Abstract
The WHO has declared the COVID-19 epidemic on January 31, 2020. This virus has infected millions of people worldwide in just a few months. Shortly afterwards, the National Medical Products Administration (NMPA) announced nucleic acid testing as the gold standard for virus detection. Antibody testing is used as well as a supplementary test for suspected cases where nucleic acid detection was negative. In short, nucleic acid–based polymerase chain reaction (PCR) is the mainstream detection method for clinical samples as well as for the detection of SARS-CoV-2 in wastewaters. First data collected around the globe were reported in the last few months being part of the so-called Wastewater-Based Epidemiology (WBE) approach. Selection of concentration methods and primers, laboratory inter-comparison and various modalities of PCR detection of the virus in complex wastewater matrices were flagged up as main bullets that require urgent improvement. Novel approaches to enhance sensitivity, speed and automate streamlined virus detection will be discussed here as well. This list comprises devices mainly used for clinical purposes like Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), Digital PCR, Lab-on-a-chip (LOC) and related platforms as well as Biosensors. The last part will be devoted to the identification of biomolecules to target Covid-19 outbreak based on inflammatory response biomarkers among others. To this end this opinion paper brings for discussion the issue of PCR detection and its limitations as well as new diagnostic methods in WBE.
Keywords: Wastewater-based epidemiology, SARS-CoV-2, COVID-19, Biosensors, Biomarkers, Lab-on-a-chip (LOC)
1. Introduction
Wastewater-based epidemiology (WBE) can be used as early detection system and to determine the scale of COVID-19 outbreak [1,2]. Determination of SARS-CoV-2 is generally carried out with nucleic acid–based polymerase chain reaction (PCR) assay, and used for confirmation of COVID-19 patients around the globe. PCR determinations do offer high sensitivity and specificity, but require complicated sample handling in the laboratory, skilled personnel, and a long period of data processing and analysis (4–6 h). First data on COVID-19 in sewage using PCR were reported for a variety of countries, among them the Netherlands [3], Italy [4,5], Australia [6], Spain [7] France [8], Japan [9], USA [10,11], Ecuador [12], India [13] and Germany [14]. This PCR methodology relies on the fact that an average of 15–83% of patients infected with SARS-CoV-2 have detectable viral RNA in feces, even in the absence of gastrointestinal symptoms or diarrhea [15]. Samples may continue to remain positive in the stool, even when respiratory tract samples become negative whereas urine is often negative.
Another key step in the PCR determination are the concentration methods. There is a variety of them in wastewaters. In one of the US studies [10] ultrafiltration and adsorption-elution in electronegative membranes were used for wastewater concentration. It pointed out that no one of the secondary and final treated effluents samples with chlorine were tested positive for SARS-CoV-2. The second US [11] study from Massachusetts did use a different concentration method based on unfiltered sewage precipitated with polyethylene glycol 8000 (PEG). Remarkably, viral titers observed were significantly higher than expected based on clinical cases, being attributed to asymptomatic patients not measured in clinical tests. In Italy [5] SARS-CoV-2 RNA was detected in raw, but not in treated wastewaters (four and two samples, respectively, sampled in two dates). Viral RNA was not detected in the Milano and Monza wastewater treatment plants (WWTPs), equipped with tertiary treatments, being in agreement with the case of the Murcia region (Spain) using as well tertiary treatments [7]. Interesting to remark that levels of SARS-CoV-2 detected in urban rivers of Quito receiving direct discharges of wastewaters are similar to those of Valencia and Paris, with 5000–10000 hospitalized cases. The main difference was that in Quito [12] only 750 Covid-19 clinical cases were reported, suggesting a lack of PCR-based diagnosis. With this said, the first explanation could be related to the fact that Ecuador is a low sanitation country, with not many facilities to perform the clinical tests. Another point could be attributed to a high level of asymptomatic cases, as reported in one of the US cases [11]. Another relevant example of a first study in a large country comes from India [13]. WBE surveillance with reverse transcription (RT)-qPCR was performed at the Old Pirana WWTP at Ahmedabad, Gujarat, which has 106 million liters per day and receives effluent from Civil Hospital treating COVID-19 patients. Several genes of SARS-CoV-2 were detected only in the influent with no genes detected in effluent. Increasing levels of SARS-CoV-2 genetic loading in the wastewater did correspond to an increase in the number of active COVID-19 patients in the city. The number of gene copies was comparable to those reported in untreated wastewaters of Australia [6]. Lastly, the most recent example is from Germany [14]. Nine municipal WWTPs from different cities of the Federal State of North Rhine-Westphalia were sampled. A set of SARS-CoV-2-specific genes, as well as pan-genotypic gene sequences covering other coronavirus types, were detected using RT-qPCR.
