Abstract
Background: Previous studies have cross-sectionally described amenorrhea in cohorts of transgender men on intramuscular or subcutaneous testosterone injections. It remains uncertain which testosterone preparations most effectively suppress vaginal bleeding and when amenorrhea occurs after testosterone initiation.
Aim:To investigate the clinical effects of various testosterone preparations on vaginal bleeding and spotting in transgender men.
Methods: This prospective cohort study was part of the European Network for the Investigation of Gender Incongruence (ENIGI). Data on the persistence and intensity of vaginal bleeding and spotting, serum sex steroid levels and body composition were prospectively and cross-sectionally assessed in 267 transgender men during a three-year follow-up period, starting at the initiation of various testosterone preparations.
Results: After three months of testosterone, 17.9% of transgender men reported persistent vaginal bleeding and 26.8% reported spotting. The percentages reporting vaginal bleeding and spotting decreased over the first year of testosterone (bleeding 4.7% and spotting 6.9% at 12 months, respectively), with no participants reporting vaginal bleeding or spotting after 18 months of testosterone. Factors associated with vaginal bleeding or spotting included lower serum testosterone levels and being on testosterone gel as compared to injections (e.g., esters or undecanoate preparations). If vaginal bleeding persisted, starting progestogens at three months resulted in a decrease in the intensity of vaginal bleeding and spotting.
Discussion: Transgender men and hormone-prescribing providers can be reassured that vaginal bleeding and spotting usually stop within three months after testosterone initiation. If not, serum testosterone levels should be measured and testosterone dose adjusted to achieve serum testosterone levels in the physiologic male range. Adding a progestin can be considered after three to six months if bleeding persists. Providers should be aware that cessation of bleeding can be more difficult to achieve in transgender men with lower serum testosterone levels or those on testosterone gel.
Keywords: Transgender men, testosterone therapy, amenorrhea, bleeding, prospective cohort study, ENIGI
Introduction
Transgender people may request gender affirming hormone therapy (GAHT) to alleviate their gender dysphoria (Hembree et al., 2017), with the aim of suppressing the secondary sex characteristics of the birth-assigned gender and inducing the secondary sex characteristics of the experienced gender. GAHT in transgender men (TM) consists of testosterone (T), which can be administered intramuscularly, subcutaneously or transdermally (Hembree et al., 2017). Serum T levels in TM on GAHT are aimed at the physiologic male reference range (T’Sjoen, Arcelus, Gooren, Klink, & Tangpricha, 2018).
If cessation of vaginal bleeding is desired in TM on T, serum T levels should be measured to ensure they are in the physiologic male reference range. T dose and administration frequency can be increased if subphysiologic levels are measured and it is deemed safe (Nakamura et al., 2013). Another option is the addition of progestogens (T’Sjoen et al., 2018). Persistent vaginal bleeding can be psychologically stressful (Armuand, Dhejne, Olofsson, & Rodriguez-Wallberg, 2017) and incongruent with gender identity in TM. In addition, studies on anger after the initiation of T in TM revealed that feelings of anger were associated with the persistence of vaginal bleeding, rather than T administration (Defreyne et al., 2019; Motta et al., 2018).
To date, knowledge on the role of exogenous T in the cessation of vaginal bleeding in TM is limited. Two previous prospective cohort studies revealed that vaginal bleeding and spotting usually end within six months of starting T (Nakamura et al., 2013; van Dijk et al., 2019). There appears to be a dose-dependent response of amenorrhea to T administration, with a greater effect from higher doses of intramuscular T injections, though in some cases spotting is still observed (Nakamura et al., 2013). However, these previous studies failed to account for other factors, including different T treatment modalities, the use of cycle-suppressing hormone therapy (HT), body composition, gonadectomy, etcetera.
The aim of this study was to prospectively examine the effect of various T preparations on persistence and intensity of vaginal bleeding and spotting. We aimed to assess if and when vaginal bleeding or spotting stop and whether these are dependent on serum sex steroid levels or type of exogenous T. Additionally, we aimed to identify other factors influencing vaginal bleeding and spotting, including use of cycle-suppressing HT, body composition and age.
Methods
Cohort
This study was part of the European Network for the Investigation of Gender Incongruence (ENIGI), a multicenter prospective cohort study conducted at four European treatment centers (Ghent, Oslo, Florence, and Amsterdam) (Dekker et al., 2016). Participants were included in the ENIGI endocrine protocol at the initiation of GAHT. After receiving oral and written information about the ENIGI endocrine protocol, written informed consent was obtained according to the institutions’ guidelines. Inclusion criteria were people aged 17 years or older and having a diagnosis of gender dysphoria before initiating GAHT (Dekker et al., 2016). After the baseline visit, GAHT was initiated in accordance with the World Professional Association for Transgender Health Standards of Care, edition 7 (Coleman et al., 2012). Exclusion criteria were previous use of GAHT and insufficient knowledge of the native languages (Dutch, French, Italian or Norwegian).
For the present study, only data from Ghent and Amsterdam were selected because of logistical reasons. From February 2010 until February 2018, 1651 transgender persons were included in the Belgian–Dutch cohort of the ENIGI study, of which 809 were TM. A data lock was then performed and a database containing 692 TM was analyzed. After applying additional exclusion criteria (e.g., missing data, not adhering to the treatment protocol, history of hystero-oophorectomy at baseline, TM aged 50 years old and older), data on vaginal bleeding and spotting were available in 267 TM. After a baseline visit, follow‐up occurred at 3, 6, 9, 12 and 36 months in Amsterdam and at 3, 6, 9, 12, 18, 24 and 36 months in Ghent. A venous blood sample was obtained at each visit, independent of the time from last T administration. Undergoing hystero-oophorectomy after initiation of GAHT or using cycle-suppressing HT were not exclusion criteria, as we aimed to include a sample representative of this clinical population.
Questionnaires
The persistence of vaginal bleeding and spotting were assessed using an investigator-designed (non-validated) questionnaire to evaluate side-effects of GAHT (Dekker et al., 2016). Vaginal bleeding and spotting intensity were scored on a scale ranging from 0 to 3, with 0 indicating “no,” 1 indicating “mild,” 2 indicating “moderate” and 3 indicating “severe” spotting or bleeding. This was self-reported by participants, without quantification by a physician. As vaginal bleeding was assessed as “persistent menstrual bleeding,” baseline scores were not analyzed in this prospective study. At each study visit, the use of progestogens and undergoing surgery (e.g., gonadectomy) were logged.
