Abstract
Bacillus anthracis, the causative agent for anthrax, is a dangerous pathogen to humans and has a history as a bioterrorism agent. While sampling methods have been developed and evaluated for characterizing and clearing contaminated indoor sites, the performance of these sampling methods is unknown for use in outdoor environments. This paper presents surface sampling data for Bacillus atrophaeus spores, a surrogate for B. anthracis, from a 210-day outdoor study that evaluated the detection and recovery of spores using five different sampling methods: sponge sticks, 37-mm vacuum filter cassettes, residential wet vacuums, robotic floor cleaners, and grab samples of soil, leaves, and grass. The spores were applied by spraying a liquid suspension onto the surfaces. Both asphalt and concrete surfaces were sampled by all the surface sampling methods, excluding grab sampling. Stainless-steel coupons placed outdoors were additionally sampled using sponge sticks. Sampling methods differed in their ability to collect detectable spores over the duration of the study. The 37-mm vacuums and sponge sticks consistently detected spores on asphalt through day 37 and robots through day 99. The wet vacuums detected spores on asphalt for days 1 and 4, but not again until day 210. On concrete, all samplers detected spores until day 210 except for sponge stick samplers that detected spores only up until the day 99 time point. For all sampling methods, spore recoveries were higher from concrete than from asphalt surfaces. There was no statistically significant difference in recoveries of sponge sticks and 37- mm vacuums from either asphalt or concrete surfaces. Processing of grab samples was challenging due to non-target background microorganisms resulting in high detection limits for the samples.
Keywords: Bacillus anthracis, spore, surface sampling, recovery efficiency, Bacillus atrophaeus
Introduction
Bacillus anthracis spores have been weaponized by individuals and governments due to their lethal properties and environmental persistence (Leitenberg, Zilinskas et al. 2012). In 2001, a United States’ Senator’s office was contaminated by B. anthracis-laden letters, and the United States Environmental Protection Agency (EPA) executed a large-scale remediation effort in concert with federal and local partners. During the remediation process, sampling was used to detect and quantify spore contamination levels in the affected buildings (Franco and Bouri 2010). Results provided investigators critical information for identification of contaminated areas (Teshale, Painter et al. 2002). A 2005 review of this effort by the U.S. Government Accountability Office determined that validated sampling methods for B. anthracis lacked scientific rigor (U.S. Government Accountability Office 2005). Since 2001, many methods have been developed for indoor sampling of B. anthracis spores (Hodges, Rose et al. 2010, Piepel, Amidan et al. 2012, Lee, Calfee et al. 2013). However, weaponized B. anthracis spores could also contaminate a wide outdoor area containing many different material types and environmental conditions than what is present indoors. Validated sampling methods for B. anthracis spores, optimized for outdoor surfaces, do not exist nor does a comparative study of different techniques. The objective of the study presented here is to evaluate the detection of spores by several sampling methods on different outdoor surfaces contaminated with Bacillus atrophaeus over a six-month time period.
Sampling methods
Existing indoor sampling methods include wipe-based methods for nonporous surfaces, vacuum-based methods for porous surfaces, and composite methods (e.g., residential wet vacuums and robotic floor cleaners) for sampling larger areas. Several researchers have performed detailed literature reviews and summarized historical efforts with recovery efficiency by sampling device and material type (Piepel, Amidan et al. 2012, U.S. EPA 2017a, Rastogi and Wallace 2020). In brief, the United States Centers for Disease Control and Prevention (CDC) developed surface sampling methods for indoor nonporous surfaces that include procedures for macrofoam swabs, cellulose sponge sticks, and gauze wipe sampling (CDC 2012). Swabs are intended for use on small surfaces (less than approximately 97 cm2), the standard template for sponge sticks is 25.40 × 25.40 centimeters, and for gauze wipes the standard template is 30.48 × 30.48 centimeters. During development of these methods, spore recovery data were collected using stainless-steel coupons inoculated with the B. anthracis surrogate B. anthracis Sterne and tested by the United States Laboratory Response Network (LRN). The macrofoam swab protocol achieved 15–55% percent recovery from stainless steel depending on the presence of dust on the sampled surfaces. The average recovery from dusty surfaces was reported as 41.6% (Hodges, Rose et al. 2010). The cellulose sponge sticks achieved an average of 30.1% recovery from stainless-steel coupons that were inoculated with −104 of B. anthracis Sterne spores (Rose, Hodges et al. 2011). Others have reported similar recovery ranges, 31–39%, using the surrogate Bacillus atrophaeus (ATCC 9372; formerly Bacillus subtilis var. niger and previously “Bacillus globigii”) and polyester-rayon blend wipes on stainless steel with surface loadings of 102-105 CFU/cm2 (Brown, Betty et al. 2007). Gauze wipe sampling on stainless steel was also reported to have similar average recovery efficiencies of 31% (Piepel, Amidan et al. 2012).
Vacuum sampling is generally recommended for porous materials because of its potential to draw particles out of crevices. A comparative study of different vacuum-based methods for sampling concrete, carpet, and upholstery examined recovery efficacies for 37-mm filter cassettes, vacuum socks, and 3M forensic filters (Calfee, Rose et al. 2013). With an average of 49%, mixed cellulose ester 37-mm filter cassettes were observed to have significantly higher relative recoveries than the other vacuum methods when sampling concrete.
Robotic floor cleaners and wet vacuums are composite sampling methods that have been studied to provide technologies that assess a larger area than traditional methods (and in the case of the robots provide an autonomous option). Different robots have been tested indoors with B. atrophaeus loaded carpet and laminate floor surfaces and observed to have variable recovery depending on robot manufacturer and surface material type. For carpet, a Neato Robotics® vacuum with air filter was reported to have a 162% higher average recovery when compared to vacuum sock sampling. (Lee, Calfee et al. 2013). In a widely dispersed contamination test on carpet, sampling efficacies using the Neato were approximately 1–10% (Lee, Calfee et al. 2014). Wet vacuum sampling uses off the shelf residential vacuums that have clean and dirty water tanks. They are filled with a surfactant solution rather than tap water or cleaning chemicals. One study that used a residential vacuum cleaner (Hoover F7452900) had a mean recovery of 49% of B. atrophaeus from concrete surfaces (U.S. EPA 2018).
Studies related to sampling B. anthracis outdoors have focused primarily on aerosol, water, and soil grab/composite sampling. Aerosol and water sampling are outside the scope of this paper, but have focused on concentrating large volumes of water (Gallardo, Morris et al. 2019) and testing different impingers, impactors, and filter materials such as gelatin, mixed cellulose, and Teflon® for aerosol samples (Emanuel, Roos et al. 2008). Soil sample collection procedures have been developed for surface samples (<5.08 centimeters deep) for naturally occurring B. anthracis, but have not been developed for bioterrorism incident response (U.S. EPA 2014).
