Abstract
Brain metastases of breast and other cancers remain resistant to chemotherapeutic regimens that are effective systemically, in part due to the blood–brain barrier. We report that TPI-287, a new microtubule-stabilizing agent, displays in vitro cytotoxic activity similar to taxanes and epothilones. Unlike the taxanes, TPI-287 is permeable through the blood–brain barrier. Brain-to-plasma ratios of TPI-287 after a single injection typically exceeded one and were as high as 63.8 in the rat and 14.1 in the mouse. A brain-tropic derivative of the MDA-MB-231 triple-negative breast cancer cell line, 231-BR, was used to test whether TPI-287 may be efficacious at preventing or treating brain metastases. TPI-287 had growth inhibitory effects comparable with paclitaxel when 231-BR tumor cells were injected into the mammary fat pad. Brain metastatic colonization was determined by intracardiac injection of 231-BR cells, with treatment beginning on day 3 to 4 postinjection, culminating in a histologic count of brain metastases in brains necropsied days 25 to 28 postinjection. In this assay, paclitaxel, ixabepilone, and nab paclitaxel did not have significant inhibitory activity. TPI-287 was ineffective in the same assay using a 6 mg/kg every week schedule; however an 18 mg/kg dose delivered on days 3, 7, and 11 significantly reduced the outgrowth of brain metastases (55% reduction, P = 0.028) and reduced proliferation in brain metastases (16% reduction, P = 0.008). When TPI-287 treatment was delayed until days 18, 22, and 26 postinjection, efficacy was reduced (17% reduction, not significant). These data suggest that TPI-287 may have efficacy when administered early in the course of the disease.
Introduction
Paclitaxel, discovered in 1967 and first introduced into the clinic in 1993, continues to be one of the most active antineoplastic agents against a wide spectrum of malignancies, including ovarian, breast, lung, prostate, head and neck cancers (reviewed in refs. 1, 2). The success of this microtubule-stabilizing agent has led to the development of synthetic taxane derivatives, such as docetaxel, and more recently the epothilones, exemplified by ixabepilone (reviewed in ref. 3). On-going efforts continue to develop alternative molecules, which target the tubulin cytoskeleton, as not all cancers respond to treatment with these agents. Even among susceptible cancers, the majority of patients eventually develop resistance. There are multiple pathways involved in acquired resistance to taxanes, including detoxification, alterations in tubulin dynamics, chromosomal instability, and antiapoptotic pathways (4-9). Another common cause of resistance in cancer cells is the expression of ATP-dependent drug efflux pumps, such as P-glycoprotein (P-gp), also known as ABCB1, the product of the multidrug-resistance-1 gene (MDR1; reviewed in ref. 10). P-gp and other efflux pumps may also represent a major cause of the inability of taxanes to cross the blood–brain barrier (BBB), limiting their efficacy for central nervous system malignancies (11).
We have studied the problem of brain metastasis of breast cancer. This complication was once a late aspect of disease progression but, increasingly, is now a first site of disease progression after otherwise successful treatment in the metastatic setting, and is only treated by palliation (reviewed in refs. 12-16). Multiple reasons may account for the lack of efficacy of traditional chemotherapeutics in the brain. Detailed pharmacokinetic studies using 2 experimental brain metastasis models showed that permeability to either tracers or radiolabeled drugs (paclitaxel, doxorubicin) was increased in brain metastases relative to normal brain, but the magnitude of increase was just several fold and far lower than needed to reach effective drug concentrations; only 10% of experimental lesions exhibited sufficient paclitaxel uptake to show a cytotoxic response (17). Other components of the BBB such as astrocytes may also limit drug efficacy (18). To date, we have tested 16 approved or experimental drugs in an experimental brain metastasis assay and have found efficacy in only a minority (19-21), suggesting that a distinct, brain-permeable armamentarium will be needed for this site.
In this report, a new microtubule stabilizer, TPI-287, is described. TPI-287 readily crossed the normal BBB. While paclitaxel and TPI-287 showed similar cytotoxicity to breast cancer cells in vitro, and in a model of primary breast tumors, only TPI-287 was able to significantly reduce metastatic colonization of breast cancer in the brain.
Materials and Methods
Drugs and administration
Paclitaxel was purchased from Sigma-Aldrich. TPI-287 was obtained from Archer Biosciences. Stock solutions of TPI-287 and paclitaxel were prepared in a 1:1 mixture of ethanol and cremophor EL (Sigma). For in vivo studies, drugs were diluted 1:10 in saline (0.9% sodium chloride) immediately before intravenous injection (i.v.) into mice. Clinical preparations of nab paclitaxel (Abraxane, Abraxis Bioscience, powdered), docetaxel (Taxotere, Sanofi Aventis), ixabepilone (BMS-247550, Bristol-Meyers Squibb), and paclitaxel [Taxol, Bristol-Myers Squibb (BMS), 6mg/kg stock], were obtained from the NIH Clinical or Veterinary Pharmacies.
Cell lines and viability assays
Derivation and maintenance of the brain metastatic breast cancer line MDA-MB-231-BR (231-BR) was described previously (22). The 231-BR cell line was obtained from Toshiyuki Yoneda (University of Texas, San Antonio, TX) and was authenticated by our laboratory most recently in August 2011 to the MDA-MB-231 line by short tandem repeat profiling conducted at the American Type Culture Collection.
The in vitro cytotoxicity of compounds was assessed in 231-BR cells by a tetrazolium-based colorimetric (MTT) assay as previously reported (22). Cells were seeded into replicate wells of a 96-well plate at a density of 2,000 cells per well, 24 hours before compound addition. Drug stocks were prepared in ethanol, and serial dilutions of compounds into culture media were added to triplicate wells. Results represent a minimum of 3 independent experiments.