To this end it should be pointed out that other ways to measure COVID-19 outbreak were already highlighted in two opinion papers [1,2] such as rapid ELISA/biosensors/Paper-based tests and monitoring of exposure biomarkers. Paper-based devices would be certainly one of the best measurement solutions for the rapid and on-site detection of COVID-19 in sewage waters and humans as well [2,16] and also the use of other biomarkers of exposure [1]. Recent literature reported the use of lab-on-a-chip (LOC) procedure [17] to analyze SARS-Cov-2 outbreak mainly for clinical purposes.
In short, it is obvious that qPCR is the method of choice in most of the laboratories involved in WBE to detect SARS-CoV-2. This opinion paper wants to bring attention to the reader first that PCR methods should be improved in terms of comparability and sensitivity when applied to WBE mainly due to the complexity of the wastewater matrix. Most importantly is to encourage the scientists involved in virus detection in WBE to think outside the PCR box by considering other complementary ways to detect COVID-19 outbreak in wastewaters.
2. Direct virus concentration and detection methods- PCR based
Detection of SARS-CoV-2 in sewage has been employed as a complementary method to clinical test. It is an early warning indicator of virus spreading in communities, covering both symptomatic and asymptomatic cases. As already mentioned, RT-qPCR analysis is the most commonly used method to determine the concentration of viral RNA in wastewater. To notice that not only PCR detection is relevant when analyzing wastewaters, but concentration methods and the RNA extraction protocol are key steps of this methodology. Generally similar protocols for the influent, secondary-treated sewage are used and a volume of 100 mL of untreated wastewater samples is sufficient to detect enteric viruses. Most of the methods developed were used for non enveloped enteric viruses such as norovirus, and hepatitis A virus. There is a comprehensive list of methods for concentrating viruses from wastewater like electropositive or electronegative membranes, ultrafiltration, polyethylene glycol (PEG) precipitation, ultracentrifugation, skimmed-milk flocculation, monolithic adsorption filtration columns among others.
Excellent review papers on concentration methods of viruses from wastewater were recently published [15,[18], [19], [20], [21], [22]]. D. Lu [21] did highlight that PEG-based separation method is the most used for the COVID-19 in WBE. The authors indicated as well that the electronegative membrane filtration method may have problems with the preferential adsorption of organic matter on the charged membrane surface and the potential risk of clogging when handling turbid wastewater samples.
Standardization of the analytical protocols for determining SAR-CoV-2 in wastewaters by different PCR platforms is as well a matter of concern. To investigate a bit more in this direction De La Rosa and co-workers [ 4 ] did analyze the presence of SARS-CoV- 2 using three different nested RT-PCR assays and one real-time qPCR assay. Primers were also indicated to be very relevant using this methodology. From all the different methods used. a novel nested PCR assay specific for SARS-CoV-2 detection was chosen.
As drawbacks it should be indicated that important information on the analytical approach is often lacking, while there is still no optimization of the whole protocol: sampling, sample storage and concentration, RNA extraction and detection/quantification. A recent critical review paper [20] identified the main issues for consideration, i.e., the development of validated methodological protocols for the virus quantitative analysis in WBE. The last review on this topic from MVA Corpuz et al. [22] highlights the efforts to improve efficiency of virus detection and quantification methods in the complex wastewater and sludge matrices.
Novel approaches based on RNA detection like CRISPR and Digital PCR (dPCR) were used for clinical and aquatic environment applications [[21], [22], [23]]. CRISPR is a powerful technology, mainly employed in gene editing. CRISPR is based on RNA detection and it can achieve low level detection within 30–40 min. A low-cost and accurate CRISPR-Cas12 based lateral flow assay for detection of SARS-CoV-2 was already reported [23]. The entire time of this assay is less than 40 min. In contrast, RT-qPCR needs 4 h.