Gender-affirming hormone therapy
In Ghent, GAHT for TM consisted of intramuscular long‐acting T undecanoate (TU, Nebido® 1000 mg once every 12 weeks). In Amsterdam, TM could choose between injectable T, either as short-acting mixed testosterone esters (TE, Sustanon® 250 mg every 2–3 weeks) (further referred to as “testosterone esters,” TE) or as TU (Nebido® 1000 mg every 12 weeks), or transdermal T gel (TG, 50 mg daily). Data on type of T were collected within the ENIGI study protocol and reassessed at each study visit, in addition to anthropometric measurements (Dekker et al., 2016). A progestin (e.g., oral lynestrenol 5 mg once daily, injectable medroxyprogesterone acetate 150 mg once every 3 months) could be added to the treatment regimen if suppression of vaginal bleeding was desired or if vaginal bleeding did not cease (Jeppsson, Johansson, & Sjöberg, 1973). Some participants used combined oral contraceptives at baseline. Combined oral contraceptives were stopped or switched to progestogens at T initiation. The use of cycle-suppressing HT (progestogens and/or combined oral contraceptives) was logged at each visit.
Laboratory analyses
In Ghent, competitive chemiluminescent immunoassays were run for estradiol (E2, E170 Modular, Roche, Gen III, Mannheim, Germany, LOQ 25 pg/mL, interassay CV 3.2%), T (E170 Modular, Roche, Gen II, Mannheim, Germany, LOQ 10 ng/dL (0.4 nmol/L), interassay CV 2.6%), luteinizing hormone (LH, E170 Modular, Roche, Gen III, Mannheim, Germany, LOQ 0.1 mIU/mL, interassay CV 3.48%), and follicle stimulating hormone (FSH, E170 Modular, Roche, Gen III, Mannheim, Germany, LOQ 0.1 mIU/mL, interassay CV 3.3%); a sandwich‐type chemiluminescent immunoassay was employed for sex hormone-binding globulin (SHBG, E170 Modular, Roche, Gen III, Mannheim, Germany, LOQ 0.35 mIU/mL, interassay CV 4.06%).
Before March 19, 2015, E2 was measured using an E170 Modular (Gen II; Roche Diagnostics). To convert E2 values measured before March 19, 2015, the formula Gen III = 6.687940 + 0.834495 × Gen II was used (E170 Modular; Roche Diagnostics).
In Amsterdam, E2 was measured using a competitive immunoassay (Delfia, PerkinElmer, Turku, Finland, LOQ 20 pmol/L, interassay CV 10–13%) until July 2014. After July 2014, E2 was measured using a LC‐MS/MS (VUmc, Amsterdam, the Netherlands, LOQ 20 pmol/L, interassay CV 7%). To convert Delfia values, the formula LC‐MS/MS = 1.60 × Delfia–29 was used. T was measured using a radioimmunoassay (RIA, Coat‐A‐Count, Siemens, Los Angeles, CA, USA, LOQ 1 nmol/L, interassay CV 7–20%) until January 2013. Thereafter, T was measured using a competitive immunoassay (Architect, Abbott, Abbott Park, IL, USA, LOQ 0.1 nmol/L, interassay CV 6–10%). The RIA values were converted to the competitive immunoassay values. For T levels below 8 nmol/L, the formula Architect = 1.1 × RIA + 0.2 was used; for T levels above 8 nmol/L, the formula Architect = 1.34 × RIA – 1.65 was used. LH, FSH and SHBG were measured using chemiluminescent microparticle immunoassay (Architect System, Abbott, Abbott Park, IL, USA, LOQ 2 U/L, interassay CV 4%).
Body composition
Body composition was measured in a subset of the study population using whole-body dual-energy X-ray absorptiometry (DXA) at baseline and after one year of T. In Amsterdam and Ghent, Hologic Discovery A was used. Body fat mass and body fat percentage were measured using DXA. All scans were analyzed using software, version 13.5.3. More information can be found in Klaver et al. (2018).
Data analysis
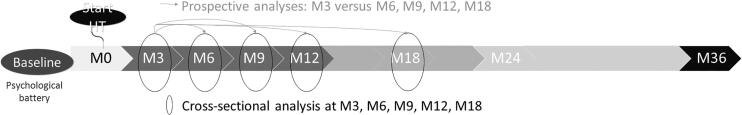
Data were analyzed using IBM SPSS 25.0 (SPSS, Chicago, IL, USA). Data were verified for normal distribution using the Shapiro–Wilk test. Normally distributed values are shown as mean ± standard deviation (SD), whereas non-normally distributed values are described as median [percentile 25–percentile 75]. The current manuscript describes both the intensity (continuous variables) and incidence (categorical variables) of persistent vaginal bleeding and spotting. Data were analyzed prospectively (individual mean changes with 95% confidence intervals [CI]) and cross-sectionally (Figure 1). Data were also analyzed as a continuous (intensity of vaginal bleeding and spotting, 0 = none, 1 = mild, 2 = moderate, and 3 = severe) as well as a dichotomous variable (incidence, any = 1 or no = 0). We performed both prospective and cross-sectional analyses since both are clinically relevant. While many TM ask about changes in persistence of vaginal bleeding and/or spotting after initiating T (prospective analysis), some may wonder why they still experience vaginal bleeding and/or spotting at any given follow-up visit (cross-sectional analysis) while other TM don’t experience bleeding and/or spotting anymore. By using binary logistic mixed models, we explored the influence of type of T, use of progestogens, BMI, fat mass, fat percentage, age and serum sex steroid levels on the incidence of vaginal bleeding and spotting over time. Convergence was not achieved in the models for type of T and BMI for both vaginal bleeding and spotting, and for use of progestogens (spotting only). In addition, mixed models were applied to evaluate the influence of type of T, use of progestogens, BMI, fat mass, fat percentage, age and serum sex steroid levels on the intensity of vaginal bleeding and spotting over time. Prospective differences in the incidence and intensity of vaginal bleeding and spotting, BMI values and serum sex steroid levels were calculated (value x – baseline or three-month value). Prospective changes are shown as individual mean delta (Δ) with 95% CI. To control for use of cycle-suppressing HT and hystero-oophorectomy, subgroup analyses were run in groups with versus without cycle-suppressing HT and in groups with versus without hystero-oophorectomy. Differences between groups (e.g., those using cycle-suppressing HT versus those without) were analyzed by unpaired Student’s t‐test for normally distributed data and the Mann–Whitney U‐test for non‐normally distributed data. Correlations were tested using Pearson’s R for normally distributed data and Spearman’s ρ for non-normally distributed data. Bonferroni-Holm correction was applied to all p values to limit the chance of type II error (Holm, 1979).