Microtubule polymerization assays
The determination of drug effects on tubulin polymerization were measured using a fluorescence-enhanced tubulin polymerization assay kit (Cytoskeleton, cat# BK011P). The assay determines the effects of drugs on tubulin polymerization in a kinetic assay, originally described by Heusele and colleagues (23) and Bonne and colleagues (24). Polymerization is followed by fluorescence enhancement due to the incorporation of a fluorescent reporter into microtubules as polymerization occurs. The standard assay uses neuronal tubulin, which generates a polymerization curve representing the 3 phases of microtubule formation, namely nucleation, growth, and steady state equilibrium. In this assay, paclitaxel at 3 μmol/L final concentration eliminates the nucleation phase and enhances the Vmax of the growth phase, reaching steady state in a much shorter time than control (no drug). Assays were conducted in standard 96-well flat bottom black plates and read on the TECAN machine, SpectroFluor Plus, excitation wavelength 340 to 360 nm and emission 410 to 460 in kinetic mode. Samples were done in quadruplicate, and ED50 determined to be the amount of drug necessary to reduce the time to achieve Vmax by 50%.
Pharmacokinetic studies
Pharmacokinetic studies were conducted at Quest Pharmaceutical Services. All animal studies were conducted under approved Institutional Animal Care and Use Committee protocols. TPI-287 was prepared fresh each day. For rat studies, male Sprague Dawley rats weighing between 225 and 275 g were dosed with 20 mg/kg TPI-287 by tail vein. At 15 minutes, 2 and 6 hours post-dose, samples (5 rats per time sample) were collected. Cerebrospinal fluid (CSF) was collected under isoflurane anesthesia from the cisterna magna. A terminal blood sample was collected from each animal by cardiac puncture using K2EDTA as an anticoagulant and plasma processed immediately. Whole brains were removed by dissection, collected on wet ice, weighed, and stored at −20°C with all other samples until assayed. For mouse experiments, male CD-1 mice weighing 20 to 30 grams were dosed at 20 mg/kg TPI-287, i.v. via tail vein. Samples were collected at 5 minutes, 15 minutes, and 2, 8, 32, 48, 72, and 96 hours post-dose (4 mice per sample time). Terminal blood samples were collected and brains processed in identical fashion to the rat study. No attempts were made to quantify TPI-287 in mouse CSF. The quantification of TPI-287 was carried out with a liquid chromatography/tandem mass spectrometry method with internal standards for plasma and brain homogenates. CSF samples were run against the plasma standard curve. Duplicate standard curves were run before and after sample analysis. Calibration curves consisted of standards prepared in blank plasma and blank brain homogenates. A minimum of 5 standards were run for each curve with lower limit of quantitation at 3 nmol/L (~2.6 ng/mL).
Breast primary tumor model
Experiments were conducted under an approved Animal Use Agreement with the National Cancer Institute (NCI). Five- to 7-week-old female NCr nu/nu mice (Charles River Laboratories) were anesthetized under isoflurane/O2 and 5 × 106 231-BR cells were inoculated in the fourth mammary fat pad. Treatment started when the average tumor size was approximately 200 mm3. Mice were then randomized into 3 groups (n = 5 per group) and received 18 mg/kg vehicle, paclitaxel, or TPI-287 on every fourth day for a total of 3 injections (every 4 days ×3). Caliper measurements of the tumor size and mouse weight were calculated twice weekly. For tumor volume determination: Two-dimensional measurements were taken twice per week with a caliper and tumor volume (V) calculated using the following formula: V = a × b2 × 0.52, where a is the longest diameter, b is the shortest one, and 0.52 is a constant to calculate the volume of an ellipsoid.
Experimental brain metastasis model
Experiments were conducted under an approved Animal Use Agreement with the NCI as previously reported (19, 22, 25). Briefly, female NCr nu/nu mice (Charles River Laboratories) were inoculated in the left cardiac ventricle with 1.75 × 105 human 231-BR cells. Treatments began on days 3 to 4 postinjection unless noted. At necropsy, generally on days 25 to 28 postinjection, the brain was removed for histologic analysis. Ten micron cryosections were prepared and processed for histology and immunofluorescence; and metastases were quantified by manual counts in 10 step sections through one hemisphere as previously published (25).
Immunofluorescence analyses
Immunofluorescence analyses were conducted as previously described (25). The following antibodies were used: anti-Ki67 (Vector Laboratories); anti-acetylated Tubulin (Sigma-Aldrich). Secondary antibodies were conjugated to Alexa568 (Molecular Probes). The number of Ki67-positive cells was enumerated in photographs using DAPI (4′,6-diamidino-2-phenylindole, dilactate; Molecular Probes) to counterstain all nuclei.
Statistical methods
A variety of ANOVAs were conducted on the raw or transformed data (as indicated by the Box–Cox transformation). One-way ANOVAs were used to analyze simple fixed effect models. Two- or 3-factor factorial ANOVAs were used to analyze more complex fixed effect models. Repeated measures ANOVA was used to analyze longitudinal data. Mixed models were used as necessary to model random variation due to repeated experiments or other random effects. Analysis of covariance was used to account for important covariates. A binomial distribution using a logit link function was used to analyze percentage data (events per trials). Finally, for all ANOVAs, residuals were examined for normality and homogeneity and residuals were partitioned if found to be heterogeneous. For pairwise comparisons, Holm method was used to adjust pairwise P values.