Methodology
Inoculation
The study was conducted at EPA’s Urban Watershed Facility located in Edison, New Jersey. The complex was originally designed to study green infrastructure and can capture and contain large volumes of stormwater runoff. A sampling grid of 1.52 × 1.52 meter cells was constructed over an un-trafficked parking lot (Figure 1A). For the sampling study, approximately 74 m2 of asphalt and 74 m2 of concrete were inoculated with B. atrophaeus spores as well as approximately 7.4 m2 each of soil, leaves, and grass. Additionally, 18 0.09 m2 stainless steel coupons were placed in the asphalt test area where sealants had previously been applied for an unrelated research study. Other portions of the asphalt and concrete pad were inoculated for a rainfall washoff study which is outside the scope of this paper.
Figure 1.
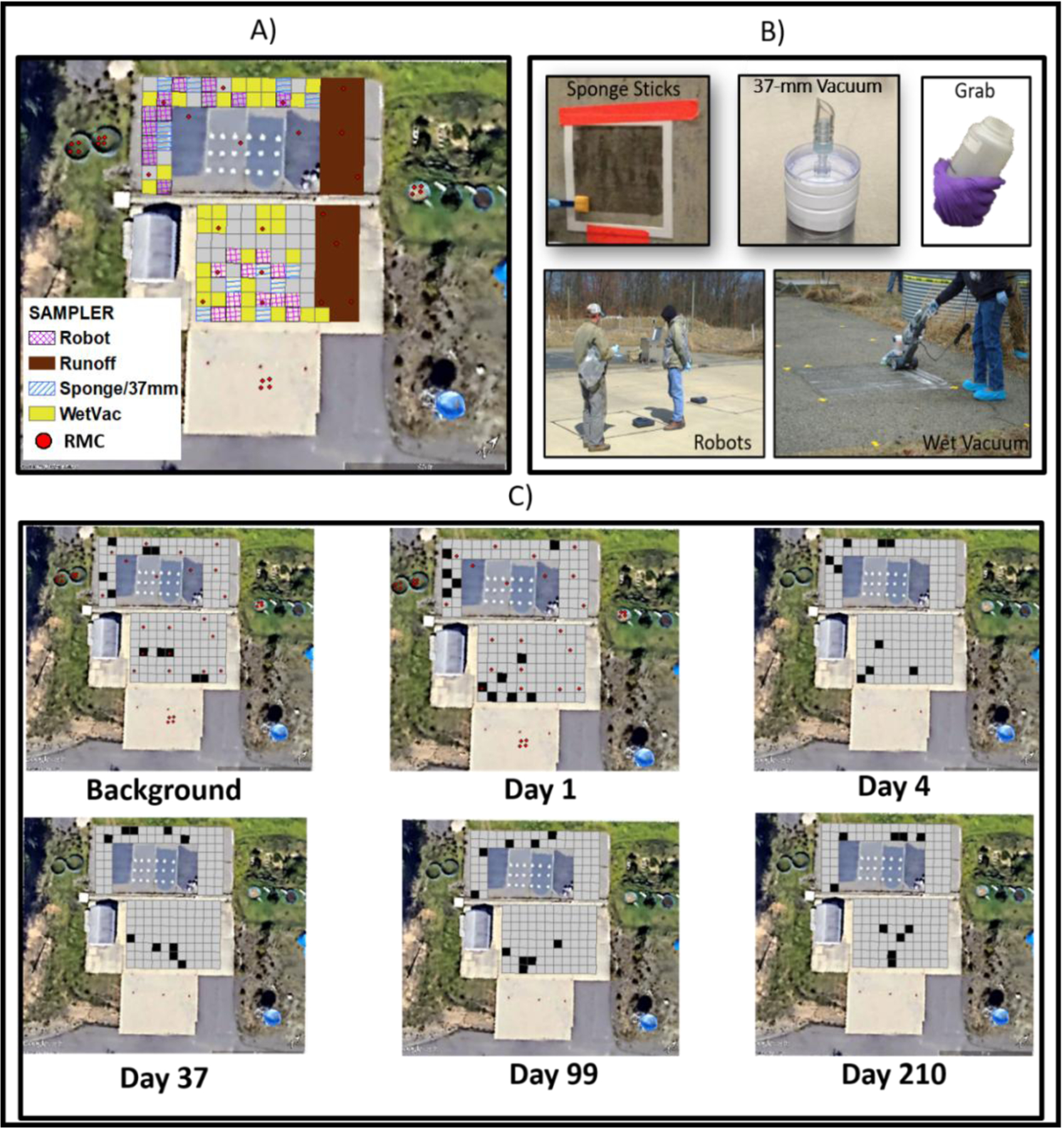
A) study site and locations of samples collected by different sampling methods (note, the runoff area was for a different study), B) sampling devices used in the study, C) temporal sample locations
Dry spores of B. atrophaeus from the United States Army Laboratory, Dugway Proving Ground, (Lot #: 2803349) were suspended in solution and applied by wet deposition using a NorthStar ATV Spot sprayer with Agitation Kit (Item #s: 26810011, 28520, NorthStar, Burnsville, MN). A stock solution of 10 mL of 109 CFU (Colony Forming Units)/mL in sterile 0.05% phosphate-buffered saline with Tween 20 (PBST) was diluted with a total of 49.970 L of deionized (DI) water for spray application. Two 10 mL vials of sterile PBST were used to rinse the stock solution tube and added to the sprayer for 50 L of total inoculum solution. The flow rate of the sprayer was set to 10 mL/s and checked prior to and following application using a 100 mL graduated cylinder and National Institute of Standards and Technology (NIST) stopwatch. The inoculum was sprayed evenly across the test area at approximately 10 mL/ft2 with each pass starting adjacent to the area that was visibly wetted from the previous pass. Reference material coupons (RMCs) were used to determine the initial surface inoculation levels. The RMCs were made of 316 super corrosion resistant stainless-steel rectangles with a #8 polished finish (Item # 9759K51, McMASTER-CARR, Robbinsville, NJ). They were autoclaved prior to use and measured 2.54 × 5.08 centimeters in area and were 0.76 mm thick. RMCs (36 total) were placed throughout the site prior to inoculation and collected into sterile 50 mL conical tubes the morning after inoculation.
Experimental design
Five different sampling methods were studied over the course of this study: sponge sticks, 37-mm vacuum filters, grab samples of grass, leaves, and soil, robotic floor cleaners, and wet vacuum samplers (Figure 1B). The study area was delineated into a grid consisting of 1.52 × 1.52 meter sampling cells. Each cell was assigned an alpha-numeric identifier and a sampling method using a random number generator (Excel, Microsoft Corporation) (Figure 1A). Background samples were collected prior to inoculation, then test samples were collected at post-inoculation time points of 1, 4, 37, 99, and 210 days (Figure 1C). The RMCs were collected on day one instead of time zero to permit the inoculated surfaces to dry prior to walking on them. No grid surface was sampled twice, as such evaluation of cross-contamination over time was not the focus of this study. The section of asphalt previously used for surface sealant tests unrelated to this research was avoided in the current study. Sterility check swabs and field blanks were also collected during each sampling campaign to ensure that cross contamination did not occur. The randomness of the locations over time attempted to ensure that samples were not biased by the slope of the parking lot or other environmental conditions. Because of space constraints at the site, the wet vacuum and robots were collected in duplicate and all other sample types were collected in triplicate (Table 1).
