Abstract
Background:
HIV infection is now largely a chronic condition as a result of the success of antiretroviral therapy. However, several comorbidities have emerged in people living with HIV (PLWH), including alcohol use disorders (AUD) and musculoskeletal disorders. Alcohol use has been associated with lower bone mineral density, alterations to circulating bone turnover markers and hypocalcemia. The pathophysiological basis of bone loss in the PLWH population is unclear but has been suggested to be linked to oxidative stress and inflammation. To test the hypothesis that PLWH consuming excessive alcohol have altered markers of bone turnover and/or calcium homeostasis in association with oxidative stress, we correlated measurements of alcohol consumption with markers of oxidative stress and inflammation, serum calcium concentrations and measurements of bone turnover, including c-terminal telopeptide crosslinks (CTX-1) and osteocalcin.
Methods:
Data were drawn from cross-sectional baseline data from the ongoing New Orleans Alcohol Use in HIV (NOAH) study, comprised of 365 in care PLWH. Alcohol consumption measures (Alcohol Use Disorders Test (AUDIT), 30-day timeline followback (TLFB) calendar and phosphatidylethanol (PEth)) were measured in a sub-cohort of 40 subjects selected based on highest and lowest PEth measurements. Multivariate linear regression was performed to test the relationships between alcohol consumption and systemic oxidative stress (4-hydroxynonenal; 4-HNE) and inflammation (c-reactive protein; CRP).
Results:
Serum calcium and CTX-1 did not differ significantly between the high and low PEth groups. Individuals in the high PEth group had significantly lower serum osteocalcin (median low PEth group: 13.42 ng/mL, IQR 9.26–14.99 ng/mL; median high PEth group 7.39 ng/mL, IQR 5.02–11.25 ng/mL; p = 0.0005, Wilcoxon rank-sum test). Osteocalcin negatively correlated with PEth (Spearman r = −0.45, p = 0.05) and self-reported measures after adjusting for covariates. Alcohol consumption showed mild, but significant, positive associations with serum 4-HNE, but not with CRP. Osteocalcin did not correlate with either 4-HNE or CRP.
Conclusions:
In this sub-cohort of PLWH, we detected significant associations between at-risk alcohol use and osteocalcin, and at-risk alcohol use and serum 4-HNE, suggesting suppression of bone formation independent of increased systemic oxidative stress with increasing alcohol consumption.
Keywords: HIV, Alcohol, Bone, Oxidative Stress
Introduction
The use of anti-retroviral therapy (ART) among people living with HIV/AIDS (PLWH) has effectively reduced mortality from the disease and extended lifespans, but as a result, the incidence rates of chronic and age-related diseases have increased among this population. Osteoporosis and osteopenia (low bone density) rates are higher among PLWH compared to the general population (Paton et al., 1997; Bruera et al., 2003; Dolan et al., 2006). These conditions heavily contribute to the higher rates of bone fracture observed in PLWH (Triant et al., 2008, Shiau et al., 2013, Sharma et al., 2015).
Bone health is maintained via a balance of bone formation by osteoblasts and bone resorption by osteoclasts (Henriksen et al., 2009). The dominant mechanism by which HIV directly affects bone tissue to disrupt this homeostasis and compromise bone health is not known. It has been hypothesized that HIV-associated chronic inflammation contributes by indirectly stimulating osteoclast differentiation and resorptive activity (Fakruddin and Laurence 2003, Gazzola et al., 2013; Hileman et al., 2014). Although HIV infection independently is associated with reduced bone mass (Bruera et al., 2003), ART has been found to accelerate bone loss (Tebas et al., 2000), potentially by exacerbating chronic inflammation coincident with immune cell restoration and promoting bone osteoclastogenesis (Pan et al., 2004; Ofotokun et al., 2015). In addition, in vitro studies have shown HIV to directly impair osteoblast differentiation and function (Cotter et al., 2007). Together or independently, these mechanisms could act to shift the balance of bone turnover to favor resorption and a net loss of bone.