Results
Characterization of TPI-287
TPI-287, (2′R,3′S)-2′hydroxy-N-carboxy-3′-amino-5′-methyl-hexanoic, N-tert-butyl ester, 13 ester 5β-20-epoxy-1,2α,4,7β,9α,10α,13α-heptahydroxy-4,10-diace-tate-2-benzoate-7,9-acrolein acetal-tax-11(15 → 1)-abeotaxane, is a derivative of taxol, and is part of a class of structures known as abeotaxanes. Its structure, shown in Fig. 1A, differs significantly from the taxanes not only in that the core baccatin (A) ring is a 5-membered versus a 6-membered ring in all other taxanes, but also in that several side chain substitutions and changes make it more lipophilic (see paclitaxel and docetaxel, Fig. 1A). A tubulin polymerization assay showed that TPI-287 stabilized microtubules with kinetics similar to or exceeding those of paclitaxel and docetaxel (Table 1).
Figure 1.
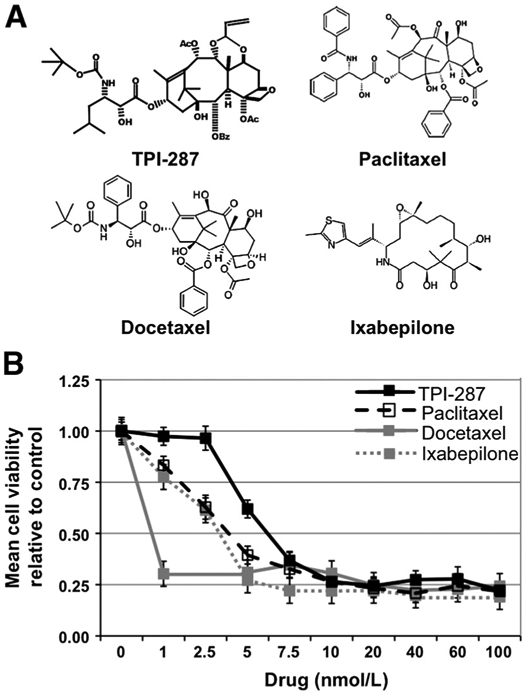
TPI-287 is a cytotoxic microtubule-stabilizing agent. A, chemical structures of TPI-287, paclitaxel, docetaxel, and ixabepilone. B, comparison of the cytotoxic activities of TPI-287 and other microtubule-stabilizing agents. Adherent 231-BR breast cancer cells were exposed to the indicated doses of TPI-287, paclitaxel, docetaxel, or ixabepilone and cultured for 72 hours. Relative numbers of viable cells were determined by an MTT assay, and values normalized to the number of viable cells at the experimental endpoint in the absence of drug.
Table 1.
Microtubule polymerization kinetics with TPI-287 and taxanes, in vitro
| Compound | Mean ± SEM ED50a, μmol/L |
ED50 compound/ ED50 paclitaxel |
|---|---|---|
| TPI-287 | 1.58 ± 0.46 | 0.53 |
| Paclitaxel | 2.97 ± 0.50 | 1.00 |
| Docetaxel | 3.18 ± 0.45 | 1.07 |
The ED50 is the amount of drug required to reduce the time required to reach Vmax of tubulin polymerization in the standard reaction mixture as described in the kit instructions. Each value is the mean of 4 reaction wells ± the SEM.
The cytotoxicity of TPI-287 was determined using 231-BR brain-tropic breast cancer cells, in comparison to paclitaxel, docetaxel, and ixabepilone (Fig. 1B). All 4 compounds exhibited IC50 values and maximal toxicity in the low (0–10) nanomolar range. All 4 drugs also resulted in a similar phenotype in treated cells, with the appearance of micronuclei, apoptotic cell bodies, and an increase in mitotic figures indicative of microtubule stabilization (data not shown).
Brain permeability of TPI-287
Experiments using normal rats and mice were carried out using a single intravenous injection of 20 mg/kg TPI-287. The plasma and brain concentrations of TPI-287 over time are plotted for both species on Fig. 2 (A, rats; B, mice). Calculated pharmacokinetic parameters for TPI-287 in mice are listed on Supplementary Table S1; Supplementary Tables S2 and S3 detail the brain/plasma concentration data for both species. In both time curves, a typical drug elimination profile with limited sampling time was observed. Brain concentrations of TPI-287 exceeded those of plasma throughout most (rat) or all (mouse) of the time points tested (Fig. 2). The mean fold elevation of TPI-287 distribution in brain compared with plasma increased progressively in the mouse from 1.2 just after injection to 63.8 at 96 hours (Supplementary Table S2); in the rat, the brain/plasma ratio was 3.7 at 4 hours and rose to 14.1 at 96 hours (Supplementary Table S3). TPI-287 distribution into the brain was also more favorable than into plasma in terms of maximum drug concentration achieved (Cmax), area under the curve (AUC), and mean residence time (MRT), with reduced clearance (CL; Supplementary Table S1). CSF concentrations were investigated in the rat study and were only detectable 15 minutes after injection (Supplementary Table S3). These data indicate a potentially high distribution of TPI-287 into brain tissue, suggesting activity in the brain metastatic setting.
Figure 2.