Table 1.
Sample summary
| Material (# of Samples) | ||
|---|---|---|
| Reference Material Coupons (RMCs) | 1 Day | Background (4)* |
| Asphalt (10) | ||
| Concrete (10) | ||
| Soil (4) | ||
| Leaves (4) | ||
| Grass (4) | ||
| Wet Vacuum, Robots | Background | Asphalt (2), Concrete (2), Field Blank (1) |
| 1 Day Sampling | Asphalt (2), Concrete (2), Field Blank (1) | |
| 4 Days Sampling | Asphalt (2), Concrete (2), Field Blank (1) | |
| 37 Days Sampling | Asphalt (2), Concrete (2), Field Blank (1) | |
| 99 Days Sampling | Asphalt (2), Concrete (2), Field Blank (1) | |
| 210 Days Sampling | Asphalt (2), Concrete (2), Field Blank (1) | |
| Sponge Stick | Background | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) |
| 1 Day Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 4 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 37 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 99 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 210 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 37-mm Vacuum | Background | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) |
| 1 Day Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 4 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 37 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 99 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| 210 Days Sampling | Stainless Steel (3), Asphalt (3), Concrete (3), Field Blank (1) | |
| Grab | Background | Grass (3), Leaves (3), Soil (3), Field Blank (1) |
| 1 Day Sampling | Grass (3), Leaves (3), Soil (3), Field Blank (1) | |
| 4 Days Sampling | Grass (3), Leaves (3), Soil (3), Field Blank (1) | |
| 37 Days Sampling | Grass (3), Leaves (3), Soil (3), Field Blank (1) | |
| 99 Days Sampling | Grass (3), Leaves (3), Soil (3), Field Blank (1) | |
| 210 Days Sampling | Grass (3), Leaves (3), Soil (3), Field Blank (0) |
The materials listed in the RMC row refer to the areas in which the RMCs were placed, and the materials that were sampled. All RMCs were stainless steel.
Sponge sticks
Asphalt, concrete, and stainless-steel coupons were sampled using pre-moistened 3M sponge stick wipes (Item #: SSL10NB, 3M, St. Paul, MN) in triplicate at three different locations for each time point of the study. Sponge stick samples were collected according to methods previously described (CDC 2012). In brief, clean nitrile gloves were used for each sample and a sterile 25.4 × 25.4 centimeters template was used to demarcate the sampling area. An ‘S’ stroke pattern was used in different directions for all sides of the sponge stick. The head of the sponge stick was then placed into a sterile specimen container, bagged, shipped overnight to a microbiology laboratory, stored at 4°C ± 2°C, and analyzed in a microbiological laboratory within 30 days by methods described previously and summarized below (Abdel-Hady, Calfee et al. 2019).
37-mm Vacuums
Asphalt and concrete were sampled using 37-mm vacuum cassettes loaded with 0.8 μm pore-size mixed cellulose ester filters (Item # 225-3-01, SKC Inc., Eighty Four, PA) according to procedures adapted from (Calfee, Rose et al. 2013, U.S. EPA 2017b). In brief, Tygon® tubing (Item # 225–1345, SKC Inc., Eighty Four, PA) was attached via adapters (Item # 225–132A, SKC Inc., Eighty Four, PA) to both ends of a filter cassette. The end used for sampling was cut at a 45⁰ angle to facilitate surface contact. The other end of the tubing was attached to a vacuum pump (Item # 100–3002, SKC Inc., Eighty Four, PA) and operated at 5–10 liters per minute. A 30.48 × 30.48 centimeters sterile template was used to vacuum a standardized sized surface area. Horizontal ‘S’-stroke movements were used for 5 minutes (or until the filter clogged). After sample collection the tubing was removed, and manufacturer-supplied plastic plugs were used to seal the cassette. The plugged cassette was placed into a sterile bag, shipped overnight to a microbiology laboratory, stored at 4°C ± 2°C and analyzed within 30 days by the methods described by (Calfee, Rose et al. 2013). At each time point of the study, the triplicate samples were collected in the same test cell as the triplicate sponge stick samples, each collected from a unique section of the cell.
Wet vacuums
Asphalt and concrete were sampled using Hover® Max Extract wet vacuum cleaners (Item # F7452900, The Hoover Company, North Canton, Ohio). New wet vacuums were used for each sample, so sterilization was not performed. However, sterility checks with swabs were still performed prior to use. The vacuums were filled with a 2 L solution of 0.05% Tween® 20 and operated in rinse and power scrub modes as detailed in (U.S. EPA 2018). The initial vacuum stroke was performed while the liquid dispensing trigger was in the “on” position. The initial stroke was followed by a vacuum-only stroke covering the same area. For each new pass of the vacuum, the vacuum position was shifted to cover 50% new area and 50% of the area just vacuumed. Vacuuming proceeded in this manner, one wet stroke followed by one dry stroke, until the entire 2.3 m2 cell area was covered. Immediately following completion of the wet vacuuming, a homogenized 1 L aliquot from the dirty water tank was transferred to a sterile bottle, shipped overnight to a microbiology laboratory, stored at 4 °C ± 2 °C, and analyzed within 30 days using methods described previously(U.S. EPA 2018).
Robotic floor vacuums
Asphalt and concrete were sampled using Neato Botvac D3 robotic floor cleaners (Item #: 945–0211, Neato Robotics, Newark, CA). Prior to use in tests, robot sterilization was performed using vaporized hydrogen peroxide with swab sterility checks according to procedures previously described in (Lee, Calfee et al. 2013). The Neato Botvac D3s were equipped with the device’s standard mapping and navigation technology that returns the robot to a starting position after covering the entire floor surface of an enclosed sampling area. This automatic setting was utilized in the designated 2.3 m2 sampling cell delineated by magnetic strips. After completion of sampling, the contents of the robot’s dirt collection bin and particle filter were bagged and shipped overnight to a microbiology laboratory, stored at 4°C ± 2°C, and analyzed within 30 days using methods described previously (Lee, Calfee et al. 2013).
Grab samples
The inoculated grab sampling areas were demarcated by a galvanized metal ring for each sample type. Over the course of the study samples were randomly collected from within these areas. Grass, leaves, and soil grab samples were each collected by samplers wearing a fresh pair of nitrile gloves and using a sterile 1 L HDPE bottle. Sterile scissors were also provided should the sampler choose to use them for cutting the grass above the roots. Most samplers collected samples by tearing off the vegetation with their gloved hands rather than using the scissors. The samples were collected up to the half-way point of the 1 L bottle, double bagged, shipped overnight to a microbiology laboratory, stored at 4°C ± 2°C, and analyzed within 30 to 95 days (soil).