Alcohol use disorders (AUDs) are prevalent among a rising incidence of chronic conditions affecting PLWH (Galvan et al., 2002; Conigliaro et al., 2003; Williams et al., 2016). Alcohol overuse is also a known risk factor for low bone mass and fracture in healthy populations (Berg et al., 2008; Santori et al., 2008; Gonzales-Reimers et al., 2011). Disruptions to calcium homeostasis have also been reported among people consuming hazardous levels of alcohol (Laitinen et al., 1992; Miguez et al., 2012). Mechanistically, animal models have demonstrated that alcohol-induced oxidative stress in osteoblasts suppresses pro-osteoblastic signaling while increasing osteoclast differentiation to promote resorption (Chen et al., 2010; Mercer et al., 2014), and dietary antioxidants protect against ethanol-induced bone loss (Chen et al., 2011; Alund et al., 2017).
Osteocalcin and c-terminal telopeptide crosslinks (CTX-1) are soluble biomarkers of bone formation and bone resorption, respectively, commonly used to assess bone homeostasis in human serum and in animal models of bone pathology (Szulc et al., 2017; Greenblatt et al., 2017). Circulating levels of CTX-1 have been found to be unaltered or decreased in the case of mild alcohol consumption (Sripanyakorn et al., 2009; Marrone et al., 2012) but increased in cases of high consumption (or diagnosis of alcoholism) (Nyquist et al., 1996; Alvisa-Negrin et al., 2009). Animal models of alcohol exposure have linked decreases in circulating osteocalcin to changes in bone structure (e.g. cortical thinning and loss of trabecular bone) (Mercer et al., 2014; Shankar et al., 2008), and heavy alcohol use in humans is associated with decreases in serum osteocalcin (Laitinen et al., 1991b; Nyquist et al., 1996; Santori et al., 2008), implying a suppressive effect of alcohol on bone formation. Serum osteocalcin has also been reported to be decreased in PLWH, negatively correlating to disease severity (Serrano et al., 1995; Teichmann et al., 2000).
Although both HIV and AUD increase the incidence of adverse skeletal outcomes, it is not known mechanistically how these two conditions interact to exacerbate risk. The objective of this cross-sectional study was to determine whether PLWH consuming excessive alcohol (representing AUD) had altered calcium and/or altered levels of bone turnover markers, and whether these were associated with changes in systemic oxidative stress and markers of inflammation. Data were collected from baseline participant visits in the New Orleans Alcohol Use in HIV (NOAH) study, a prospective cohort study of 365 predominately African-American, low-income in care PLWH in the New Orleans, Louisiana area (Welsh et al., 2019). A sub-cohort of 40 PLWH with lowest and highest levels of alcohol consumption as measured by phosphatidylethanol (PEth), a plasma marker of recent ethanol consumption (Isaksson et al., 2011) was selected for analysis. Alcohol consumption was also estimated by self-report (AUDIT, time-linked follow back) (Saunders et al., 1993, Sobell and Sobell, 1992).
Methods
Study Population
The New Orleans Alcohol Use in HIV study (NOAH) is a bi-directional investigation of alcohol use disorder, HIV/AIDS, and ART in aging and exacerbated comorbid conditions in an underserved cohort of PLWH. A total of 365 HIV-infected individuals, under care at a HIV specialty clinic, were enrolled in NOAH. The study population was oversampled towards individuals with Alcohol Use Disorders Identification Test (AUDIT) scores ≥ 8, suggestive of potentially hazardous drinking habits. Exclusion criteria included acute illness within the preceding 6 weeks (defined by unscheduled healthcare utilization for a new or exacerbated illness), nonprophylaxis administration of antibiotics, pregnancy, or acute intoxication. Louisiana State University Health Sciences Center, New Orleans Human Research Protection Program and Institutional Review Board approved and oversee the study. Further study details are described in Welsh et al. (2019).
As a pilot analysis to detect effects at the extreme ends of the alcohol consumption spectrum, a subset of the study population based on recent alcohol consumption was selected for analyses of bone turnover markers. This consisted of the 20 participants with the highest phosphatidylethanol (PEth) values and a random sample of 20 participants from 128 NOAH study subjects with PEth values < 8 ng/mL, equivalent to a negative PEth result.