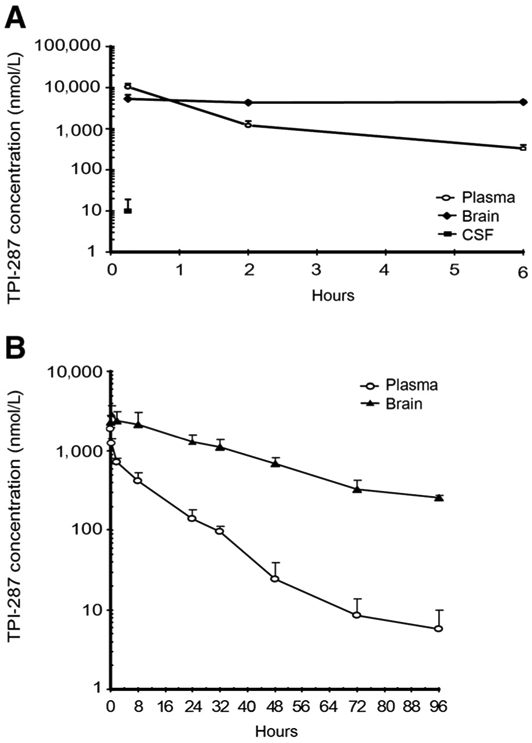
Brain permeability of TPI-287 in rats (A) and mice (B). The mean levels of TPI-287 in the blood (open circles) and brain (black symbols) were quantified over a period of 96 hours following a single i.v. injection of 20 mg/kg. CSF levels at a single point in the rat are shown in the black square in A.
Primary tumor growth
Primary tumors were generated in the mouse mammary fat pad via inoculation of 231-BR breast cancer cells. When the tumors reached a mean volume of 200 mm3, the mice were divided into 3 treatment groups. Three doses of paclitaxel (18 mg/kg), TPI-287 (18 mg/kg), or vehicle (5% cremophor, 5% ethanol, 90% saline) were delivered i.v. every 4 days, starting on day 37 of the experiment (Fig. 3A). Mean tumor volume in the paclitaxel- and TPI-287–treated animals declined beginning on day 41 postinjection, whereas vehicle-treated tumors continued to grow (Fig. 3B). The growth rates (slopes) in tumor volume between days 41 and 48 of the experiment were estimated using transformed data (log10(y)). The estimated slope for the vehicle treatment (0.0345 ± 0.00743) was significantly higher than both the paclitaxel- and TPI-287–estimated slopes (both P < 0.0001). Paclitaxel (slope = −0.0450 ± 0.00743) and TPI-287 (−0.0332 ± 0.00743) were not significantly different (P = 0.26). Immunofluorescent stain for Ki67 revealed a reduction in proliferation in both paclitaxel- and TPI-287–treated tumors relative to vehicle-treated control tumors (Supplementary Fig. S1A). An increase in the amount of acetylated tubulin-α, a marker for stabilized microtubules, was also visible following treatment with either drug (Supplementary Fig. S1B). Thus, TPI-287 has comparable antitumor properties to paclitaxel in a primary tumor setting.
Figure 3.
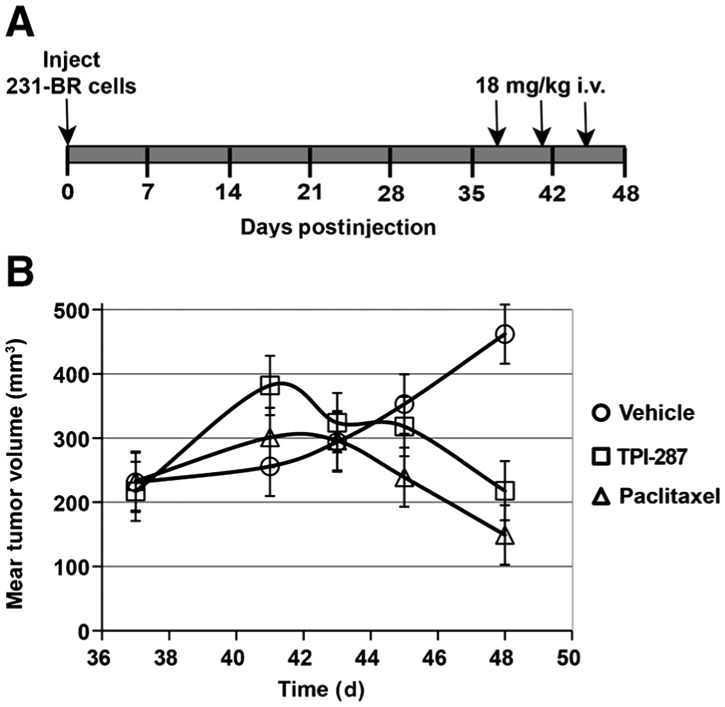
Effect of microtubule-stabilizing drugs on 231-BR primary tumor growth in the mammary fat pad. A, mice were inoculated with 231-BR breast cancer cells into the mammary fat pad. Arrows illustrate the every 4 days ×3 dose schedule for paclitaxel, TPI-287, or vehicle, starting on day 37 postinjection, beginning when the tumors reached a mean volume of 200 mm3. B, tumor volumes were measured by caliper measurement. Final tumor measurements were taken 3 days after the last dose (day 48). Ancova on log10(y) transformed data was used to compare slopes on days 41 to 48. The estimated tumor growth slope for the vehicle treatment was significantly higher than that for paclitaxel and TPI-287 (both P < 0.0001). Paclitaxel and TPI-287 tumor growth slopes were not significantly different (P = 0.26).