Microbiological analysis
All samples were processed using the following general steps: extraction using agitation, heat treatment (as needed at 80 ⁰C for 30 minutes to reduce background non-target organisms), serial dilution, spread plating on Trypticase Soy Agar (TSA), incubation at 35 ± 2 ⁰C, and CFU enumeration. For samples with expected low count levels, filter plating with a 0.45 μm micro-funnel (Item # 4804, PALL, Port Washington, NY) was implemented. Agitation method varied by sample type. Sponge stick samples utilized a stomacher; grass, leaves, and robots an orbital shaker; soil samples a vortex and cell strainer; and wet vacuum samples were hand shaken. For the 37-mm vacuum samples, 10 mL of PBST was used to dampen the filter prior to opening the cassette so that dust was not aerosolized and for rinsing the inside walls of the cassette. The rinse eluent and filter were then placed in a sterile polypropylene jar and sonicated for three minutes. Level of detection calculations were determined by using a value of 1 CFU divided by the largest volume analyzed and multiplied by the total sample volume. This formula is displayed in Equation 1. The largest volumes analyzed were dependent on the amount of debris and heat-resistant background contamination present in each individual sample. The total sample volume analyzed for wet vacuum corresponded to the volume that was returned from the field (180–1,008 mL). For the other samples, the total sample volume analyzed corresponded to the extraction volume, which was 11 mL for 37-mm vacuums, 100 mL for sponge sticks, 180 mL for robots, 400 mL for grass and leaves, and 5 g in 20 mL for soil samples.
| (1) |
Percent recovery was calculated as mean recovery for replicates from a specific day-material-sampler combination divided by the mean RMC recovery normalized to the equivalent surface area of the sampling method multiplied by 100.
Statistical Analysis
For sampling methods where duplicate data were collected at each time point (wet vacuums and robots), analysis was limited to simple descriptive statistics. Where data were collected in triplicate for each time point, multilevel modeling was used for significance testing. Multilevel modeling was employed due to the repeated measures and nested nature of the dataset. Computations were performed using the lme4 (Bates, Maechler et al. 2019) and lmerTest (Kuznetsova, Brockhoff et al. 2017) packages in the open source software R version 3.6.1. Analysis of the sponge stick data used the formula displayed in Equation 2 and comparison of the sponge sticks to 37-mm vacuums for concrete and asphalt surfaces used the formula displayed Equation 3.
| (2) |
| (3) |
LogCFU refers to the logarithm of the spores recovered, Day to the sampling time point, Sample_ID to the unique data point collected, Material to the surface (stainless steel, asphalt, or concrete), and sampling method to the sponge stick or 37-mm vacuum methods. The small size of the data set does not allow for incorporation of interactions between sampling methods and materials in the statistical analysis. A p-value less than 0.05 was considered significant for this study.
Results
Inoculation and sterility
The RMC extraction results indicated that the study area was inoculated with an average of 2.2 × 107 CFU/m2 (standard deviation of 9.5 × 106 CFU/m2 and relative standard deviation of 44%, n = 36). The inoculation of B. atrophaeus spores was spatially consistent across the different surfaces (Figure 2). No spores were detected on the RMC control coupons that were placed in the un-inoculated concrete area. Additionally, no growth of B. atrophaeus spores was detected on any sterility swab samples collected throughout the study, and only one field blank detected spores: the day 99 wet vacuum sample, which had 25 CFU. Since the wet vacuum results from this day were over two orders of magnitude above this value, the field blanks were kept in the final dataset.
Figure 2.
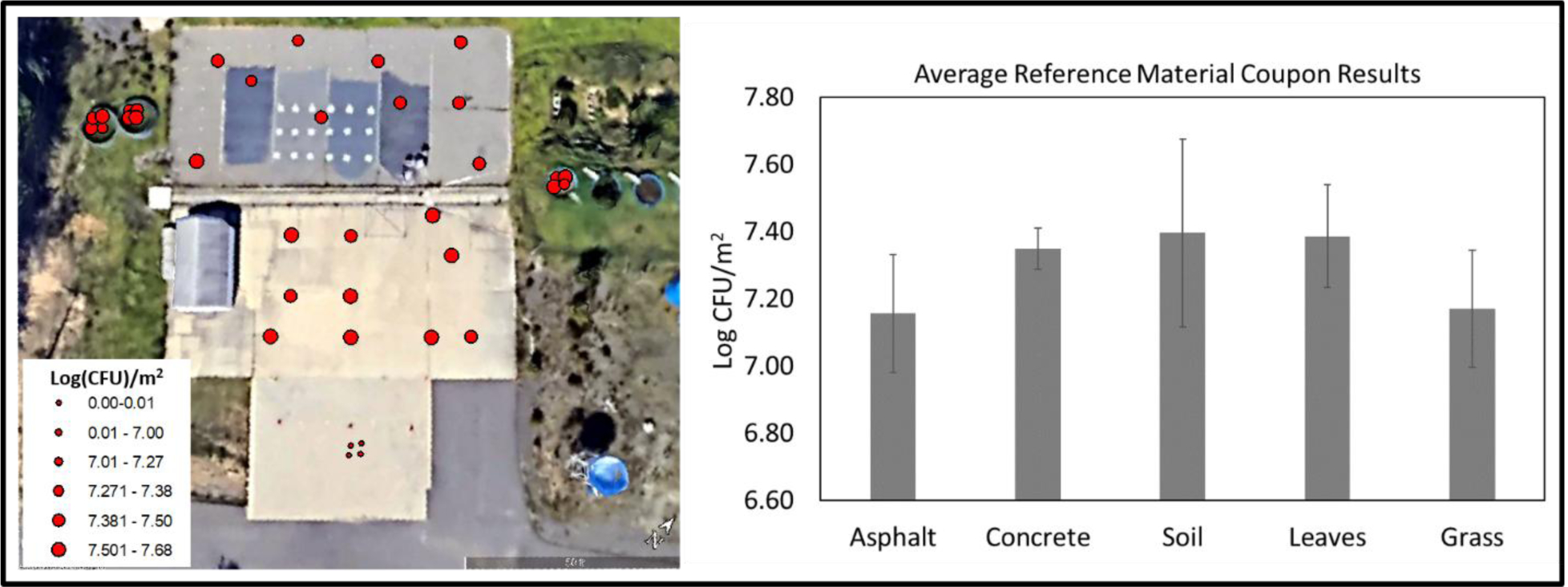
Spatial distribution of B. atrophaeus on RMCs at study site. The error bars represent standard deviation of the replicates.