Figure 1.
Methodology of the cross-sectional and prospective analyses over the study follow-up duration (months).
Results
Data on persistent vaginal bleeding and spotting were available in 267 (33.0%) TM. At baseline, 92 people (34.5%) were using cycle-suppressing HT (either contraceptive agents or progestogens). The type of HT used to suppress the menstrual cycle at baseline was not logged in Amsterdam, whereas 77.1% of those using cycle-suppressing HT at baseline in Ghent (n = 70) were using progestogens (50.0% oral progestogens, 27.1% injectable progestogens) and 22.9% were using oral contraceptives containing ethinyl estradiol.
Characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population (n = 267).
| TM (n = 267) | |
|---|---|
| Age (years) | 22.0 [20.0–27.0] |
| Body mass index (kg/m2) | 23.2 [21.0–27.5] |
| Serum testosterone levels (nmol/L) | 1.2 [0.9–1.5] |
| Serum estradiol levels (pg/mL) | 45.6 [24.0–102.2] |
| Serum luteinizing hormone levels (U/L) | 5.2 [2.9–8.4] |
| Serum follicle stimulating hormone levels (U/L) | 5.2 [3.2–7.2] |
| Serum sex hormone binding globulin levels (nmol/L) | 56.4 [34.1–82.8] |
| Total body fat percentage (%) | 36.5 [31.5–40.3] |
| Total body fat mass (g) | 17139.3 [14647.6–22001.0] |
| On menstrual cycle-suppressing hormonal therapy, n = yes (%) | 92 (34.5) |
| Mastectomy, n = yes (%) | 31 (11.6) |
For values that are not normally distributed, median values and IQR [P25 and P75] are shown.
Serum levels of sex steroids
After initiating GAHT, individual mean serum T levels increased by 23.4 (95% CI 21.5–25.3, p < 0.001) nmol/L, from median 1.2 [0.9–1.5] nmol/L to 19.0 [12.0–30.0] nmol/L over the first three months, with comparable levels at 36 months (p = not significant [NS]).
Serum E2 levels decreased from 45.6 [24.0–102.2] pg/mL at baseline to 37.6 [28.3–51.2] pg/mL after three months of T (mean Δ –16.5, 95% CI –26.2 to –6.9, p < 0.001), remaining stable over the first year with a second decrease between 12 and 18 months, from 42.0 [30.5–53.7] pg/mL to 28.7 [25.0–36.4] pg/mL (mean Δ –19.1, 95% CI –24.4 to –13.8, p < 0.001).
Vaginal bleeding and spotting
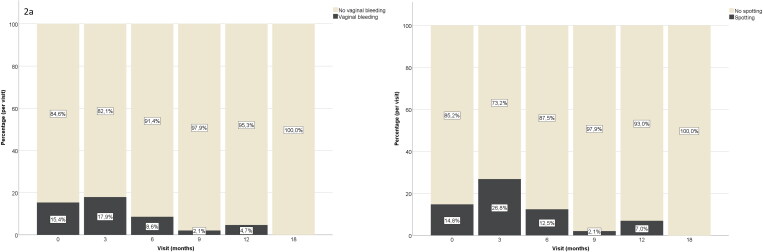
After three months of T, only 17.9% still reported persistent vaginal bleeding, whereas 26.8% reported spotting (Table 2).
Table 2.
Distribution of vaginal bleeding and spotting intensity in transgender men, after initiation of testosterone therapy.
| Vaginal bleeding | |||||
|---|---|---|---|---|---|
| Months | None | Moderate | Mild | Severe | Total vaginal bleeding |
| 3 (n = 262) | 215 (82.1%) | 11 (4.2%) | 26 (9.9%) | 10 (3.8%) | 47 (17.9%) |
| 6 (n = 256) | 234 (91.4%) | 6 (2.3%) | 13 (5.1%) | 3 (1.2%) | 22 (8.6%) |
| 9 (n = 143) | 140 (97.9%) | 1 (0.7%) | 2 (1.4%) | 0 | 3 (2.1%) |
| 12 (n = 234) | 223 (95.3%) | 5 (2.1%) | 6 (2.6%) | 0 | 11 (4.7%) |
| 18–36 (n = 43–101) | 43–101 (100%) | 0 | 0 | 0 | 0 |
| Spotting | |||||
| Months | None | Moderate | Mild | Severe | Total spotting |
| 3 (n = 272) | 199 (73.2%) | 44 (16.2%) | 22 (8.1%) | 7 (2.6%) | 73 (26.8%) |
| 6 (n = 271) | 232 (87.5%) | 18 (6.8%) | 12 (4.5%) | 3 (1.1%) | 33 (12.2%) |
| 9 (n = 145) | 142 (97.9%) | 1 (0.7%) | 1 (0.7%) | 1 (0.7%) | 2 (2.1%) |
| 12 (n = 244) | 227 (93.0%) | 12 (4.9%) | 2 (0.8%) | 3 (1.2%) | 17 (6.9%) |
| 18–36 (n = 43–101) | 43–101 (100%) | 0 | 0 | 0 | 0 |
Frequencies are shown as n (%). The total number of people experiencing vaginal bleeding or spotting are shown in the “total spotting” and “total vaginal bleeding” columns.
After six months of T, there was a continued decrease in the number of TM with persistent vaginal bleeding and spotting (8.6%, p = 0.001 and 12.2%, p < 0.001, respectively), with further decreases between six and nine months (2.1%, p = 0.010 and 2.1%, p < 0.001, respectively) (Figure 2(a,b)). Between nine and 12 months, there was an increase in the incidence of spotting (6.9%, p = 0.035), whereas vaginal bleeding remained stable (p = NS). Between 12 and 18 months, the incidences of spotting and vaginal bleeding decreased further (both to 0%, p = 0.022 and p = 0.004, respectively). None of the participants experienced persistent vaginal bleeding or spotting between 18 and 36 months of T. Only one person with persistent spotting after 12 months of T underwent gonadectomy between the second and third year of T, and another person with persistent vaginal bleeding after 12 months of T underwent gonadectomy between 12 and 18 months of T.