Approved microtubule stabilizers fail to prevent 231-BR brain metastatic colonization
The taxanes are known to be relatively brain impermeable, due to the action of efflux pumps such as P-gp, the MRP family (ABCC 2 and 7), and the OATP family (1 and 4; ref. 12), whereas several of the epothilones were reported to be more brain permeable. We asked whether approved microtubule-stabilizing drugs were effective in the 231-BR experimental brain metastasis assay. Briefly, 231-BR cells were injected into the left cardiac ventricle. Drug dosing began on days 3 to 4 postinjection and continued weekly through the 26 to 28 days of the experiment. Drug doses were 6 mg/kg i.v. for paclitaxel. For ixabepilone, the initial 2 doses of 5 mg/kg of ixabepilone showed significant toxicity, therefore, the subsequent 2 doses were delivered at 2.5 mg/kg. At necropsy, each brain was cryosectioned; hematoxylin and eosin-stained large metastases (>300 μm in one dimension) and micrometastases (smaller) were quantified in 10 sections every 300 μm through one hemisphere. Mean large metastases and micrometastases are graphed on Fig. 4. The absolute number of brain lesions per histologic section varied from experiment to experiment but, in each case, neither paclitaxel, nor nab paclitaxel or ixabepilone significantly prevented the development of brain metastases as compared with vehicle. These data confirm the need for microtubule-stabilizing drugs with in vivo brain efficacy.
Figure 4.
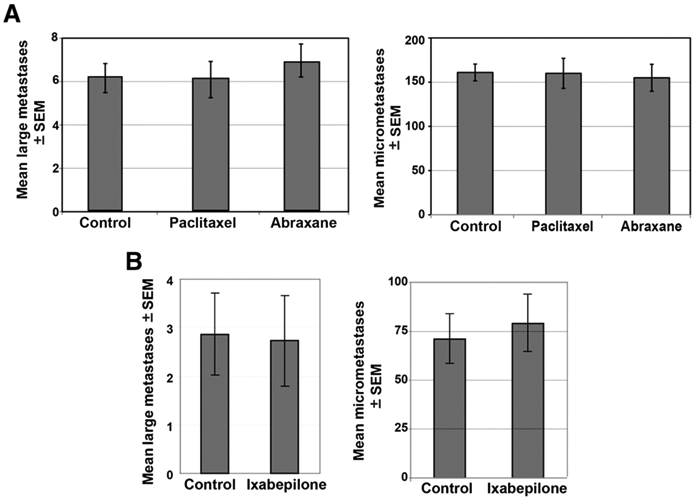
Effects of approved microtubule-stabilizing drugs in an experimental brain metastasis model of breast cancer. A, mice were inoculated by intracardiac injection of 231-BR breast cancer cells and were subsequently randomized to receive weekly treatments (administered i.v.) with the following: vehicle control (5% ethanol:5% cremophor:90% saline), nab paclitaxel (6 mg/kg), or paclitaxel (6 mg/kg). An additional group of mice received no treatment; the no treatment and vehicle control groups were combined. Following 28 days, serial sections of mouse brain were examined histologically and the number of large brain metastases (>300 μm) and micrometastases (smaller) determined. Graphs indicate the mean number of large brain metastases and micrometastases counted per brain section. n = 7 mice for no drug, n = 15 for vehicle, n = 17 for nab paclitaxel, and n = 14 for paclitaxel for combined data from 2 independent experiments. B, number of large brain metastases formed by 231-BR cells after 27 days in untreated mice (no drug, control) or following weekly treatments with ixabepilone (5 mg/kg ×2, 2.5 mg/kg ×2). n = 10 mice for control and n = 8 for ixabepilone. All data not significantly different from vehicle arms.
TPI-287 activity in 231-BR experimental brain metastasis assays
Supplementary Fig. S2 presents a series of experiments in which TPI-287 was administered to mice in a schema similar to that shown in Fig. 4. In this experiment, TPI-287 was dosed twice weekly, at 6 mg/kg, starting on day 4 postinjection, as shown in Fig. 4A. Using this dose regimen, there was no significant effect on large brain metastases or brain micrometastases (Fig. 4B). However, in a preliminary experiment using higher doses of TPI-287 (18 mg/kg, ×3), we found evidence that the number of macrometastases were reduced compared with vehicle treatment (P = 0.047; data not shown).
On the basis of the hypothesis that an elevated dose of drug, delivered in fewer injections, may be more efficacious, mice received 18 mg/kg TPI-287 or paclitaxel 3 times. In this experiment, the effect of treatment timing was also investigated. In the experiment shown on Fig. 5, mice were randomized to paclitaxel, TPI-287 or vehicle, with the drugs delivered either early (days 3, 7, and 11 postinjection) or late (days 18, 22, and 26 postinjection). Paclitaxel at this higher intensity dose continued to be ineffective in preventing brain metastatic colonization (all points not significant). Early administration of TPI-287 reduced the number of large brain metastases by 55%, from 4.25 ± 0.48 in vehicle-treated mice to 1.92 ± 0.55 in the TPI-287–treated mice (P = 0.025). However, for the large metastases there was a marginally significant timing effect (early vs. late, P = 0.039), if these data were pooled over the 2 drugs. There was also a numerical, but not statistical, trend in the reduction of micrometastases, from 115 ± 10.7 in the vehicle group to 72 ± 12.3 in the TPI-287–treated group. In addition, for the micrometastases there was a drug effect (paclitaxel vs. TPI-287, P = 0.018) if these data were pooled over the 2 timings. Thus, TPI-287 showed efficacy at this dose and schedule in contrast to paclitaxel. Later administration of TPI was less effective, resulting in a 17% decrease in large metastases, to 3.54 (not significant).
Figure 5.