Data Collection
Enrolled participants completed a baseline visit where data were collected via survey questionnaires, physical exam, function performance tests, and specimen and blood collection. Interviewer-administered surveys were collected through LSUHSC-NO’s online REDCap (Research Electronic Data Capture) System by trained research staff. Fasting blood samples were collected as part of a baseline examination for clinical laboratory tests (including total calcium (as part of a comprehensive metabolic panel), 4-hydroxynonenal (4-HNE, via ELISA, Cell BioLabs, Inc, San Diego, CA, cat# STA-838), c-reactive protein (CRP, via clinical lab panel), and PEth (measured by USDTL, Inc., Des Plaines, IL) for recent alcohol use). A complete list of sample collection and analysis protocols is provided in Welsh et al. (2019) (supporting information).
Multidimensional characterization of alcohol exposure and psychosocial stressors was carried out by interviewer-administered questionnaire, which included the Alcohol Use Disorder Identification Test (AUDIT) (WHO, 2001) and a 30-day timeline followback (TLFB) calendar (Sobell and Sobell, 1992). The AUDIT questionnaire is a 10-item tool and responses are summed for an overall score ranging from a possible 0‒40 (Saunders et al., 1993). The first three questions’ scores were summed for an AUDIT-C score ranging from 0‒12.
Serum analysis
Serum was separated by centrifugation and stored at −80 until analysis. Samples were assayed for total osteocalcin (Invitrogen, Waltham, MA cat# KAQ1381), and c-terminal telopeptide crosslinks (CTX-1) (Immunodiagnostic Systems, Tyne & Wear, UK, cat# AC-02F1) by enzyme-linked immunosorbent assay (ELISA). Serum biomarkers levels were interpolated from curves generated by manufacturer-provided osteocalcin or CTX-1 standards, respectively, included in the assay.
Data analysis
Data were analyzed using Microsoft Excel 2016, Prism v.8.0 (GraphPad, Inc., San Diego, CA) and SAS v.9.4 (Statistical Analysis Systems, Cary, NC). Pairwise comparisons of circulating calcium, c-terminal telopeptide crosslinks (CTX-1), and osteocalcin were performed by PEth category. Wilcoxon ranked sum tests were used due to the lack of normality to compare the means of the continuous alcohol measures by PEth category. Additional covariates (age, gender, education, race, smoking status, viral load and CD4 count) were adjusted for in multivariate linear regression analyses based on known association to alcohol and oxidative stress and/or bone health, as well as preliminary analyses of alcohol consumption in the study population. Serum 4-HNE and C-reactive protein data were log-transformed prior to analysis.