Figure 2.
Percentages for the occurrence of vaginal bleeding (a) and spotting (b) after the initiation of testosterone therapy in transgender men, by study visit.
In those experiencing vaginal bleeding at the three-month visit (n = 47), median bleeding intensity decreased between three and nine months of T (mean Δ –2.0, 95% CI –3.9 to –0.1, p = 0.037), from 2.0 [2.0–2.0] to 0.0 [0.0–0.0]. In those experiencing spotting at the three-month visit (n = 71), spotting intensity decreased between three and six months (mean Δ –1.2, 95%CI –1.4 to –1.0, p < 0.001), from 1.0 [1.0–2.0] to 0.0 [0.0–0.0], remaining stable thereafter (p = NS).
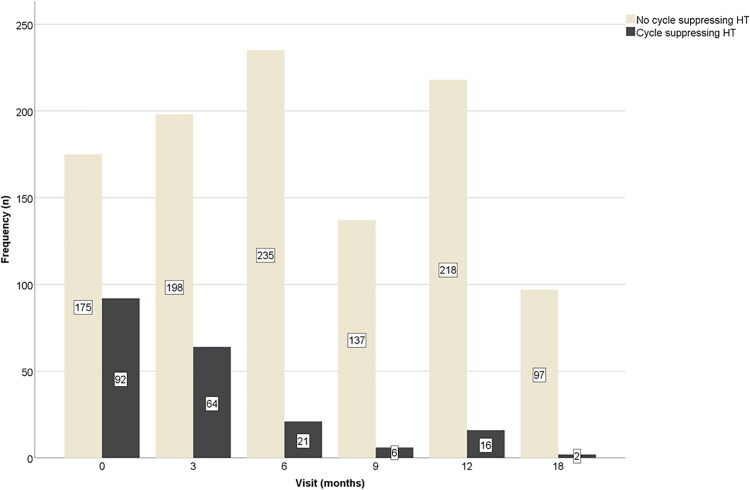
At three months of T therapy, 64 people were using cycle-suppressing HT (24.4%), which significantly decreased between three and six months (p < 0.001), as only 21 (8.2%) were still using cycle-suppressing HT at the six-month visit. This remained stable after nine months (six people; 4.2%) and after 12 months (16 people, 6.8%) (p = NS). After 18 months of T, only 2 people were using cycle-suppressing HT (2.0%) (Figure 3). The median duration of cycle-suppressing HT use was three months [0–3].
Figure 3.
Frequencies of the use of cycle-suppressing HT after the initiation of testosterone therapy in transgender men, by study visit.
Explanatory multiple regression models
During the first year, type of T, use of cycle-suppressing HT, BMI, body fat mass, body fat percentage, age and serum sex steroid levels did not significantly influence prospective changes in the incidence of vaginal bleeding (all p = NS) or spotting (all p = NS).
However, the multiple regression model revealed a slightly higher mean score for intensity of vaginal bleeding in people using cycle-suppressing HT (mean Δ + 0.147, 95% CI 0.056–0.238, p = 0.002).
Prospective analysis
Serum sex steroid levels
People who experienced spotting at three months had a smaller prospective increase in serum T levels between baseline and three months compared to those without spotting (mean Δ + 14.5 nmol/L, 95% CI +10.5 to +18.5, versus mean Δ + 21.0 nmol/L, 95% CI +17.6 to +24.3, p = 0.045, respectively), whereas prospective changes in serum E2 levels were comparable (p = NS).
In addition, people reporting vaginal bleeding at six months also had a smaller prospective increase in serum T levels between baseline and six months compared to those without vaginal bleeding (mean Δ + 16.7 nmol/L, 95% CI +11.3 to +22.2, versus mean Δ + 20.0 nmol/L, 95% CI +18.7 to +21.3, p = 0.005, respectively).
Prospective changes in the severity of vaginal bleeding or spotting did not correlate with prospective changes in serum sex steroid levels at any visits (p = NS).
Cycle-suppressing hormonal therapy
Starting progestogens at the three-month visit resulted in a larger decrease in vaginal bleeding and spotting intensity between three and six months, compared to people without progestogens (vaginal bleeding: mean Δ –1.00, 95% CI –0.30 to –0.07 versus mean Δ –0.16, 95% CI –0.30 to –0.07, p < 0.001, respectively, and spotting: mean Δ –0.67, 95% CI –1.20 to –0.12 versus mean Δ –0.19, 95% CI –0.30 to –0.07, p < 0.001, respectively). After initiation of progestogens at three months, vaginal bleeding intensity decreased more between three and 12 months, compared to those without progestogens (mean Δ –0.83, 95% CI –2.06 to 0.39 vs. mean Δ –0.20, 95%CI –0.31 to –0.09, p = 0.011, respectively). In addition, people with persistent vaginal bleeding who were prescribed progestogens at the three-month visit were more likely to report cessation of vaginal bleeding after six (100.0%) and 12 (87.5%) months, compared to those who were not on progestogens (66.7% and 50.0%, p = 0.002 and p = 0.035, respectively). The incidence of spotting did not change after prescribing progestogens at three, six or nine months (p = NS). The type of T did not influence prospective changes in the intensity of vaginal bleeding or spotting after the third month of T (p = NS).
Body composition
A prospective decrease in vaginal bleeding intensity was positively correlated with a decrease in fat percentage over 12 months (ρ = 0.261, p = 0.005), but not body fat mass (p = NS). Changes in spotting intensity were not correlated with changes in body fat percentage or mass (p = NS). Prospective changes in the severity of spotting or vaginal bleeding after the third month of T were not correlated with BMI values or prospective changes in BMI (p = NS).
Age
Prospective changes in the incidence as well as the intensity of spotting or vaginal bleeding were not correlated with age (p = NS).
Cross-sectional analysis
Serum sex steroid levels
Probably due to limited sample size during individual follow-up visits, cross-sectional analysis did not reveal consistent findings. However, serum E2 levels were positively correlated with severity of vaginal bleeding at the 6-month visit (ρ = 0.247, p = 0.006) and severity of spotting at the 12-month visit (ρ = 0.196, p = 0.003). Serum T levels were negatively correlated with spotting severity at the three-month visit (ρ = –0.184, p = 0.003). Spotting and vaginal bleeding intensity were not correlated with serum levels of LH or FSH (p = NS).