In recent years, dPCR has gained attention as a novel approach to detect and quantify nucleic acids [24,25]. The major benefit of dPCR over qPCR is the direct absolute quantification of virus genome copy numbers in a sample without the necessity of external calibration. dPCR platforms can generally be divided into two groups: droplet dPCR (emulsion based) and chip-based dPCR (microfluidic). The limit of detection of dPCR is at least 10-fold lower than that of RT-qPCR. The major advantage of dPCR over qPCR is that it performs absolute quantification, and hence, no standards are required.
3. Lab-on-a-chip (LOC) and related platforms
An emerging field of microfluidics also known as the lab-on-a-chip (LOC) technologies or micro total analysis system includes a wide range of diagnostic devices. LOC technologies advanced from original devices that can conduct a single task to integrated systems capable of performing complex jobs. Each integrated LOC platform typically contains sets of microfluidic elements, dedicated to single operations such as reagent storage, fluid transport and mixing, detection, and possibly collection. Currently, the systems consist of complex devices with interconnected micro-channel networks provided of, valves, mixers, pumps, reaction chambers, and detectors. In short, they are able to perform many laborious benchtop protocols with minimal operator handling in clinical diagnostics and with a low-cost mass production. LOC-based techniques are widely used for viral detection in clinical applications like in the cases of Ebola virus disease, dengue fever hepatitis and human immunodeficiency virus (HIV) and others [[26], [27], [28]]. The LOC concept has been expanded using different types of configurations, called LionX systems, adopting miniaturized fluidic manipulation platforms and automated virus detection. As an example LOC-related platforms are depicted in Fig. 1 and include Lab-in-a Tube, Lab-in-a Box and even Lab on-a-Drone systems among others [28].
Fig. 1.
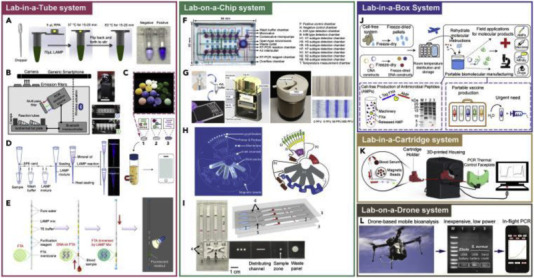
The LionX Systems for NA-Based Virus Detection (Reproduced with permission from Quin et al., 2020 ([28]).
PCR systems for Point of Care (POC) or POC testing (POCT) contributed to diagnosis during pandemics such as the 2014 Ebola virus outbreak. In this respect, Bill & Melinda Gates Foundation, developed the GeneXpert Ebola Assay based on the GeneXpert system. The GeneXpert Ebola Assay is fully automated and only requires the placing of the patient sample into the cartridge and inserting the cartridge into a compact desktop-level instrument. It takes ≈2 h for an entire assay. The COVID-19 global pandemic has greatly speed up the development of POCT systems, as well as the number of companies prepared to fund such work. There is a long list of companies that have developed systems for the diagnosis of COVID-19-like outbreaks (i) Eiken Chemical Co. in Japan based on multiple testing using a microfluidic cartridge with 25 wells, (ii) Roche created a POCT system called cobas® Liat®, a fast and fully automated sample-to-answer system based on a PCR capable of testing samples in 20 min or less.(iii) ID NOW, from Abbott Laboratories, is a fully integrated sample-to-answer that is currently available with modified primers to diagnose the COVID-19 virus, (iv) Biofire® Filmarray®, from BioMerieux, uses microfluidic technology integrating nucleic acid extraction, purification and PCR amplification into a single chip and resulting in sequential and accurate detection. It was previously used for the detection of Ebola virus and, at present, a COVID-19 test kit has been approved by the FDA (v). GeneXpert® developed by Cepheid™ integrates sample preparation, nucleic acid amplification and detection into a small detection kit. (vi) RTisochip® proposed by CapitalBio™ in China can detect 6 common respiratory viruses including COVID-19 in a single chip within 1.5 h. This system not only detects SARS-CoV-2, but also effectively identifies patients with influenza and COVID-19.
Compared to the SARS-CoV and MERS-CoV outbreaks, LOC has played a crucial role in the COVID-19 outbreak. Countries including the USA, China and Japan have approved the use of this technology, which fully demonstrates the application value of LOC. Main advantage is that most of these technologies can be used without professional skills.