Sampling
Limits of detection were calculated for each sample individually and summarized according to the maximum, minimum, and average for each method and material type (Table 2). With higher averages, the results demonstrate that some samples contained more debris and heat-resistant bacteria (grab samples and wet vacuums). They also demonstrate only slight differences in processing volumes from concrete vs. asphalt samples. Spore recovery was highest for all methods during the day 1 sampling event and was reduced for samples collected on later dates. In addition, average recovery was slightly higher from concrete than asphalt for all sampling methods, except on day 37 sampling with robots (Figure 3). Initially, sponge sticks recovered the most spores from the stainless-steel coupons and the fewest spores from the asphalt surfaces, but by the second sampling point (day four), there was no consistent surface with higher recovery (Figure 3A). By day 99, spores were detected on concrete surfaces with sponge sticks, but not on asphalt or stainless-steel surfaces. Overall, there was not a statistically significant difference for sampling the different material surfaces with sponge sticks (concrete vs. asphalt p-value = 0.098, stainless steel vs. asphalt p-value = 0.091, and stainless steel vs. concrete p-value = 0.884). There was, however, a statistical difference for 37-mm vacuum sample recoveries on concrete vs. asphalt (p-value = 0.013). This result is consistent with the observation that spores were detected on concrete until day 210, but detected on asphalt only until day 37 (Figure 3B). Further, the 37-mm vacuum samples were not statistically different from the sponge-stick samples in detecting spores (grouped asphalt and concrete p-value = 0.225). For concrete, wet vacuums collected more spores per sample across all time points than the other sampling methods, but they also sampled a larger area than the 37-mm vacuums and sponge stick methods (Figure 3C). For asphalt, wet vacuums collected samples with detectable spores intermittently throughout the duration of the sampling study and at a level that was not appreciably higher than other methods for the same time points. Similar to the wet vacuums, the robots collected samples with detectable spores on asphalt and concrete throughout the duration of the study, albeit at lower levels over time than the wet vacuums (Figure 3D). The robots were the only sampling method that demonstrated a slight increase in recovery at later time points. Recovery from grass, leaves, and soil remained similar and appreciable through the third time point (37 days), but by day 99, only the soil samples had recoverable spores, and by day 210, none of the grab-type samples contained detectable spores (Figure 3E). Since the methods sampled different sized areas, all results were normalized to a standard 2.3 m2 (25 ft2) for comparison (Figure 4). When compared in this way, for most of the time points, the robots had the lowest normalized recovery for both asphalt (Figure 4A) and concrete (Figure 4B) of all sampling methods. Sponge sticks, 37-mm vacuums, and wet vacuums had similar recovery efficiencies. Although all surface sampling methods collected detectable spores, only a small percentage of the inoculated spores were recovered in any one sample (Table 3). Note, since grab samples had an unknown surface area inoculation per sample collected, the percent recovery was not estimated for this method. The sample size for each calculation in Table 3 corresponds to the number of samples outlined in Table 1, with one exception as noted in the Table 3 (day 4, robot, asphalt).
Table 2.
Limit of detection (CFU/sample)
| Method | Material | Min | Average | Max |
|---|---|---|---|---|
| 37-mm Vacuum | Asphalt | 1.5 | 6.4 | 11 |
| Concrete | 1.6 | 11 | ||
| Sponge Sticks | Asphalt | 5.0 | 7.4 | 20 |
| Concrete | 5.0 | 10 | ||
| Stainless Steel | 1.1 | 20 | ||
| Robots | Asphalt | 4.0 | 6.3 | 6.0 |
| Concrete | 9.0 | 9.0 | ||
| Wet Vacuums | Asphalt | 19 | 28 | 32 |
| Concrete | 34 | 35 | ||
| Grab Samples | Grass | 20 | 319 | 267 |
| Leaves | 20 | 533 | ||
| Soil | 208 | 604 |
Figure 3.
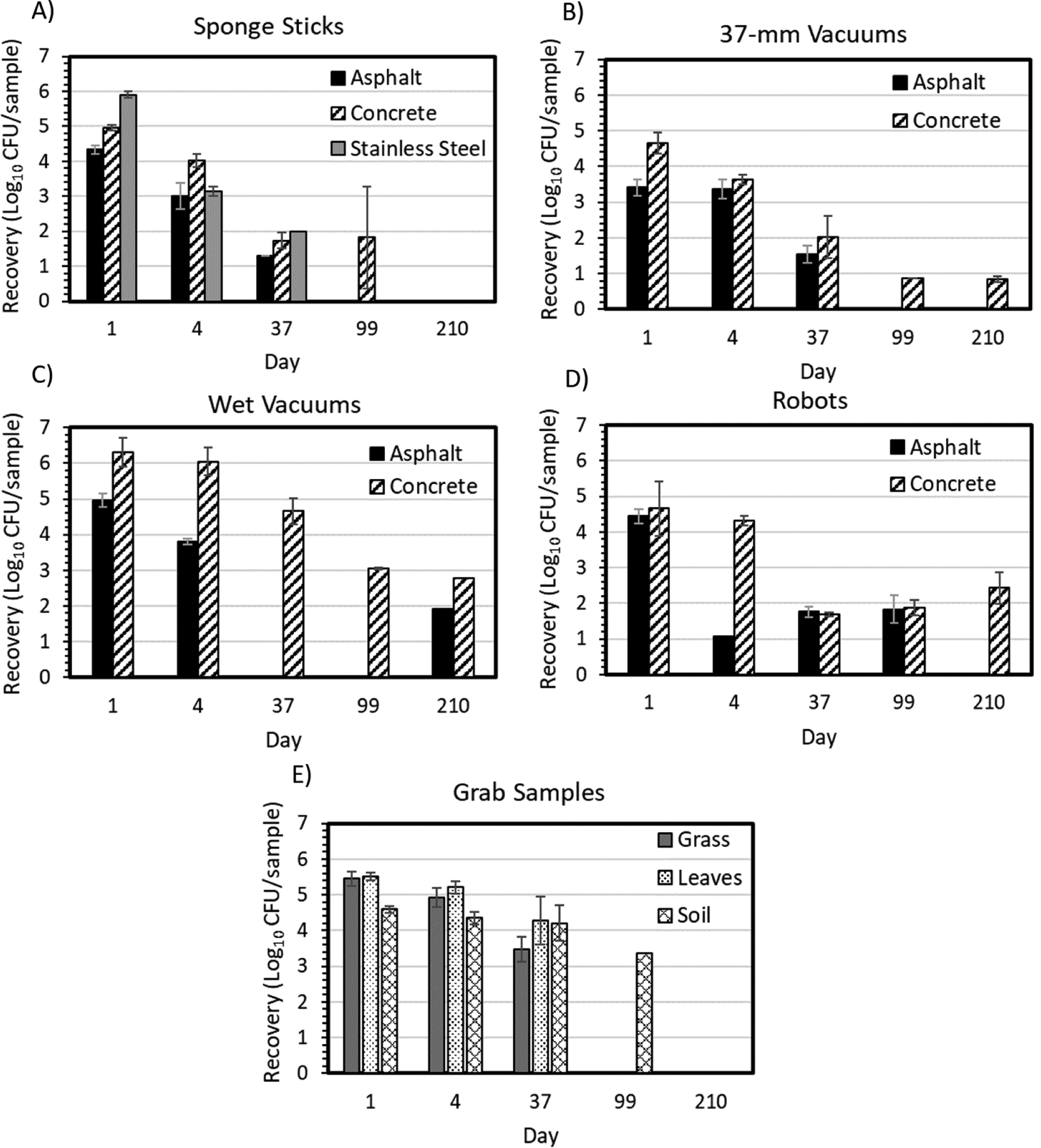
Recovery of B. atrophaeus spores over time from different surfaces by A) sponge sticks, B) 37- mm vacuums, C) wet vacuums, D) robots, and E) grab samples. The error bars represent standard deviation of replicates
Figure 4.