Cycle-suppressing hormonal therapy
The use of cycle-suppressing HT was associated with a higher incidence of persistent vaginal bleeding after 12 months of T, compared to people not using cycle-suppressing HT (18.8% vs. 3.7%, p = 0.036, respectively), but not at any of the other visits (p = NS). People on cycle-suppressing HT were also more likely to report more severe vaginal bleeding after 9 and 12 months compared to those without (mean ± SD 0.17 ± 0.41 vs. 0.03 ± 0.24, p = 0.012, and 0.38 ± 0.81 versus 0.06 ± 0.30, p = 0.007, respectively). People taking cycle-suppressing HT at baseline were equally likely to present with persistent vaginal bleeding or spotting at three months (p = NS).
People on cycle-suppressing HT were more likely to report persistent and heavier spotting after six and 12 months of T, compared to those without cycle-suppressing HT (six months incidence: 38.1% vs. 10.6%, p = 0.002, severity: 0.67 ± 1.02 vs. 0.15 ± 0.49, p < 0.001; 12 months incidence: 25.0% vs. 6.0%, p = 0.018, severity: 0.50 ± 1.03 vs. 0.07 ± 0.34, p = 0.003).
The incidence of persistent vaginal bleeding was higher in people on TG compared to TU and TE after three months (32.6% vs. 17.1%, p = 0.021 for TU and 8.6%, p = 0.002 for TE). The incidence of persistent vaginal bleeding did not differ between groups on TE versus TU (p = NS). Serum T levels after three months were lower in the TU group (13.7 [9.6–19.3] nmol/L, compared to the TE (25.0 [15.0–37.0] nmol/L, p < 0.001) and TG (21.0 [13.0–33.5] nmol/L, p < 0.001) groups.
People on TU and TE experienced less severe vaginal bleeding and spotting after 12 months, compared to those receiving TG (bleeding: 0.03 ± 0.24 and 0.11 ± 0.45 versus 0.16 ± 0.49, p = 0.015, respectively; spotting: 0.04 ± 0.19 and 0.09 ± 0.30 versus 0.26 ± 0.75, p = 0.021, respectively). The incidence of spotting did not differ by T modalities (p = NS). Serum T levels after 12 months were lower in the TG (20.0 [11.0–37.0] nmol/L) and TU (18.6 [11.9–25.6] nmol/L) groups, compared to the TE (25.0 [15.0–39.0] nmol/L, p < 0.001) group. Serum T levels did not differ between the TG and TU group (p = 0.053).
Body composition
TM who still experienced vaginal bleeding after 12 months of T (n = 11) had a lower body fat percentage compared to those without (25.0% [19.1–30.3] vs. 31.6% [26.4–36.9], p = 0.038, respectively), although incidence of spotting did not differ between these groups (p = NS). BMI was not different between groups with versus without vaginal bleeding or spotting (p = NS). There was no correlation between BMI, body fat mass or percentage and severity of vaginal bleeding or spotting (p = NS).
Age
There was no difference in age between groups with versus without spotting or with versus without persistent vaginal bleeding (p = NS). However, intensity of persistent vaginal bleeding was lower in older TM at the six-month visit (ρ = –0.141, p = 0.021), but not at other visits (p = NS). Age was not correlated with spotting intensity (p = NS).
Subgroup analyses: people not using cycle-suppressing HT
In TM not using cycle-suppressing HT, 15.9% experienced vaginal bleeding after three months of HT, whereas 25.6% experienced spotting. The incidence of vaginal bleeding and spotting decreased between three and six months to 7.8% (p = 0.008) and 10.2% (p < 0.001), respectively. These incidences decreased further between six and nine months to 1.4% (p = 0.009) and 2.2% (p = 0.003), respectively. The incidence of vaginal bleeding and spotting did not change between nine and 12 months (3.9%, p = 0.218 and 5.7%, p = 0.122, respectively). None of the participants not taking cycle-suppressing HT experienced vaginal bleeding or spotting after 18 months of GAHT.
People who still experienced spotting after three months of GAHT had a lower prospective increase in serum T levels compared to those no longer experiencing spotting (mean Δ + 24.4 nmol/L, 95% CI 20.5–28.2 vs. mean Δ + 17.0 nmol/L, 95% CI 13.5–20.5, p = 0.007, respectively), but not at the other time points (p = NS). The incidence of spotting during the follow-up of the study was not associated with prospective changes in other serum sex steroid levels. There was no difference in prospective changes in serum sex steroid levels between people still experiencing vaginal bleeding after three, six, nine and 12 months, versus those no longer experiencing vaginal bleeding (p = NS).
In the cross-sectional analysis, lower serum T levels were associated with more severe spotting (ρ = –0.177, p = 0.013) after three months. Higher serum E2 levels after six months were associated with more severe vaginal bleeding (ρ = 0.262, p = 0.004).
The use of TG was associated with a higher incidence of vaginal bleeding after three months (36.1%), compared to those on TU (14.5%, p = 0.006) and TE (4.3%, p < 0.001). Type of GAHT did not influence the incidence of vaginal bleeding at other time points nor the incidence of spotting at any of the study time points (all p = NS).
A prospective decrease in vaginal bleeding intensity was positively correlated to a decrease in fat percentage (ρ = 0.236, p = 0.017) over 12 months, but not body fat mass or BMI (p = NS). Changes in spotting intensity were positively correlated to changes in BMI (ρ = 0.193, p = 0.019) over 12 months, but not body fat percentage or mass (p = NS). Cross-sectionally, there were no associations between body composition and vaginal bleeding or spotting intensity (p = NS).
Prospective changes in the incidence and intensity of spotting or vaginal bleeding were not correlated with age (p = NS). There was no difference in age between groups with versus without spotting (p = NS). Groups with persistent vaginal bleeding at the six-month visit were younger compared to those without vaginal bleeding (20.0 [18.0–23.0] vs. 22.0 [20.0–30.0] years, p = 0.015, respectively). Age was not correlated with vaginal bleeding or spotting intensity (p = NS).