Hopefully at certain moment applications to detect SARS-CoV-2 and other viruses in wastewater will be developed based on these LOC/POCT systems that will enable simple, fast and sensitive virus detection. Such technology is generally equipped for both home and clinical use.
4. Biosensors
The global risk of viral disease outbreaks emphasizes the need for rapid, accurate, and sensitive detection techniques to speed up diagnostics allowing early intervention [2,[29], [30], [31], [32], [33]]. Biosensors are simple and cost-effective smart sensing systems for rapid, high-sensitivity detection with an integrated sample preparation and flexible to detect different targets using the same platform (such as CRISPR-powered systems), and able of simultaneous detection of different analytes.
Main types of biosensor used for clinical applications are based on electrochemical reactions (EC), surface enhanced Raman scattering (SERS), field-effect transistor (FET), or surface plasmon resonance (SPR) detection. Genosensors and immunosensors are also used for this purpose. Few applications of biosensors such those based on SERS (Fig. 2 ) and FET (Fig. 3 ) will be reported in more detail below.
Fig. 2.
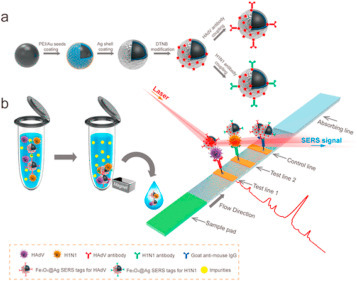
(a) Synthetic Route for Antibody-Modified Fe3O4@Ag Magnetic Tags and (b) Schematic Diagram of the Magnetic SERS Strip for Detecting Two Respiratory Viruses-Reproduced with permission from Chongwen Wang et al., 2019 [32].
Fig. 3.
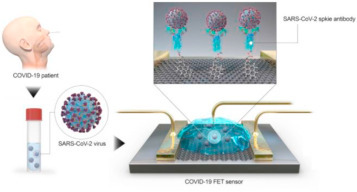
Schematic diagram of COVID-19 FET sensor operation procedure. Graphene as sensing material is selected and SARS-CoV-2 spike antibody is conjugated onto the graphene sheet via 1-pyrenebutyric acid n hydroxysuccinimide ester, which is an interfacing molecule used as a probe linker. Reproduced with permission from (Seo et al., 2020 [31]).
A Raman scattering-based lateral flow immunoassay (SERS-based LFIA) strip for simultaneous detection of influenza A H1N1 virus and human adenovirus (HAdV), that uses Fe3O4@Ag nanoparticles, was recently reported [32] (see Fig. 2). This system allows specific recognition and magnetic enrichment of target viruses in the solution and SERS detection of the viruses on the strip. Based on this strategy, the magnetic SERS strip can directly be used for the analyses of biological samples without any sample pretreatment steps. Further, this strip is easy to operate, rapid, stable, and can achieve high throughput.
EC biosensors have been widely used to detect nucleic acids, proteins, small molecular antibodies, and viruses. The work of Seo et al. [31]reported a FET biosensor for detecting SARS-CoV-2 in clinical samples (see Fig. 3). This sensor was produced by coating the gate of the transistor made up of graphene sheets, with an antibody that was specific against the SARS-CoV-2 spike protein. Desired performance of the sensor was identified with tests conducted with the SARS-CoV-2 spike protein antigen, cultured virus, and nasal swab specimens from COVID-19 patients. This biosensor was further used for the successful detection of viral strains in culture medium.
One of the questions when working with new technological devices like biosensors is to know the limits of detection (LODs). Since this paper targets an environmental audience, the readers may not be familiar with the terminology used in biosensors applied to virus detection. One of the most commonly used measures to express the LOD is the Plaque Forming Units (PFU)/mL or μL. PFU is a measure used to describe the number of virus particles capable of forming plaques per unit volume. Generally is expressed as PFU/mL or PFU/μL and indicates how much viruses infect a target cell. In the case of SERS (see Fig. 2) LODs for Lassa fever, West Nile Fever and Influenza were 230, 195 and 10 PFU/μL respectively. As regards to FET-biosensor devices (see Fig. 3) LODs for SARS-CoV-2 are in the range of 16 PFU/mL or 16 × 10−3 PFU/μL. In short, sensitivity with FET-biosensors is much better than with SERS-biosensors.