Theoretical number of B. atrophaeus spores recovered over time by different sampling methods from A) asphalt and B) concrete when normalized to a 2.3 m2 (25 ft2) collection area. The error bars represent standard deviation of replicates.
Table 3.
Estimated average percent recovery* by sampling method over the 210 day study.
| Material | 37-mm Vacuums | Robots | Sponge Sticks | Wet Vacuums |
|---|---|---|---|---|
| Day 1 | ||||
| Asphalt | 0.11% | 0.054% | 1.7% | 0.20% |
| Concrete | 2.0% | 0.15% | 6.7% | 4.2% |
| Stainless Steel | - | - | 61% | - |
| Day 4 | ||||
| Asphalt | 0.11% | only one data point | 0.094% | 0.013% |
| Concrete | 0.18% | 0.032% | 0.83% | 2.8% |
| Stainless Steel | - | - | 0.11% | - |
| Day 37 | ||||
| Asphalt | 0.0020% | 0.0% | 0.0010% | 0.000% |
| Concrete | 0.0070% | 0.0% | 0.0040% | 0.11% |
| Stainless Steel | - | - | 0.0020% | - |
| Day 99 | ||||
| Asphalt | 0.0% | 0.0% | 0.0% | 0.0% |
| Concrete | 0.0% | 0.0% | 0.074% | 0.0030% |
| Stainless Steel | - | - | 0.0% | - |
| Day 210 | ||||
| Asphalt | 0.0% | 0.0% | 0.0% | 0.0% |
| Concrete | 0.0% | 0.0010% | 0.0% | 0.0010% |
| Stainless Steel | - | - | 0.0% | - |
Percent recovery calculated as mean Recovery at Dayx / mean RMC Recovery normalized to equivalent surface area of sampling method × 100
Discussion
Outdoor environments are dynamic systems that experience various weather conditions. During this study, a snowstorm hit the area two days after inoculation, blanketing the site in approximately 13 centimeters of snow, which had melted by the day 4 sampling time point. Although the percentage recovery difference is a drastic decrease between day 1 and 4, the log recovery trends do not indicate a decline between day 1 and day 4 samples that is larger than between the day 4 and day 37 samples (Figure 3). It is possible that the evaporative melting of the snow left spores to puddle on the surface of the parking lot and did not cause significant removal of spores, but this remains an unknown variable in the study. Additionally, a large rain event occurred prior to the day 99 sampling event (Figure 5), which may have contributed to some sampling methods no longer detecting spores due to removal by washoff or spores being driven deeper into the soil or pavement and no longer accessible to the sampling devices. Further, inoculum concentration has an impact recovery efficiency. Sampling methods see lower recovery efficiencies at lower inoculum concentration. As such, the estimated percent recovery values in Table 3 must be interpreted cautiously, because they were calculated using the initial inoculation values, not what was on the surface after weathering or at the time of sample collection. Currently, sophisticated fate and transport models have not been developed to provide estimates of these values.
Figure 5.
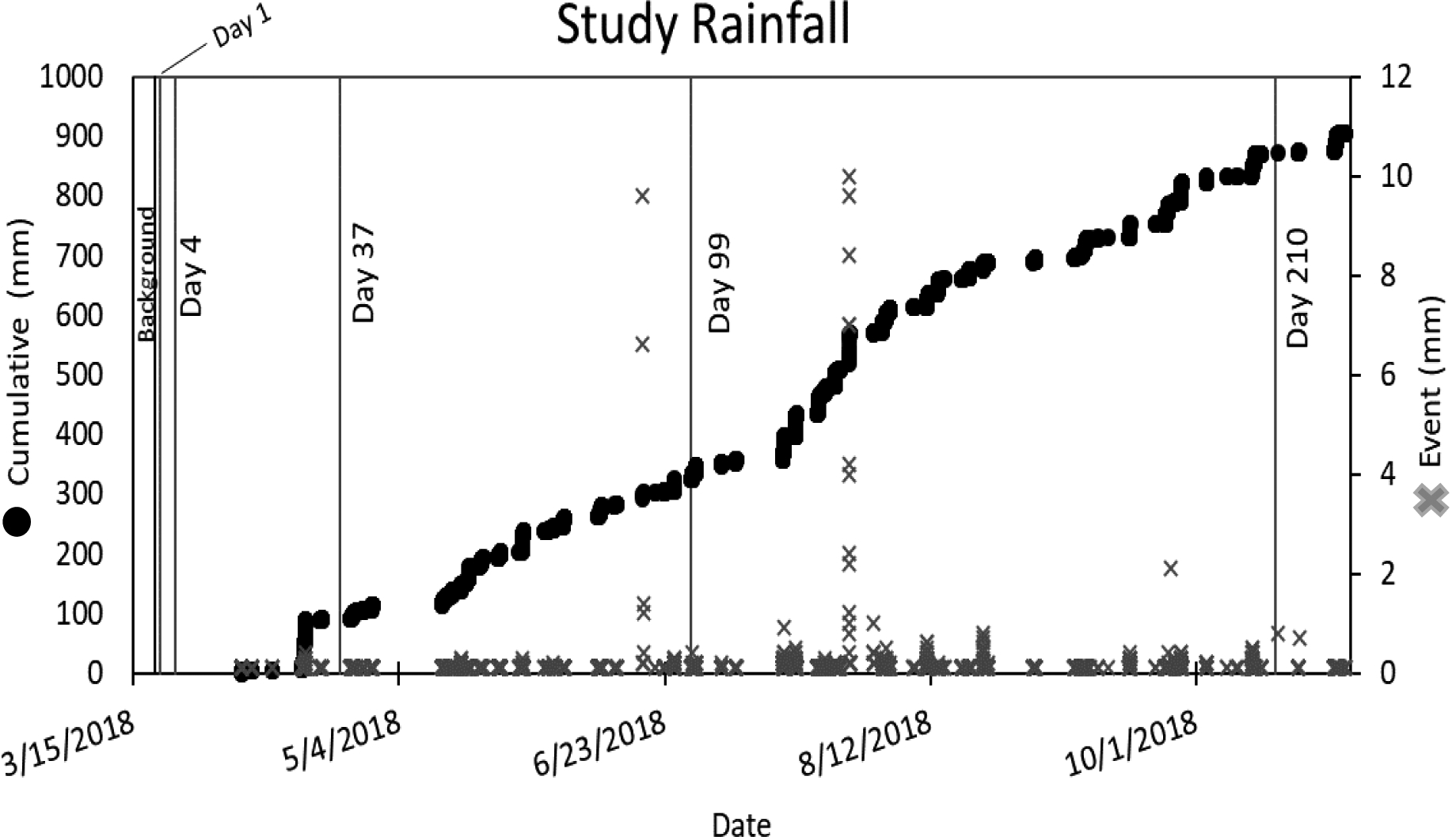
Rainfall measurements from onsite gages
The inoculation procedure used in this study was simple to execute and resulted in high surface loading values comparable to the Amerithrax postal equipment, which was contaminated up to 8 × 106 CFU/100 cm2 (Edmonds, Collett et al. 2009). While indoor sampling studies have reported 30% sponge stick recovery from stainless-steel coupons, 49% recovery for 37-mm vacuums from concrete, 1–10% robot recovery from carpet, and 49% wet vacuum recovery from concrete, aside from stainless steel on day 1, the outdoor study demonstrated markedly lower recovery percentages (Rose, Hodges et al. 2011, Calfee, Rose et al. 2013, Lee, Calfee et al. 2014, U.S. EPA 2018). The highest percent recoveries in this outdoor study were obtained using a sponge stick (1.7% for asphalt, 6.7% for concrete, and 61% for stainless steel) on the day after inoculation. Later sampling time points recovered just a fraction of the initially inoculated spores, either because they had already been removed due to weathering (including transport or inactivation due to UV, temperature, and relative humidity) or because the collection efficiencies of the sampling methods were too low. Despite this unknown, the sampling devices continued to collect detectable spores over the course of the entire study (collectively) and at different levels depending on sampling device.
Not all methods require the same amount of time and strain on the sampler. Sponge stick sampling and 37-mm vacuums require the sampler to squat low to the ground (for the 37-mm vacuums, up to five minutes per sample), which can be physically strenuous, especially when wearing personal protective equipment. Both methods also cover a small area relative to the robots/wet vacuums. During the study, one robot malfunctioned resulting in loss of data and several others had to be restarted to ensure the entire area was covered. At this stage, they are not fully autonomous sampling devices. Further, their automated collection routine does not have a standardized collection time across runs of the robot. Further, the flowrate of the 37-mm vacuum pumps was reduced on several occasions due to clogging. The rest of the equipment was observed to be robust despite sampling outdoors.