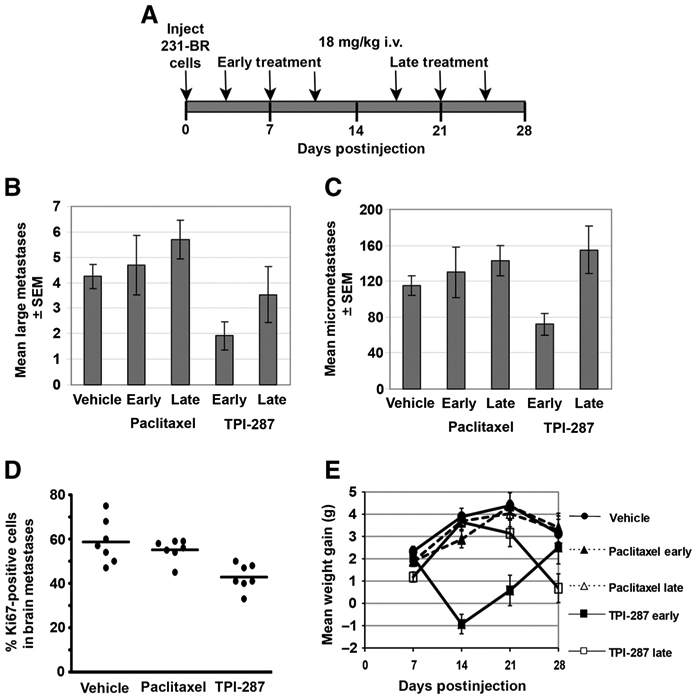
TPI-287, but not paclitaxel, prevented the formation of large 231-BR brain metastases. A, experimental schema in which mice were inoculated with 231-BR tumor cells in the left cardiac ventricle. Mice were randomized to receive an every 4 days ×3 schedule of 18 mg/kg paclitaxel or TPI-287, delivered either early (days 3, 7, and 11 postinoculation) or late (days 18, 22, and 26 postinoculation). B, number of large brain metastases (>300 μmol/L) in mice 28 days postinoculation of 231-BR cells following the treatments described in A. C, number of brain micrometastases (<300 μmol/L) in mice 28 days postinoculation of 231-BR cells. n = 17 for vehicle, n = 13 for TPI-287 early, n = 9 for TPI-287 late, n = 8 for paclitaxel early, and n = 7 for paclitaxel late for the results in B and C. D, immunoflourescence for Ki67 was used to determine proliferation rate in brain metastases on day 28, 48 hours after the final treatment with paclitaxel (late schedule) or TPI-287 (late schedule). Each dot represents the percentage of Ki67-positive 231-BR cells counted in a mouse brain, P = 0.0002 in a comparison of TPI-287 and vehicle-treated groups. E, mean (±SEM) animal weight differences from baseline (day 0) through the experiment described in A.
As a biomarker for drug activity in the brain, immunofluorescence for Ki67 was carried out on day 28 brains, 48 hours after the final (late) treatment with paclitaxel or TPI-287 (Fig. 5). 231-BR cells in the brain showed a significant reduction (P = 0.0002) in proliferation (mean = 40.6% ± 1.92%) when treated with TPI-287 as compared with vehicle-treated mice (mean = 56.0% ± 2.22%), supporting the trend in metastasis treatment. In contrast, paclitaxel did not significantly affect the rate of brain metastasis proliferation (mean = 54.4% ± 1.91%). Acetylated tubulin staining of brain metastases was not quantifiable in the heavily stained brain microenvironment. Of the 2 experiments using the 18 mg/kg dose, no animal deaths were recorded in experiment 1; in experiment 2, 3 mice in the early TPI-287 treatment group died after the third dose. Animal weights for this experiment are graphed on Fig. 5E. Early administration of TPI-287 resulted in weight loss, which recovered to those of mice on the late treatment schedule or vehicle after treatment ended. Collectively, these data indicate that TPI-287 can reduce brain metastatic colonization in the 231-BR model.
Discussion
The taxanes have been shown to provide a clinical benefit in both the adjuvant setting (26) and in early treatment of metastatic (27) breast cancer. Recently, several epothilones have been developed, which bind a similar site on tubulin but differ from the taxanes in specific binding characteristics, potency, and cross-resistance (28-30). A number of microtubule-stabilizing drug regimens are under investigation for refractory metastatic disease (31, 32). The current report describes a new drug related to the taxanes, TPI-287.
Salient features of TPI-287 include a structure similar to the taxanes, microtubule stabilization in vitro, and significant brain permeability. Brain distribution was determined after a single injection into mice and rats. Multiple-fold elevation was observed in brain as compared with plasma in both species, in terms of Cmax, AUC, and other pharmacologic parameters. Additional pharmacologic characteristics such as protein binding, metabolite concentrations, and tissue levels remaining after perfusion of the vasculature, remain to be established. Unpublished data show that TPI-287 is a poor substrate for the P-gp efflux pump. These data support the interesting hypothesis that TPI-287 may have efficacy in brain metastatic breast cancer.
Using an experimental model of brain metastasis of triple-negative breast cancer, we report a 55% reduction in the formation of large metastases using 3 intravenous injections of 18 mg/kg TPI-287, beginning on day 3 postinjection. This level of brain metastasis inhibition is comparable with that previously reported for lapatinib using Her-2–transfected 231-BR cells (19), but less than that using either vorinostat or a polo-like kinase inhibitor (21, 25). A trend of reduced micrometastases was observed. Micrometastases as defined in this study were less than 300 μm in length in a single dimension, and would correspond to occult lesions in a human brain. In previous studies, gene transfections and drug delivery altered the formation of large metastases without significant effects on micrometastases, suggesting that these are biologically distinct entities (19, 22). The significance of micrometastases remains unknown, as they could remain occult or grow out to detectable lesions. Using a similar total dose, but a twice weekly injection schedule, TPI-287 was ineffective, suggesting that Cmax may be a significant factor for activity. When administered later in the approximate 1 month assay, TPI-287 was less effective, although a trend in metastasis reduction was supported by Ki67 staining. This characteristic was also observed in vorinostat experiments (25), suggesting that relapse free survival rather than the shrinkage of large lesions may be a more relevant measure of clinical efficacy.