Results
Table 1 reports select characteristics of the NOAH cohort and the 40-subject subsample used in the current analysis to examine the relationship between bone turnover markers and alcohol consumption. The distributions of the variables in the subsample reflected the distributions of each variable in the full study population, with the exception of smoking status: the sub-cohort drawn herein contained a higher proportion of individuals who are former smokers, whereas the study population as a whole (at baseline) is predominately current smokers (p < 0.0001, chi-square test). Greater than 90% of the study participants reported taking ART.
Table 1.
Demographic distributions and alcohol measures of the NOAH cohort (N = 365) and subsample of 40 individuals selected based on low and high phosphatidylethanol (PEth) levels.
| Full study (N=365) | Cohort subsample (n=40) | |||
|---|---|---|---|---|
| Demographics | Frequency | Percent | Frequency | Percent |
| Gender | ||||
| Women | 113 | 31.0 | 10 | 25.0 |
| Men | 252 | 69.0 | 30 | 75.0 |
| p-value † | 0.4702 | |||
| Education | ||||
| < High School | 149 | 40.8 | 12 | 30.0 |
| High School Graduate | 114 | 31.2 | 17 | 42.5 |
| Some College/Community College, Vocational/Trade School | 81 | 22.2 | 10 | 25.0 |
| College Graduate or Graduate School | 21 | 5.7 | 1 | 2.5 |
| p-value † | 0.2508 | |||
| Race | ||||
| Black | 305 | 83.6 | 34 | 85.0 |
| Other | 60 | 16.4 | 6 | 15.0 |
| p-value † | >0.9999 | |||
| Smoking Status | ||||
| Non-Smoker | 84 | 23.0 | 7 | 17.5 |
| Former Smoker | 59 | 16.2 | 29 | 72.5 |
| Current Smoker | 222 | 60.8 | 4 | 10.0 |
| p-value † | <0.0001 | |||
| Age | ||||
| 20–29 | 19 | 5.2 | 0 | 0.0 |
| 30–39 | 60 | 16.4 | 12 | 30.0 |
| 40–49 | 88 | 24.1 | 9 | 22.5 |
| 50–59 | 152 | 41.6 | 15 | 37.5 |
| ≥ 60 | 46 | 21.6 | 4 | 10.0 |
| p-value † | 0.0976 | |||
| Viral Load (copies/mL) | ||||
| < 20 | 247 | 67.7 | 26 | 65.0 |
| ≥ 20 | 118 | 32.3 | 14 | 35.0 |
| p-value † | 0.7219 | |||
| cd4 count (cells/μl) | ||||
| < 200 | 47 | 13.2 | 5 | 12.5 |
| 200–350 | 47 | 13.2 | 7 | 17.5 |