Discussion
The primary aim of this study was to assess the effect of T administration on vaginal bleeding and spotting in TM. After initiation of T, we observed a decrease in both the incidence and the severity of vaginal bleeding and spotting. After three months of T, the majority no longer reported vaginal bleeding (82.1%) or spotting (72.9%). In TM who never used cycle-suppressing HT, 84.1% no longer experienced vaginal bleeding and 74.4% no longer experienced spotting after three months of T. None of the participants reported spotting or vaginal bleeding after more than one year of T. This could not be explained by hystero-oophorectomy, as only one person with persistent spotting after 12 months of T underwent gonadectomy between the second and third year of T, and another person with persistent vaginal bleeding after 12 months of T underwent gonadectomy between 12 and 18 months of T.
Cross-sectionally, we observed a positive correlation between serum E2 levels and severity of vaginal bleeding and spotting. Conversely, serum T levels were negatively correlated to spotting and vaginal bleeding intensity. The inverse correlation between serum T and E2 levels in TM on T therapy has been previously reported (Chan, Jolly, Liang, Weinand, & Safer, 2018). Although both the incidence and severity of vaginal bleeding and spotting were not related to serum LH and FSH levels, we hypothesize that effectively suppressing serum E2 levels by exogenous T therapy in TM will result in cessation of vaginal bleeding and spotting in most TM. This hypothesis is supported by the higher incidence of vaginal bleeding and higher intensity of both vaginal bleeding and spotting in people using TG, compared to people using TE or TU. As previously described, TG has a shorter half-life compared to TE and TU (Srinivas-Shankar & Wu, 2006) and is less efficient at suppressing serum E2 levels in TM (unpublished data). Serum T levels were not lower in people on TG, compared to those on TU. It is possible, however, that TG therapy leads to lower daily mean serum T levels compared to TU and TE. Serum T levels were measured in the morning and this could also be a possible confounder.
Analogous to high serum T levels due to exogenous administration of T, people with endogenous hyperandrogenism due to underlying polycystic ovary syndrome (PCOS) exhibit an increased prevalence of oligo- and amenorrhea compared to those without PCOS (Baptiste, Battista, Trottier, & Baillargeon, 2010; Jonard & Dewailly, 2004; Lerchbaum, Schwetz, Rabe, Giuliani, & Obermayer-Pietsch, 2014). Burgers et al. (2010) reported higher serum T levels and a higher free androgen index in anovulatory patients presenting with amenorrhea (serum T levels >2.0 nmol/L) compared to patients with oligomenorrhea. However, these T levels are much lower than the levels seen in TM on exogenous T. Of note is that the prevalence of PCOS among treatment naive TM was reported to be as high as 14.4% to 58% compared to the generally accepted prevalence of 5–10% among females of reproductive age (Baba et al., 2007; Vujovic, Popovic, Sbutega-Milosevic, Djordjevic, & Gooren, 2009).
These results are in line with previously reported effects of high T levels on endometrial atrophy and amenorrhea (Grynberg et al., 2010). This can be explained by suppression of serum E2, either by negative feedback of the hypothalamic-pituitary-gonadal (HPG) axis or by changes in body composition. It is well known that a higher BMI as well as a higher body fat percentage may result in a higher concomitant rise in serum E2 levels through peripheral conversion of T in adipose tissue. An unpublished study by our team in TM observed a decrease in serum E2 levels after the initiation of T in TM, with a positive correlation between serum E2 levels and total body fat percentage, but a negative correlation with BMI. It was hypothesized that the decrease in serum E2 levels could be predominantly attributed to negative feedback of the HPG axis. In addition, various studies have described an increase in muscle and a decrease in fat mass in TM on T therapy (Moore, Wisniewski, & Dobs, 2003; Mueller, Kiesewetter, Binder, Beckmann, & Dittrich, 2007; Slagter, Gooren, Scorilas, Petraki, & Diamandis, 2006). In the current study, a decrease in body fat percentage was associated with a decrease in vaginal bleeding intensity, although no correlations with changes in BMI were observed. This can be explained by the increasing lean mass and decreasing fat mass after initiation of T in TM, which can result in unaltered or increased BMI, as previously described in Klaver et al. (2018). However, cross-sectionally, the incidence of vaginal bleeding was associated with a lower body fat percentage after 12 months of T, which may seem counter-intuitive. This observation was merely cross-sectional and therefore not explanatory. Based on cross-sectional analyses, the use of cycle-suppressing HT was associated with increased vaginal bleeding and spotting in TM after 12 months of T. It is unlikely that prescribing progestogens will induce spotting and/or menstruation in TM receiving T. This apparent discrepancy may be explained by the fact that TM, who still experience spotting and/or menstruation after six and 12 months of T, were more likely to be started on progestogens in order to suppress the menstrual cycle. In contrast, prescribing progestogens at the three-month visit resulted in cessation of vaginal bleeding in 100% after six months of T. However, we do not recommend routinely administering progestogens to all TM at the initiation of T, as this would result in unnecessary use of progestogen therapy in approximately 80% of people.
As previously mentioned, persistent vaginal bleeding can be perceived as psychologically stressful (Armuand et al., 2017) and can result in feelings of anger (Defreyne et al., 2019). Therefore, we suggest informing TM about the high probability of cessation of vaginal bleeding and spotting during the first three months of T, but also offering the option to initiate a progestogen after three months of T, if necessary. Based on the observation that the use of cycle-suppressing HT decreased over the first year of T, with none of the participants experiencing vaginal bleeding or spotting at the 18-, 24- and 36-month follow-up visits, we recommend that progestogens can be stopped between 12 to 18 months of T. If cessation of vaginal bleeding does not occur after initiation of T, the hormone prescribing provider should first assess serum T levels. If serum T levels are below the physiologic male reference range, we suggest increasing the dose or administration frequency of T if safe to do so. If vaginal bleeding and/or spotting occur when physiologic male serum T levels are measured, we do not suggest altering the T dose. Adding a progestogen should then be discussed.
The current literature does not suggest that T induces metabolic derangement in TM, as in PCOS (Chan, Liang, Jolly, Weinand, & Safer, 2018; Shadid et al., 2020). Unfortunately, information on PCOS diagnoses was not recorded in the current cohort. However, Grynberg et al. (2010) observed endometrial atrophy in 45% of TM after at least six months of T. Importantly, high serum T levels in TM do not necessarily correlate with high levels of intra-ovarian T (Obedin-Maliver & Makadon, 2016). In a recent observational prospective cohort study investigating ovarian tissue cryopreservation, a normal cortical follicle distribution was seen after more than one year of T treatment (De Roo et al., 2017). Moreover, in a retrospective cohort study of TM who had given birth, 80% of the TM reported resumption of vaginal bleeding within six months after stopping T administration in order to fulfill their parental desire (Light, Obedin-Maliver, Sevelius, & Kerns, 2014).