The TPI-287 brain metastasis inhibitory data stand in contrast to similar experiments using paclitaxel and, using a different dose and schedule, nab paclitaxel and the epothilone ixebepilone. The epothilones are less subject to transport by drug efflux pumps, and several have confirmed brain permeability. Sagopilone crossed the BBB of mice, with a favorable AUC and half-life (33). It reduced the growth of intracerebrally implanted MDA-MB-435 tumor cells, and Lu7187 and Lu7466 non–small cell lung cancer cells, as compared with nonsignificant effects of paclitaxel. Weight loss was 6%. In a recently published phase II study (34), patients with breast cancer with progressive brain metastases after whole brain radiation therapy received sagopilone. Two patients (13.3%) achieved partial responses; median progression-free survival and overall survival were 1.4 and 5.3 months, respectively, and enrollment was terminated prematurely. Significant brain distribution was also reported for patupilone in mice, with a favorable half-life (35). Activity was reported in a variety of subcutaneous tumors (not breast cancer) and weight loss was noted. Patupilone has moved into clinical testing for brain metastases. A preliminary report of a phase II trial of patupilone in patients with breast cancer brain metastases progressing or recurring after whole brain radiotherapy reported toxicities of diaharrea, fatigue, nausea, vomiting, and headache, with a 27% incidence of peripheral neuropathy. Partial responses were observed in 19% of patients (6 of 31), and stable disease noted in 29% of patients (9 of 31; ref. 36). A phase I trial of patupilone combined with radiation therapy was also conducted in patients with glioma and brain metastasis (37). The epothilone for which there is the most breast cancer clinical data, ixabepilone, is approved as monotherapy for the treatment of locally advanced or metastatic breast cancer that is resistant or refractory to anthracyclines, taxanes, and capecitabine (3). To our knowledge, reductions in the growth rates of multiple subcutaneous tumors have been reported for ixebepilone, but preclinical in vivo efficacy in the brain is unavailable (38, 39). While it was anticipated that paclitaxel and nab paclitaxel would not have brain metastasis inhibitory activity in the 231-BR model due to their exclusion from the brain by multiple efflux pumps, our preclinical data with ixebepilone were disappointing. One potential reason for a lack of activity may have been toxicity, which forced dose reductions. Other potential contributors may include unique resistance pathways in the cell line used, or the effects of edema or other pharmacologic parameters.
These data suggest that TPI-287 may be of clinical use in patients with brain metastasis in the prevention of further outgrowth of established lesions and micrometastases. Shrinkage of established lesions was not an endpoint herein and would require distinct models amenable to serial imaging. A potential side effect of all microtubule-stabilizing drugs is peripheral neuropathy, and entry of these agents into the brain raises questions of cognitive deficits, untested in the current model. Such concerns would be shared with the brain-permeable epothilones. It is of interest to note that peripheral neurons and central nervous system neurons respond distinctly to injury, suggesting the hypothesis that their response to microtubule stabilizers may also be distinct (reviewed in ref. 28). Another potential limitation to the clinical use of TPI-287 is the weight loss observed upon treatment. This loss recovered after cessation of treatment in the early treatment model, and may be ameliorated by hydration (D. Emerson, personal communication).
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the Intramural Research program of the National Cancer Institute, and by the US Department of Defense Breast Cancer Research Program, Grant Number: W81 XWH-062-0033 awarded to P.S. Steeg.
Footnotes
Disclosure of Potential Conflicts of Interest
P.S. Steeg received research support from Glaxo-SmithKline and Millenium Pharmaceuticals. S. Silberman is a consultant and member of the advisory board for Archer Biosciences, Inc. No potential conflicts of interest were disclosed by the other authors.