This study did not use Polymerase Chain Reaction (PCR) to confirm negative culture results. Future studies may consider using this complementary technique to address the question of whether spores were present, but nonculturable versus not present in the sample due to environmental factors. However, not unlike culture-based methods, variability and detection limits are often high for PCR-based detection of spores in environmental samples. Enrichment of samples prior to quantitative PCR (qPCR) can increase sensitivity of the assay (Gulledge, Luna et al. 2010), however, precise quantitation of spore recovery (with or without enrichment) is not possible with current methods. Rapid Viability Polymerase Chain Reaction (RV-PCR) has demonstrated high sensitivity and specificity in challenging sample matrices, and could be used in future studies to potentially increase sensitivity of detection (Calfee, Shah et al. 2019). RV-PCR is a qualitative detection assay that relies on culture-based enrichment to allow spores to germinate and multiply prior to DNA extraction.
Several additional important observations were made in relation to the grab sampling portion of this work. The first observation is that a large number of naturally occuring background microorganisms contributed to significant interference during processing and analysis, resulting in high detection limit values for these sample types. Heat treatment was not entirely effective for removal of non-target organisms, so washing, then dilution was used to aid manual CFU enumeration. The pigmented phenotype of B. atrophaeus also helped distinguish it from colonies of background organisms. There is a great need to improve analytical processing of these types of samples to study the fate and transport of spores in soil, leaves, and grass. Secondly, to estimate recovery percentages in surface grab samples, it is recommended that a template restricting a surface area be used in future studies. This study recorded the volume/weight of the sample, but that could have been collected from different areas of inoculated surface depending on the sampler, and therefore a starting concentration could not be estimated. Both the robot and the wet vacuum methods have advantages over the more traditional wipe and vacuum methods: a larger area covered (which seems to increase their detection limits) and reduced ergonomic stress on the sampler. For those reasons there may be situations where using the robot or wet vac would improve response outcomes.
Conclusions
Liquid inoculation of spores using a sprayer produced spatially consistent target surface loading and was logistically easy to implement. Where consistent with study objectives (e.g., may not be preferred for studying re-aerosolization of spores), this method is recommended for use in outdoor field work.
Sponge sticks and 37-mm vacuums had similar recoveries over time for sampling spores on both concrete and asphalt. Even though 37-mm vacuums are the recommended method for indoor porous surfaces, they did not perform significantly better than sponge sticks outdoors on concrete and asphalt. Considering these results, it is reasonable to consider the sampling ergonomics and sample collection time to guide sampling method selection on these outdoor surfaces until a larger data set is collected.
All sampling methods recovered more spores from concrete than from asphalt throughout the duration of the study (except for day 37 using robots). Further research is needed to explain if this is a material surface with sampler interaction or if it is a result of fate and more rapid transport of spores off the asphalt.
Growth of background non-target microorganisms made grab samples challenging to analyze and resulted in high detection limits. Future work may want to include the use of RV-PCR/qPCR methods to improve analysis of samples with high background. Also, a standardized surface area template is recommended for use in future studies so that the amount of spores recovered per area sampled can be estimated and compared with other sampling methods.
The composite sampling methods, which sample a larger area as specified in their standard operating procedures, were the only sampling methods that collected spores on asphalt after three months, suggesting that in outdoor environments where the fate and transport is unknown, larger standard sampling areas are recommended to maximize detection.
Future research is needed to expand the dataset for more robust statistical analysis and to better understand the fate and transport mechanisms concurrently impacting outdoor studies.
Acknowledgments
Michael Borst and Ariamalar Selvakumar of the EPA Office of Research and Development in Edison, New Jersey, are thanked for hosting us at the Urban Watershed Facility. Alexander Korf of Jacobs Technology Inc. is acknowledged for development of the quality assurance project plan and for the project management of the inoculation and first week of sampling. Lee Brush, Chris Fuller, Stella McDonald, Brian Sechrest, Jason Colon, and Timothy McArthur of Jacobs Technology Inc. are acknowledged for their execution of sample collection over the course of the study. Kathleen May of Jacobs Technology is acknowledged for assistance in biological enumeration. Work by Jacobs Technology Inc. was performed under EPA contract number EP-C-15-008. John Lapinski and Pasquale Pozzolano of PARS Environmental are also acknowledged for their assistance with site specific equipment and utilities. Their work was performed under EPA contract number EP-C-17-009.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: This manuscript was subject to administrative review but does not necessarily reflect the view of the U.S. Environmental Protection Agency. No official endorsement should be inferred, as the EPA does not endorse the purchase or sale of any commercial products or services.