| > 350 | 263 | 73.7 | 28 | 70.0 |
| p-value † | 0.6906 | |||
| Alcohol Measures | Full study (N=365) | Low PEth (n=20) | High PEth (n=20) | |
| Phosphatidylethanol (PEth) (ng/mL) | ||||
| Median (IQR) | 34.0 (179.0) | 0 | 1269 (703.7) | |
| AUDIT (score, 0–40 points) | ||||
| Median (IQR) | 6 (10.5) | 1 (4.75) | 15 (9.5) | |
| p-value †† | <0.0001 | |||
| AUDIT-C (score, 0–12 points) | ||||
| Median (IQR) | 4.0 (4.0) | 1 (4.0) | 7.0 (4.5) | |
| p-value | <0.0001 | |||
| Time-linked follow back (TLFB), total grams (g) | ||||
| Median (IQR) | 158.2 (738.5) | 0 (182.0) | 2216 (2386.1) | |
| p-value †† | <0.0001 | |||
| Time-linked follow back (TLFB), 30 days (# of days/30 days) | ||||
| Median (IQR) | 3 (11) | 0 (2.75) | 19.5 (21) | |
| p-value †† | <0.0001 | |||
p-value for comparison between distributions of the 40-subject subsample and the remaining subjects (chi-squared test or Fisher’s exact test).
p-value for comparison between low PEth subjects and high PEth subjects (Wilcoxon rank-sum pairwise comparison)
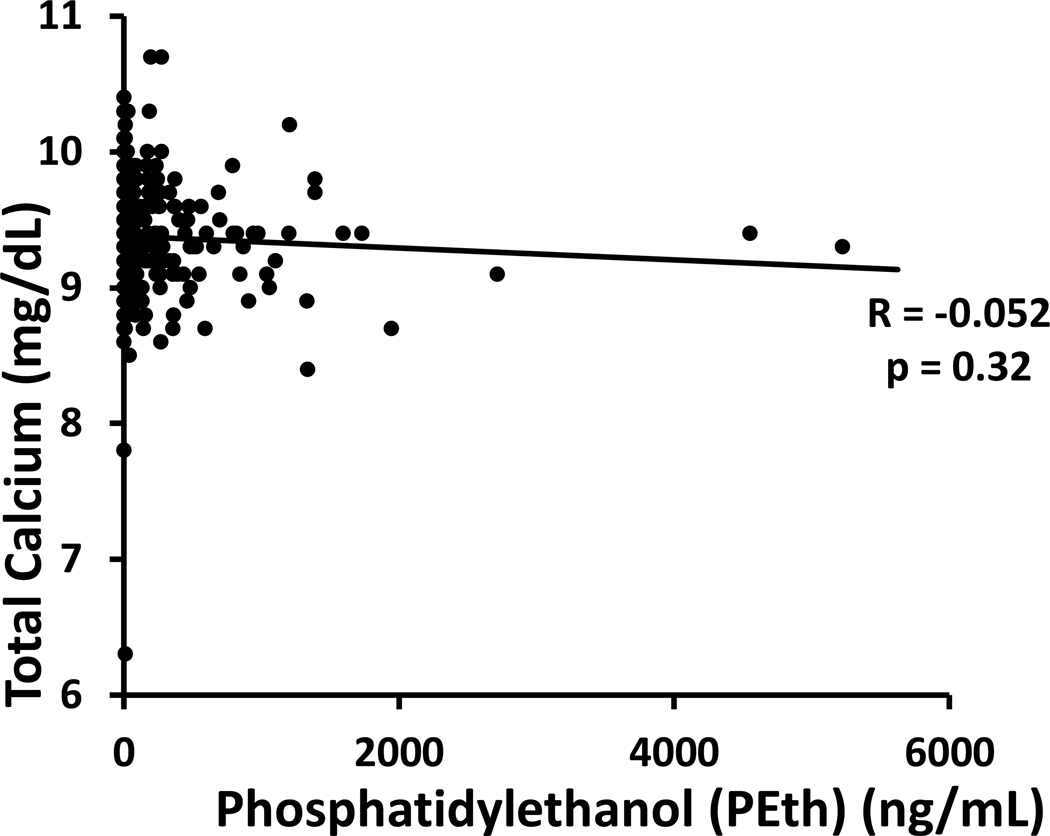
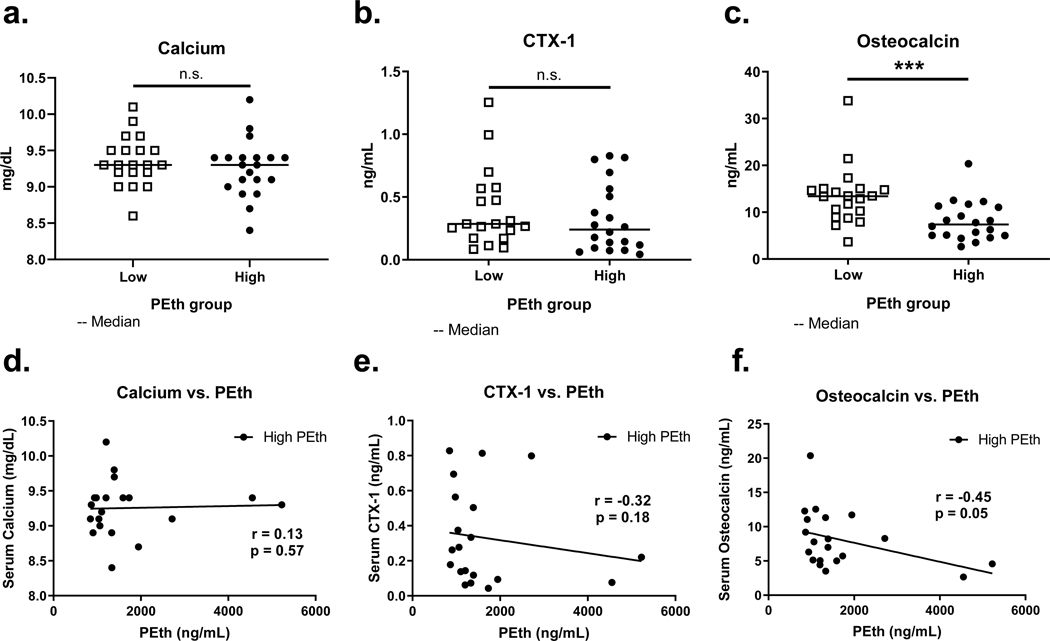
We began by determining whether increases in plasma PEth – as a measurement of recent drinking – were correlated with hypocalcemia in the total study cohort of 365 individuals. Spearman’s correlation was very weak and not statistically significant (r = −0.052, p = 0.32, Figure 1), indicating that serum calcium level was not associated with recent alcohol consumption in this study population. In the subsample, those with the highest PEth measures (n=20) had a median PEth of 1268.5 ng/mL (interquartile range 703.7 ng/mL). As expected, subsampling these two extreme groups did not reveal a significant difference in circulating calcium (mean low PEth group: 9.4 mg/dL, SD 0.34 mg/dL; mean high PEth group: 9.3 mg/dL, SD 0.39 mg/dL; p = 0.40, unpaired t-test; Figure 2a), nor did the two groups significantly differ in CTX-1 (median low PEth group: 0.29 ng/mL, IQR 0.40 ng/mL; median high PEth group: 0.24 ng/mL, IQR 0.73 ng/mL; p = 0.33, Wilcoxon rank-sum test, Figure 2b). On the contrary, we detected a significant difference in circulating total osteocalcin between the low and high PEth groups (median low PEth group: 13.42 ng/mL, IQR 5.73 ng/mL; median high PEth group 7.39 ng/mL, IQR 6.23 ng/mL; p = 0.0005, Wilcoxon rank-sum test; Figure 2c).
Figure 1.