The main question here is, similarly as in the case of LOC/POC, if any of these devices can be easily switched to also determine viruses in wastewaters. A couple of recent works [16,33] reported the possible application of paper-based sensor devices to virus detection in WBE approach. The first one [16] reports few examples on the use of paper-based biosensors as potential biosensor devices to track like influenza, HIV viruses among others in wastewaters. The results of the second paper [33] were further cross validated with a robust electrophoresis and agarose gel image assay, showing promising reliability for wastewater analysis.
As far as I am aware a paper-based device to detect SARS-CoV-2 in wastewaters is not yet available. The problem is that wastewaters represent a very complex matrix, even more than human fluids and most probably some kind of extensive clean up to remove the matrix interferences will be required prior to biosensor detection.
In short, biosensor should be a fast “sample-to-answer” analysis method which can provide quantitative monitoring of nucleic acids and genetic information through the analysis of sewage. The proposed biosensors should show advantages including affordability, rapid analysis time, good sensitivity, specificity, and low reagent/sample consumption.
5. Biomarkers
Biomarkers must be discharged through urine and feces and need to be specific to human metabolism. Biomarkers are of interest because they can be rather specific for given infectious diseases and obviously can be used for WBE. CG Daughton [34] did write a lot about biomarkers of endogenous human biochemical processes. Such biomarkers continuously undergo urinary or fecal excretion and represent the sum total contributions for the real-time population served by any given sewage system. One of the first examples used of a possible biomarker for population estimation was coprostanol, which is the predominant reduced sterol formed in the human gut. Several other endogenous biomarkers of positive health versus disease were also reported by Ch Daughton in another paper [35] like isoprostanes, desmosines, bone turnover markers (BTM), polyamines and monocyte chemoattractant protein (MCP)-1 among others.
With this said, WBE could target endogenous biomarkers that are significantly elevated in the diseased state, like in the case of COVID-19 outbreak and excreted extensively in urine. A good start to evaluate the use of biomarkers would be to make use of the fact that COVID-19 can involve remarkable inflammatory damage. A well-known biomarker for oxidative stress is the prostaglandin-like class of substances called isoprostanes [1,36]. The control of inflammatory response biomarkers for the SARS-CoV-2 infection were recommended in clinical studies in the Zhejiang University School of Medicine in China and the list includes: C-reactive protein, procalcitonin, ferritin, D-dimer, interleukins IL-4, IL-6, IL-10 and interferons-γ (IFN-γ) [37]. SARS-CoV-2 nucleocapsid protein was recently characterized and identified for diagnostic purposes at the First Affiliated Hospital of USTC, University of Science and Technology, of China, in Hefei, Anhui in China [38]. Recently our group was involved in the proteomic identification of large molecules in WWTPs being identified as disease biomarkers [39]. Such type of approach maybe useful for monitoring changes in the proteomic profile of different populations to better understand the scale of new epidemiological threats like COVID-19.
Analysis of biomarkers might have several other major advantages over the use of PCR basically on the detection side since most of the measurements for biomarkers molecules are carried out by mass spectrometry (MS) or ELISA in contrast to PCR. It is well-known that MS or ELISA provide better accuracy and detection limits and validation of results as compared to standard PCR.
Other possible ways to target SARS-CoV-2 could be via different biomolecules or biomarkers in sewage that have not been yet described and were only indicated for clinical purposes. For the COVID-19 testing, apart from the most frequently used viral RNA, novel coronavirus exhibits spike proteins which are immunogenic hence, immune system is able to produce immunoglobulins to trigger an immune response against the pathogen. Importantly, these immunoglobulins are not only valuable to detect COVID-19. Immunoglobulin M (IgM) antibodies are produced during the onset of the infectious disease (between 4 and 10 days), whereas immunoglobulin G (IgG) response is produced later (around 2 weeks).
With that being said, it maybe possible to explore the possibilities to look for the same type of biomarkers,.i.e inflammatory response biomarkers already used in clinical diagnostics for the early detection of COVID-19 outbreak in WBE. Environmental proteomics seems to be the right tool that can do this job timely and with comprehensive complementary information to PCR-based methods.
6. Conclusions and recommendations
PCR platforms like RT-qPCR are still the most widely used methods for SARS-Cov-2 detection in waste waters. As reported earlier, one of the problems is the complexity of the wastewater matrix that needs to be treated and cleaned up by using different concentration methods.