Our study results may have been affected by several limitations. The persistence of vaginal bleeding and spotting was self-assessed and not quantified by a physician. The baseline questionnaire on vaginal bleeding and spotting was not suitable to measure the incidence of people with versus without a menstrual cycle, including those in menopause regardless of etiology. Therefore, we compared the incidence of spotting and vaginal bleeding to the 3-month follow-up visit, which reflects a moment during clinical care when the persistence of vaginal bleeding and spotting is an important issue to be discussed. Follow-up in Amsterdam only consisted of visits at baseline and after three, six, nine, 12 and 36 months, leading to a decrease in sample size and power in the analyses of 18 and 24 months. In addition, due to performing a data lock, the number of cases decreased after each follow-up visit, which leads to a decrease in power in the analyses of 18, 24 and 36 months. Serum samples were obtained at fixed time points during the follow-up period, independent of the time from the last administration. This may have led to fluctuations in measured serum T and E2 levels. In addition, type of cycle-suppressing HT was not logged in Amsterdam.
Despite these limitations, this study has a number of strengths. To our knowledge, this is the largest prospective study to date that evaluated the persistence and severity of vaginal bleeding and spotting in TM after initiation of T. Our study cohorts are well defined, and participants adhered to a strict treatment regimen. Controlling for use of cycle-suppressing HT and gonadectomy during the follow-up provides results representative for everyday clinical practice. In addition, this is a larger study that directly compared the effects of TG, intramuscular TE and intramuscular TU on vaginal bleeding and spotting.
Conclusion
Testosterone administration decreased the incidence of vaginal bleeding and spotting among TM over time. T led to cessation of vaginal bleeding and spotting in the vast majority of TM after three months. Therefore, we do not suggest routinely adding progestogens at the moment of T initiation. If cessation of vaginal bleeding or spotting is not achieved within three to six months of adequately dosed T, a progestational agent may be added. Providers should be aware that cessation of bleeding can be more difficult to obtain in TM with a lower body fat percentage, those experiencing less decrease in body fat percentage after initiating T or those on TG.
Funding Statement
This collaboration was supported in part by a travel grant from the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) Lewis E. Braverman, MD, MACE, Education Fund.
Acknowledgments
We thank the following people for their valuable contributions in the ENIGI project: Inga Becker and Timo Nieder for participating as a center in the ENIGI project, the endocrinology residents (Nienke Nota, Maartje Klaver, Christel De Blok, Greet Roef, Mirra Boer, Marijn Carpentier, Liesbeth Van Huffel, Sara Vandewalle, Loes Moernaut, Sabine Vermeersch, Gert-Jan Vereecke, Charlotte Verroken, Xavier-Philippe Aers, Laurens Veldeman, Joke Marlier, Bram Vantomme and Emmanuelle Versele) for their outpatient care, Charlotte Bultynck, Charlotte Pas, Anne-Sophie De Maetelaere and Kessewa Abosi-Appeadu for their help with the dataset and our study nurse, Kaatje Toye, for handling the extensive administration of the study. We thank all participants in the ENIGI study protocol.
Disclosure statement
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Armuand, G., Dhejne, C., Olofsson, J. I., & Rodriguez-Wallberg, K. A. (2017). Transgender men’s experiences of fertility preservation: A qualitative study. Human Reproduction, 32(2), 383–390. doi: 10.1093/humrep/dew323 [DOI] [PubMed] [Google Scholar]
- Baba, T., Endo, T., Honnma, H., Kitajima, Y., Hayashi, T., Ikeda, H., … Saito, T. (2007). Association between polycystic ovary syndrome and female-to-male transsexuality. Human Reproduction, 22(4), 1011–1016. doi: 10.1093/humrep/del474 [DOI] [PubMed] [Google Scholar]
- Baptiste, C. G., Battista, M.-C., Trottier, A., & Baillargeon, J.-P. (2010). Insulin and hyperandrogenism in women with polycystic ovary syndrome. The Journal of Steroid Biochemistry and Molecular Biology, 122(1–3), 42–52. doi: 10.1016/j.jsbmb.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers, J. A., Fong, S. L., Louwers, Y. V., Valkenburg, O., De Jong, F. H., Fauser, B. C. J. M., & Laven, J. S. E. (2010). Oligoovulatory and anovulatory cycles in women with polycystic ovary syndrome (PCOS): What’s the difference? Journal of Clinical Endocrinology and Metabolism, 95(12), 485–489. [DOI] [PubMed] [Google Scholar]
- Chan, K. J., Jolly, D., Liang, J. J., Weinand, J. D., & Safer, J. D. (2018). Estrogen levels do not rise with testosterone treatment for transgender men. Endocrine Practice, 24(4), 329–333. doi: 10.4158/EP-2017-0203 [DOI] [PubMed] [Google Scholar]
- Chan, K. J., Liang, J. J., Jolly, D., Weinand, J. D., & Safer, J. D. (2018). Exogenous testosterone does not induce or exacerbate the metabolic features associated with pcos among transgender men. Endocrine Practice, 24(6), 565–572. doi: 10.4158/EP-2017-0247 [DOI] [PubMed] [Google Scholar]
- Coleman, E., Bockting, W., Botzer, M., Cohen-Kettenis, P., DeCuypere, G., Feldman, J., … Zucker, K. (2012). Standards of Care for the health of transsexual, transgender, and gender-nonconforming people. International Journal of Transgender Health, 13(4), 165–232. doi: 10.1080/15532739.2011.700873 [DOI] [Google Scholar]