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs 1998;55:5–30. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Antona C Pharmacogenomics of paclitaxel. Pharmacogenomics 2010;11:621–3. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi M, Budihardjo I, Loureiro K, Reid TR, Ma JD. Epothilones: a novel class of microtubule-stabilizing drugs for the treatment of cancer. Future Oncol 2008;4:483–500. [DOI] [PubMed] [Google Scholar]
- 4.Smoter M, Bodnar L, Duchnowska R, Stec R, Grala B, Szczylik C. The role of Tau protein in resistance to paclitaxel. Cancer Chemother Pharmacol 2011;68:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Tang K, Zhang H, Zhang Y, Zhou W, Chen X. Function of Aurora kinase A in Taxol-resistant breast cancer and its correlation with P-gp. Mol Med Report 2011;4:739–46. [DOI] [PubMed] [Google Scholar]
- 6.Lee E, Nichols P, Groshen S, Spicer D, Lee A. GRP78 as potential prodictor for breast cancer response to adjuvant taxane therapy. Int J Cancer 2011;128:726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spicakova T, O'Brien M, Duran G, Sweet-Cordero A, Sikic B. Expression and silencing of microtubule-associated protein Tau in breast cancer cells. Mol Cancer Ther 2010;9:2970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starlard-Davenport A, Lyn-Cook B, Beland F, Pogribny I. The role of UDP-glucuronosyltransferases and drug transporters in breast cancer drug resistance. Exp Oncol 2010;32:172–80. [PubMed] [Google Scholar]
- 9.Swanton C, Nicke B, Schuett M, Eckland AC, Ng C, Li QY., et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci U S A 2009;106:8671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: molecular mechanisms and development of new generation taxanes. ChemMedChem 2007;2:920–42. [DOI] [PubMed] [Google Scholar]
- 11.Fellner S, Bauer B, Miller DS, Schaffnik M, Fankhel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 2002;110:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steeg P, Camphausen K, Smith Q. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 2011;11:352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin N,Bellon J, Winer E.CNS metastases in breast cancer. J Clin Oncol 2004;22:3608–17. [DOI] [PubMed] [Google Scholar]
- 14.Patel R, Mehta M. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res 2007;13:1675–1683. [DOI] [PubMed] [Google Scholar]
- 15.Mayer M A patient perspective on brain metastases in breast cancer. Clin Cancer Res 2007;13:1623–4. [DOI] [PubMed] [Google Scholar]
- 16.Fidler I, Balasubramanian K, Lin Q, Kim S, Kim S-J. The brain micro-environment and cancer metastasis. Mol Cells 2010;30:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockman P, Mittapalli R, Taskar K, Rudraruju V, Gril B, Bohn KA, et al. Heterogeneous blood-brain barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin Cancer Res 2010;16:5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 2010;12:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gril B, Palmieri D, Bronder JL, Herring JM,Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 2008;100:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gril B, Palmieri D, Qian Y, I leva L, Bernardo M, Choyke P, et al. Pazo-panib reveals a role for tumor cell B-Raf in the prevention of breast cancer brain metastasis. Clin Cancer Res 2010;17:142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Y, Hua E, Bisht K, Woditschka S, Skordos K, Liewehr DJ, et al. Inhibition of polo-like kinase-1 prevents growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis 2011;28:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 2007;67:4190–8. [DOI] [PubMed] [Google Scholar]
- 23.Heusele C, Bonne D, Carlier M. Is microtubule assembly a biphasic process- a flurometric study using 4′,6-diamidino-2-pheynylindole as a probe. Eur J Biochem 1987;165:613–20. [DOI] [PubMed] [Google Scholar]
- 24.Bonne D, Heusele C, Simon C, Pantaloni D. 4′, 6-diamidino-2-phenylindole, a fluorescent-probe for tubulin and microtubules. J Biol Chem 1985;260:2819–25. [PubMed] [Google Scholar]
- 25.Palmieri D, Lockman P, Thomas F, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer. Clin Cancer Res 2009;15:6148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson T, Wilcken N, Vagg R, Ghersi D, Novak A. Taxanes for the adjuvant treatment of early breast cancer (Review). Cochrane Database Syst Rev 2007;CD004421. [DOI] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart M, Burzykowski T, Buyse M, Sledge G, Carmichael J, Luck H-J, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 2008;26:1980–6. [DOI] [PubMed] [Google Scholar]
- 28.Higa G The microtubule as a breast cancer target. Breast Cancer 2011;18:103–19. [DOI] [PubMed] [Google Scholar]
- 29.Bystricky B, Chau I. Patupilone in cancer treatment. Expert Opin Investig Drugs 2011;20:107–17. [DOI] [PubMed] [Google Scholar]
- 30.Denduluri N, Swain S. Ixabepilone: clinical role in metastatic breast cancer. Clin Breast Cancer 2011;11:130–45. [DOI] [PubMed] [Google Scholar]
- 31.Palmieri C, Krell J, James C, Harper-Wynne C, Misra V, Cleator S,et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol 2010;7:561–74. [DOI] [PubMed] [Google Scholar]
- 32.Vredenburg M, Ojima I, Veith J, Pera P, Kee K, Cabral F, et al. Effects of orally active taxanes on P-glycoprotein modulation and colon and breast carcinoma drug resistance. J Natl Cancer Inst 2001;93: 1234–45. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann J, Fichtner I, Lemm M, Lienau P, Hess-Stumpp H, Rotgeri A, et al. Sagopilone crosses the blood-brain barrier in vivo to inhibit brain tumorgrowth and metastases. Neuro Oncol 2008;11:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman RA, Bullitt E, Sun L, Gelman R, Harris G, Ligibel JA, et al. A phase II study of sagopilone (ZK. 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer 2011;11:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O′Reilly T, Wartmann M, Brueggen J, Allegrini PR, Floersheimer A, Maira M, et al. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol 2008;62:1045–54. [DOI] [PubMed] [Google Scholar]
- 36.Peereboom DM, Conlin AK, Seidman AD, Brewer C. Phase II trial of patupilone in patients with breast cancer brain metastases. [abstract] In: Neuro-oncology 2008;10:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fough S, Machtay M, Werner-Wasik M, Curran WJ Jr, Bonanni R, Axelrod R, et al. Phase I trial using patupilone (epothilone B) and concurrent radiotherapy for central nervous system malignancies. Int J Rad Oncol Biol Phys 2010;77:1009–16. [DOI] [PubMed] [Google Scholar]
- 38.Lee F, Borzilleri R, Fairchild C, Kim SH, Long BH, Reventos-Suarez C, et al. BMS-247550: a novel epothilone with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 2001;7:1429–37. [PubMed] [Google Scholar]
- 39.Peterson J,Tucker C, Favours E, Chesire PJ, Creech J, Bilups CA, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res 2005;11:6950–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


