K., Zuo K., Huang Y. F., Chen J. J., Li J. Y., Xue J. P. Chem. Commun. 2019;55(6):866–869. doi: 10.1039/c8cc09456j. [DOI] [PubMed] [Google Scholar]
- Zhang C. J., Hu Q. L., Feng G. X., Zhang R. Y., Yuan Y. Y., Lu X. M., Liu B. Chem. Sci. 2015;6(8):4580–4586. doi: 10.1039/c5sc00826c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaputra P., Nkepang G., Watley R., You Y. J. Bioorg. Med. Chem. 2013;21(2):379–387. doi: 10.1016/j.bmc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Chalmers S., Caldwell S. T., Quin C., Prime T. A., James A. M., Cairns A. G., Murphy M. P., McCarron J. G., Hartley R. C. J. Am. Chem. Soc. 2012;134(2):758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Y., Zhang G. X., Hu F., Jin Y. L., Zhao R., Zhang D. Q. Chem. Sci. 2016;7(12):7013–7019. doi: 10.1039/c6sc02395a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. L., Gao M., Feng G. X., Liu B. Angew. Chem., Int. Ed. 2014;53(51):14225–14229. doi: 10.1002/anie.201408897. [DOI] [PubMed] [Google Scholar]
- Jung H. S., Lee J. H., Kim K., Koo S., Verwilst P., Sessler J. L., Kang C., Kim J. S. J. Am. Chem. Soc. 2017;139(29):9972–9978. doi: 10.1021/jacs.7b04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Wang X.-Q., Zhang Y., Li C.-X., Zhang M., Cheng H., Zhang X.-Z. ACS Appl. Bio Mater. 2019;2(10):4656–4666. doi: 10.1021/acsabm.9b00739. [DOI] [PubMed] [Google Scholar]
- Chretien D., Benit P., Ha H. H., Keipert S., El-Khoury R., Chang Y. T., Jastroch M., Jacobs H. T., Rustin P., Rak M. PLoS Biol. 2018;16(1):e2003992. doi: 10.1371/journal.pbio.2003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetta A., Gamboa G., Nasseri A., Podnos Y. D., Emma D., Dorion G., Rawlings L., Carpenter P. M., Bustamante A., Patel J. Gynecol. Oncol. 1996;60(2):203–212. doi: 10.1006/gyno.1996.0026. [DOI] [PubMed] [Google Scholar]
- Lin T. K., Hughes G., Muratovska A., Blaikie F. H., Brookes P. S., Darley-Usmar V., Smith R. A. J., Murphy M. P. J. Biol. Chem. 2002;277(19):17048–17056. doi: 10.1074/jbc.M110797200. [DOI] [PubMed] [Google Scholar]
- McQuaker S. J., Quinlan C. L., Caldwell S. T., Brand M. D., Hartley R. C. ChemBioChem. 2013;14(8):993–1000. doi: 10.1002/cbic.201300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez B., Perez-Castillo A., Santos A. J. Lipid Res. 2005;46(4):736–743. doi: 10.1194/jlr.M400392-JLR200. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollon E., Pineda-Molina E., Canada F. J., Perez-Sala D. J. Biol. Chem. 2001;276(38):35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- Diers A. R., Higdon A. N., Ricart K. C., Johnson M. S., Agarwal A., Kalyanaraman B., Landar A., Darley-Usmar V. M. Biochem. J. 2010;426:31–41. doi: 10.1042/BJ20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M. Methods Enzymol. 1984;105:523–532. doi: 10.1016/s0076-6879(84)05072-2. [DOI] [PubMed] [Google Scholar]
- Robb E. L., Gawel J. M., Aksentijevic D., Cocheme H. M., Stewart T. S., Shchepinova M. M., Qiang H., Prime T. A., Bright T. P., James A. M., Shattock M. J., Senn H. M., Hartley R. C., Murphy M. P. Free Radical Biol. Med. 2015;89:883–894. doi: 10.1016/j.freeradbiomed.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Dowd P., Ham S. W., Naganathan S., Hershline R. Annu. Rev. Nutr. 1995;15:419–440. doi: 10.1146/annurev.nu.15.070195.002223. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., French J. K., Claridge R. F. C. Biochem. J. 1979;179(3):665–673. doi: 10.1042/bj1790665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira J., Amorim R., Santos K., Soares P., Datta S., Cortopassi G. A., Serafim T. L., Sardao V. A., Garrido J., Borges F., Oliveira P. J. Toxicology. 2018;393:123–139. doi: 10.1016/j.tox.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Hu Z., Sim Y., Kon O. L., Ng W. H., Ribeiro A. J. M., Ramos M. J., Fernandes P. A., Ganguly R., Xing B. G., Garcia F., Yeow E. K. L. Bioconjugate Chem. 2017;28(2):590–599. doi: 10.1021/acs.bioconjchem.6b00682. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Yang C. T., Wang J., Wang L., Li Z. B., Chen X., Liu S. J. Med. Chem. 2008;51(10):2971–2984. doi: 10.1021/jm7015045. [DOI] [PubMed] [Google Scholar]
- Taladriz A., Healy A., Flores Perez E. J., Herrero Garcia V., Rios Martinez C., Alkhaldi A. A., Eze A. A., Kaiser M., de Koning H. P., Chana A., Dardonville C. J. Med. Chem. 2012;55(6):2606–2622. doi: 10.1021/jm2014259. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Gawne P. J., Ma M. T., Blower P. J., Southworth R., Long N. J. Dalton Trans. 2018;47(43):15448–15457. doi: 10.1039/c8dt02966k. [DOI] [PubMed] [Google Scholar]
- Lu J., Jiang F., Lu A., Zhang G. Int. J. Mol. Sci. 2016;17(4):561. doi: 10.3390/ijms17040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finichiu P. G., James A. M., Larsen L., Smith R. A., Murphy M. P. J. Bioenerg. Biomembr. 2013;45(1–2):165–173. doi: 10.1007/s10863-012-9493-5. [DOI] [PubMed] [Google Scholar]
- Ong H. C., Hu Z., Coimbra J. T. S., Ramos M. J., Kon O. L., Xing B., Yeow E. K. L., Fernandes P. A., Garcia F. Inorg. Chem. 2019;58(13):8293–8299. doi: 10.1021/acs.inorgchem.8b03380. [DOI] [PubMed] [Google Scholar]
- Chang W., Liu J., Zhang M., Shi H., Zheng S., Jin X., Gao Y., Wang S., Ji A., Lou H. Nat. Commun. 2018;9(1):5102. doi: 10.1038/s41467-018-07633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]