There is an urgent need as well to evaluate RT-qPCR methods used by different laboratories with a clear target to achieve a verification/standardization status [3,19]. Several factors such as qPCR platforms, PCR inhibitors, nucleic acid extraction efficiency and low levels of targets may have contributed to the observed discrepancies between laboratories. At present most of the data reported around the globe as first detection on SARS-CoV-2 can only say that the virus was detected but in most cases it is impossible to compare the data as viral content among the laboratories in different countries. Their protocols are different and require standardization such as concentration method, PCR assay, and process controls. In addition, the large uncertainty in the viral load in feces makes it difficult to determine a typical value that could be useful in WBE and for comparison with clinical data of the COVID-19 patients, including asymptomatic ones.
With this said, the expected future of PCR in WBE needs to incorporate new technological developments from the clinical field such as digital PCR, CSRPP, LOC/POC and FET or SERS biosensors among others. Such technological devices should still be adapted to WBE and need to be economical, portable, and user-friendly. Sewage sensors, such as paper-based and smartphones for SARS-CoV-2 detection at the population level have as well a clear potential for early warning of COVID-19 pandemic. Nowadays it is possible to use smartphone-based biosensors targeting antibody/antigen targets as home POC technology. Can smartphone technology be used for detecting viruses in wastewaters? This will need certainly further developments to adapt technologies used in clinical laboratories like LOC/POC and biosensors to WBE. This has been a common problem in the field of new biosensors technologies for wastewater measurements. One of the reasons for this is that the environmental market is too small as compared to the clinical one to be able to go to mass production biosensor units for WWTPs worldwide. But not only in the detection side technologies need to be implemented. In short, WWTP plays a key role to improve virus transmission. Based on analogies with previous studies on SARS and MERS outbreaks, there are reasons to conclude that the viral content may be somewhat controlled depending on the treatment technology used at WWTPs facilities. Hopefully water utilities and water authorities will push together in this direction to develop biosensors to detect early outbreaks of COVID-19 or any other new virus that may come in the future as well as to improve WWTP technologies.
To this end the solution at present to monitor COVID-19 outbreak in WBE could be a combination of technologies and methodological strategies already in place such as PCR technologies and endogenous biomarkers measurements using ELISA and or MS. Why not using both approaches for WBE? That would help to tackle the problems in a more comprehensive and professional way. Certainly many discrepancies observed up till now could be solved. Both measurement methods got advantages and disadvantages. For biomarkers the chemical measurements are accurate and sensitive but the main question is that most of biomarkers are not specific of a given disease. For instance inflammatory response biomarkers are obviously related to SARS-CoV-2 but also to other diseases. But an advantage in case of the present pandemic situation would be that the majority of these biomarkers will be related to COVID-19 patients. Lastly, WBE seems to detect more possible cases of patients, including asymptomatic ones and also other ones who did recover form COVID-19. In this sense WBE can provide additional information not only on asymptomatic cases but also on immunized patients who did recover from COVID-19. Based on these data, epidemiologists could be able to estimate if COVID-19 outbreak would become like a common flu in the years to come.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author kindly thanks the help of Dr Bozo Zonja, Uppsala University, Sweden, for managing and depicting Fig. 1, Fig. 2, Fig. 3.