- De Roo, C., Lierman, S., Tilleman, K., Peynshaert, K., Braeckmans, K., Caanen, M., … De Sutter, P. (2017). Ovarian tissue cryopreservation in female-to-male transgender people: Insights into ovarian histology and physiology after prolonged androgen treatment. Reproductive BioMedicine Online, 34(6), 557–566. doi: 10.1016/j.rbmo.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Defreyne, J., Kreukels, B., T'Sjoen, G., Staphorsius, A., Den Heijer, M., Heylens, G., & Elaut, E. (2019). No correlation between serum testosterone levels and state-level anger intensity in transgender people: Results from the European Network for the Investigation of Gender Incongruence. Hormones and Behavior, 110, 29–39. [DOI] [PubMed] [Google Scholar]
- Dekker, M.J.H.J., Wierckx, K., Van Caenegem, E., Klaver, M., Kreukels, B.P., Elaut, E., … T'Sjoen, G. (2016). A European network for the investigation of gender incongruence: Endocrine part. The Journal of Sexual Medicine, 13(6), 994–999. doi: 10.1016/j.jsxm.2016.03.371 [DOI] [PubMed] [Google Scholar]
- Grynberg, M., Fanchin, R., Dubost, G., Colau, J.-C., Brémont-Weil, C., Frydman, R., & Ayoubi, J.-M. (2010). Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reproductive Biomedicine Online, 20(4), 553–558. doi: 10.1016/j.rbmo.2009.12.021 [DOI] [PubMed] [Google Scholar]
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., … T’Sjoen, G. G. (2017). Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 102(11), 3869–3903. ), doi: 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Jeppsson, S., Johansson, E. D. B., & Sjöberg, N. O. (1973). Plasma levels of estrogens during long-term treatment with depo-medroxyprogesterone acetate as a contraceptive agent. Contraception, 8(2), 165–170. doi: 10.1016/0010-7824(73)90122-4 [DOI] [PubMed] [Google Scholar]
- Jonard, S., & Dewailly, D. (2004). The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Human Reproduction Update, 10(2), 107–117. doi: 10.1093/humupd/dmh010 [DOI] [PubMed] [Google Scholar]
- Klaver, M., de Blok, C. J. M., Wiepjes, C. M., Nota, N. M., Dekker, M. J. H. J., de Mutsert, R., … den Heijer, M. (2018). Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: Results from a multicenter prospective study. European Journal of Endocrinology, 178(2), 163–171. doi: 10.1530/EJE-17-0496 [DOI] [PubMed] [Google Scholar]
- Lerchbaum, E., Schwetz, V., Rabe, T., Giuliani, A., & Obermayer-Pietsch, B. (2014). Hyperandrogenemia in polycystic ovary syndrome: Exploration of the role of free testosterone and androstenedione in metabolic phenotype. PLoS One, 9(10), e108263. doi: 10.1371/journal.pone.0108263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light, A. D., Obedin-Maliver, J., Sevelius, J. M., & Kerns, J. L. (2014). Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstetrics & Gynecology, 124(6), 1120–1127. doi: 10.1097/AOG.0000000000000540 [DOI] [PubMed] [Google Scholar]
- Moore, E., Wisniewski, A., & Dobs, A. (2003). Endocrine treatment of transsexual people: A review of treatment regimens, outcomes, and adverse effects. The Journal of Clinical Endocrinology & Metabolism, 88(8), 3467–3473. doi: 10.1210/jc.2002-021967 [DOI] [PubMed] [Google Scholar]
- Motta, G., Crespi, C., Mineccia, V., Brustio, P. R., Manieri, C., & Lanfranco, F. (2018). Does testosterone treatment increase anger expression in a population of transgender men? The Journal of Sexual Medicine, 15(1), 94–101. doi: 10.1016/j.jsxm.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Mueller, A., Kiesewetter, F., Binder, H., Beckmann, M. W., & Dittrich, R. (2007). Long-term administration of testosterone undecanoate every 3 months for testosterone supplementation in female-to-male transsexuals. The Journal of Clinical Endocrinology & Metabolism, 92(9), 3470–3475. doi: 10.1210/jc.2007-0746 [DOI] [PubMed] [Google Scholar]
- Nakamura, A., Watanabe, M., Sugimoto, M., Sako, T., Mahmood, S., Kaku, H., … Kumon, H. (2013). Dose-response analysis of testosterone replacement therapy in patients with female to male gender identity disorder. Endocrine Journal, 60(3), 275–281. doi: 10.1507/endocrj.EJ12-0319 [DOI] [PubMed] [Google Scholar]
- Obedin-Maliver, J., & Makadon, H. J. (2016). Transgender men and pregnancy. Obstetric Medicine, 9(1), 4–8. doi: 10.1177/1753495X15612658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadid, S., Abosi-Appeadu, K., De Maertelaere, A. S., Defreyne, J., Veldeman, L., Holst, J. J., & T’Sjoen, G. (2020). Effects of gender-affirming hormone therapy on insulin sensitivity and incretin responses in transgender people. Diabetes Care, 43, 411–417. doi: 10.2337/dc19-1061 [DOI] [PubMed] [Google Scholar]
- Slagter, M. H., Gooren, L. J. G., Scorilas, A., Petraki, C. D., & Diamandis, E. P. (2006). Effects of long-term androgen administration on breast tissue of female-to-male transsexuals. Journal of Histochemistry & Cytochemistry, 54(8), 905–910. doi: 10.1369/jhc.6A6928.2006 [DOI] [PubMed] [Google Scholar]
- Srinivas-Shankar, U., & Wu, F. C. W. (2006). Drug insight: Testosterone preparations. Nature Clinical Practice Urology, 3(12), 653. doi: 10.1038/ncpuro0650 [DOI] [PubMed] [Google Scholar]
- T’Sjoen, G., Arcelus, J., Gooren, L., Klink, D. T., & Tangpricha, V. (2018). Endocrinology of transgender medicine. Endocrine Reviews, 40(1), 97–117. doi: 10.1210/er.2018-00011 [DOI] [PubMed] [Google Scholar]
- van Dijk, D., Dekker, M. J. H. J., Conemans, E. B., Wiepjes, C. M., de Goeij, E. G.M., Overbeek, K. A., … T’Sjoen, G. (2019). Explorative prospective evaluation of short-term subjective effects of hormonal treatment in trans people—Results from the European network for the investigation of gender incongruence. The Journal of Sexual Medicine, 16(8), 1297–1309. doi: 10.1016/j.jsxm.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Vujovic, S., Popovic, S., Sbutega-Milosevic, G., Djordjevic, M., & Gooren, L. (2009). Transsexualism in Serbia: A twenty-year follow-up study. Journal of Sexual Medicine, 6(4), 1018–1023. doi: 10.1111/j.1743-6109.2008.00799.x [DOI] [PubMed] [Google Scholar]