References
- Abdel-Hady A, Calfee MW, Aslett D, Lee SD, Wyrzykowska-Ceradini B, Delafield FR, May K and Touati A (2019). “Alternative fast analysis method for cellulose sponge surface sampling wipes with low concentrations of Bacillus Spores.” Journal of microbiological methods 156: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen R and Singmann H (2019). lme4: linear mixed-effects models using “Eigen” and S4. 2017. [Google Scholar]
- Brown GS, Betty RG, Brockmann JE, Lucero DA, Souza CA, Walsh KS, Boucher RM, Tezak M, Wilson MC and Rudolph T (2007). “Evaluation of a Wipe Surface Sample Method for Collection of Bacillus Spores from Nonporous Surfaces.” Appl. Environ. Microbiol 73(3): 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee MW, Rose LJ, Morse S, Mattorano D, Clayton M, Touati A, Griffin-Gatchalian N, Slone C and McSweeney N (2013). “Comparative evaluation of vacuum-based surface sampling methods for collection of Bacillus spores.” Journal of microbiological methods 95(3): 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee MW, Shah S, Mickelsen R, Cruz F, Karim K, Ackelsberg J, Gemelli M and Hofacre K (2019). Evaluation of Analytical Methods for the Detection of Bacillus anthracis Spores: Compatibility with Real-World Samples Collected from Outdoor and Subway Surfaces EPA/600/R-19/083. Washington D.C., U.S: Environmental Protection Agency. [Google Scholar]
- CDC. (2012, April 26, 2012). “Emergency Response Resources.” Surface sampling procedures for Bacillus anthracis spores from smooth, non-porous surfaces Retrieved 1/6/2020, 2020, from http://www.cdc.gov/niosh/topics/emres/surface-sampling-bacillus-anthracis.html. [Google Scholar]
- Edmonds JM, Collett PJ, Valdes ER, Skowronski EW, Pellar GJ and Emanuel PA (2009). “Surface Sampling of Spores in Dry-Deposition Aerosols.” Appl. Environ. Microbiol 75(1): 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel P, Roos JW and Niyogi K (2008). Sampling for Biological Agents in the Environment. Washington, D.C., ASM Press. [Google Scholar]
- Franco C and Bouri N (2010). “Environmental Decontamination Following a Large-Scale Bioterrorism Attack: Federal Progress and Remaining Gaps.” Biosecurity and Bioterrorism: Biodefense Strategy, Practice, And Science 8(2): 107–117. [DOI] [PubMed] [Google Scholar]
- Gallardo VJ, Morris BJ and Rhodes ER (2019). “The use of hollow fiber dialysis filters operated in axial flow mode for recovery of microorganisms in large volume water samples with high loadings of particulate matter.” Journal of microbiological methods 160: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge J, Luna V, Luna A, Zartman R and Cannons A (2010). “Detection of low numbers of Bacillus anthracis spores in three soils using five commercial DNA extraction methods with and without an enrichment step.” Journal of applied microbiology 109(5): 1509–1520. [DOI] [PubMed] [Google Scholar]
- Hodges LR, Rose LJ, O’Connell H and Arduino MJ (2010). “National validation study of a swab protocol for the recovery of Bacillus anthracis spores from surfaces.” J Microbiol Methods 81(2): 141–146. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB and Christensen RHB (2017). “lmerTest package: tests in linear mixed effects models.” Journal of Statistical Software 82(13). [Google Scholar]
- Lee SD, Calfee MW, Mickelsen L, Clayton M and Touati A (2014). “Scenario‐Based Evaluation of Commercially Available Cleaning Robots for Collection of Bacillus Spores from Environmental Surfaces.” Remediation Journal 24(2): 123–133. [Google Scholar]
- Lee SD, Calfee MW, Mickelsen L, Wolfe S, Griffin J, Clayton M, Griffin-Gatchalian N and Touati A (2013). “Evaluation of Surface Sampling for Bacillus Spores Using Commercially Available Cleaning Robots.” Environ Sci Technol 47(6): 2595–2601. [DOI] [PubMed] [Google Scholar]
- Leitenberg M, Zilinskas RA and Kuhn JH (2012). The Soviet biological weapons program: a history, Harvard University Press. [Google Scholar]
- Piepel GF, Amidan BG and Hu R (2012). “Laboratory studies on surface sampling of Bacillus anthracis contamination: summary, gaps and recommendations.” Journal of Applied Microbiology 113(6): 1287–1304. [DOI] [PubMed] [Google Scholar]
- Rastogi VK and Wallace L (2020). Environmental sampling and bio-decontamination—Recent progress, challenges, and future direction Handbook on Biological Warfare Preparedness, Elsevier: 195–208. [Google Scholar]
- Rose LJ, Hodges L, O’Connell H and Noble-Wang J (2011). “National validation study of a cellulose sponge wipe-processing method for use after sampling Bacillus anthracis spores from surfaces.” Appl. Environ. Microbiol 77(23): 8355–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshale EH, Painter J, Burr GA, Mead P, Wright SV, Cseh LF, Zabrocki R, Collins R, Kelley KA, Hadler JL, Swerdlow DL and m. o. t. C. A. R. Team (2002). “Environmental Sampling for Spores of Bacillus anthracis.” Emerging Infectious Diseases 8(10): 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (2014). USEPA/USGS Sample Collection Protocol for Bacterial Pathogens in Surface Soil EPA/600/R-14/027. Washington DC, United States Environmental Protection Agency. [Google Scholar]
- U.S. EPA (2017a). A Review of Biological Agent Sampling Methods and Application to a Wide-Area Incident Scenario to Characterize Time and Resource Demands EPA/600/R-17/176, United States Environmental Protection Agency. [Google Scholar]
- U.S. EPA (2017b). Underground Transport Restoration (UTR) Operational technology Demonstartion (OTD) EPA/600/R-17/272. Washington, D.C., United States Environmental Protection Agency. [Google Scholar]
- U.S. EPA (2018). Evaluation of Commercial Wet Vacuums for Bacillus Spore Sampling on Surfaces EPA/600/R-18/158, United States Environmental Protection Agency. [Google Scholar]
- U.S. Government Accountability Office (2005). Anthrax Detection: Agencies Need to Validate Sampling Activities in Order to Increase Confidence in Negative Results GAO-05–251. Washington, DC. [Google Scholar]