References
- 1.Daughton C.G. Wastewater surveillance for population-wide COVID-19: The present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. Journal of Environmental Chemical Engineering. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported Covid-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 4.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi Ph M., Kitajima M., Simpson S.L., Li J., Ben Tscharke, Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz Ph, Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sanchez G. SARS-CoV-2, RNA in wastewater anticipated Covid-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurtzer S., Marechal V., Mouchel J.M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv. 2020 doi: 10.1101/2020.04.12.20062679. preprint. [DOI] [Google Scholar]
- 9.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RN in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.P Sherchan S., Shahin S., Ward L.M., Tandukar S., Tiong G. Aw, Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Acience of the Total Environment. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases (preprint) Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.04.05.200515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero-Latorre L., Ballesteros I., Villacrés I.M., Genoveva Granda M., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: Implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar Manish, Patel Arbind Kumar, Shah Anil V., Raval Janvi, Rajpara Neha, Joshi Madhvi, Chaitanya G. Joshi First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Science of the Environment. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westhaus Sandra, Weber Frank-Andreas, Schiwy Sabrina, Linnemann Volker, Brinkmann Markus, Widera Marek, Greve Carola, Janke Axel, Hollert Henner, Wintgens Thomas, Ciesek Sandra. Detection of SARS-CoV-2 in raw and treated wastewater in Germany –Suitability for COVID-19 surveillance and potential transmission risks. Science of the Environment. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., LaRosa G. SARS-CoV-2 from feces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54:3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang J., Yin J., Shaowu Lv d, Wang B., Mu Y. Advanced “lab-on-a-chip” to detect viruses – current challenges and future perspectives. Biosensors and Biolectronics. 2020;163 doi: 10.1016/j.bios.2020.112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bofill-Mas S., Rusiñol M. Recent trends on methods for the concentration of viruses from water samples. Current Opinion in Environmental Science and Health. 2020;16:7–13. doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimized protocols for SARS-CoV-2 detection and quantification. Journal of Environmental Chemical Engineering. 2020;8 doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu D., Huang Zh, Luo J., Zhang X., Sha Sha. Primary concentration – The critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: amini-review. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.Y., Guevara H., Wadford D.A., Chen J.S., Chiu Ch Y. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed W., Payyappat S., Cassidy M., Harrison N., Besley C. Interlaboratory accuracy and precision among results of three sewage-associated marker genes in urban environmental estuarine waters and freshwater streams. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140071. [DOI] [PubMed] [Google Scholar]
- 25.K. Farkas, F. Mannion, L. S. Hillary, S. K. Malham and D. I. Walker, Emerging technologies for the rapid detection of enteric viruses in the aquatic environment , Current Opinion in Environmental Science and Health, 16 (202), 1-6.
- 26.Zhuang J., Yin J., Lv Sh, Wang B., Mu Y. Advanced “lab-on-a-chip” to detect viruses –Current challenges and future perspectives. Biosens. Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H., Fohlero Z., Pekarek J., Basova E., Neuzil P. Recent advances in lab-on-a-chip technologies for viral diagnosis. Biosens. Bioelectron. 2020;153 doi: 10.1016/j.bios.2020.112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Zh, Peng R., Kolker Baravik I., Liu X. Fighting COVID-19: Integrated micro- and nanosystems for viral infection diagnostics. Matter. September 2, 2020;3:1–24. doi: 10.1016/j.matt.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui F., Zhou H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020;1165 doi: 10.1016/j.bios.2020.112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mavrikou S., Moschopoulou G., Tsekouras V., Kintzios S. Development of a portable, Ultra-rapid and Ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 SpikeProtein antigen. Sensors. 2020;20 doi: 10.3390/s20113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo G., Lee G., J Kim M., Baek S.H., Choi M., Ku K.B., Lee Ch-S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Ch Park E., Il Kim S. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor- based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 32.Wang Ch, Wang Ch, Wang X., Wang K., Zhu Y., Rong Z., Wang W., Xiao R., Wang Sh. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl.Matter. Interfaces. 2019;11:1945–19505. doi: 10.1021/acsami.9b03920. [DOI] [PubMed] [Google Scholar]
- 33.Bhalla N., Pan Y., Yang Zh, Farokh Payam A. Opportunities and challenges for biosensors and nanoscale Analytical tools for pandemics: COVID-19. ACS Nano. 2020;14:783–807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daughton C.G. Real-time estimation of small-area populations with human biomarkers in sewage. Sci. Total Environ. 2012;414:6–21. doi: 10.1016/j.scitotenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Daughton C.G. Monitoring wastewater for assessing community health: sewage Chemical-Information Mining (SCIM) Sci. Total Environ. 2018;619–620:748–764. doi: 10.1016/j.scitotenv.2017.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daughton C.G. Using biomarkers in sewage to monitor community-wide human health: Isoprostanes as conceptual proptoype. Sci. Total Environ. 2012;424:16–38. doi: 10.1016/j.scitotenv.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Morales-Narváez E., Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Weihiong, Liu Guangfeng. Biochemical characterization of SARS CoV-2 nucleocapsid protein, Biochem. Res. Commun., 2020;527:618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrascal M., Abian J., Ginebreda A., Barceló D. Discovery of large molecules as new biomarkers in wastewater using environmental proteomics and suitable polymer probes. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141145. [DOI] [PubMed] [Google Scholar]