Total serum calcium (mg/dL) by plasma PEth concentration (ng/mL) in the entire NOAH study population at baseline. Each data point represents one participant (n = 365). Solid line: linear trend; R: Spearman correlation coefficient.
Figure 2.
Pairwise differences of serum markers of bone turnover and plasma PEth in subjects with low PEth (20 random samples below LOD, open squares) and high PEth (20 highest measurements, closed circles) (a-c), and linear correlations within the high PEth group between markers and PEth (d-f). (a) and (d): total calcium; (b) and (e): c-terminal telopeptide crosslinks (CTX-1; bone resorption); (c) and (f): osteocalcin (bone formation). ***significant pairwise difference (p < 0.001), n.s.: not significant, Wilcoxon rank-sum test. r: Spearman correlation, p: p-value for Spearman correlation.
Within the high PEth group, linear increases in PEth were not significantly correlated with calcium (Pearson r = 0.04, p = 0.88, n = 20; Figure 2d) or CTX-1 (Spearman r = −0.32, p = 0.18, n = 20; Figure 2e). Consistent with the pairwise difference between high and low PEth groups, PEth was significantly, negatively correlated with circulating osteocalcin within the high PEth group (Spearman r = −0.45, p = 0.05, n = 20, Figure 2f), indicating that in this sample, recent alcohol consumption is associated with a dose-dependent suppression of bone formation.
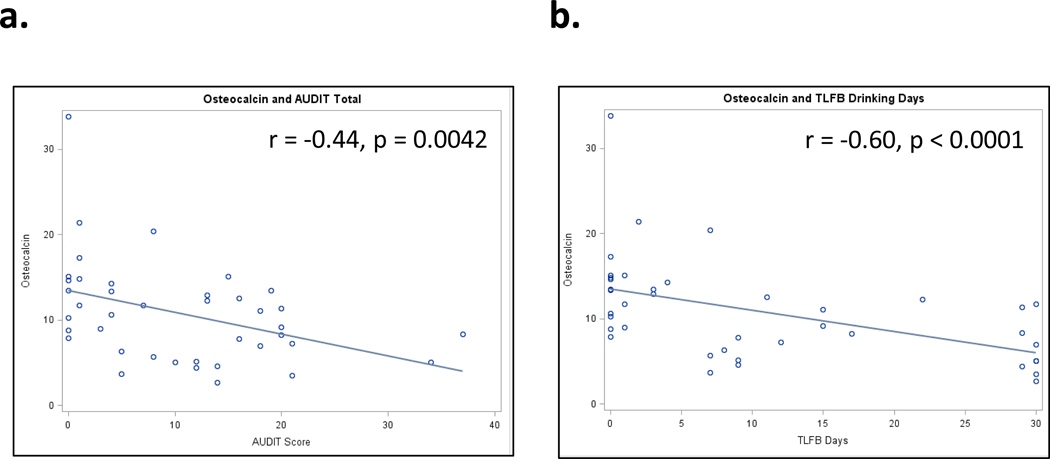
In order to account for covariates that may contribute to the observed variability between PEth and osteocalcin, and to better understand the relationship between osteocalcin and self-reported alcohol consumption, we performed multivariate linear regression analyses on the combined high and low consumption groups (n = 40). Independent variables for alcohol consumption included PEth, AUDIT (point scale of 0–40), AUDIT-C (point scale of 0–12), TLFB (number of drinks in past 30 days), and TLFB (total grams of ethanol, last 30 days) (Table 1). Each of the consumption measures was highly correlated with one another (Supp. Table 1). Additional covariates included age, gender, race, education, smoking status, viral load, and CD4 count (Table 1). Results of the crude (consumption variable only) and adjusted (consumption variable plus covariates) are presented in Figure 3 (crude analysis) and Table 2. In addition to a significant, negative association with PEth (β = −0.00291, p = 0.0007), each of the self-reported measures of alcohol consumption were associated with osteocalcin (Table 2).
Figure 3.
Linear correlations between serum osteocalcin (ng/mL) and (a) AUDIT score (0–40), (b) time-linked follow back (TLFB), number of drinking days in the past 30 days. Each data point is one subject within the 40-sample low/high PEth subsample. r: Spearman correlation, p: p-value for Spearman correlation.
Table 2.
Multivariate linear regression of serum osteocalcin vs. alcohol consumption measures.
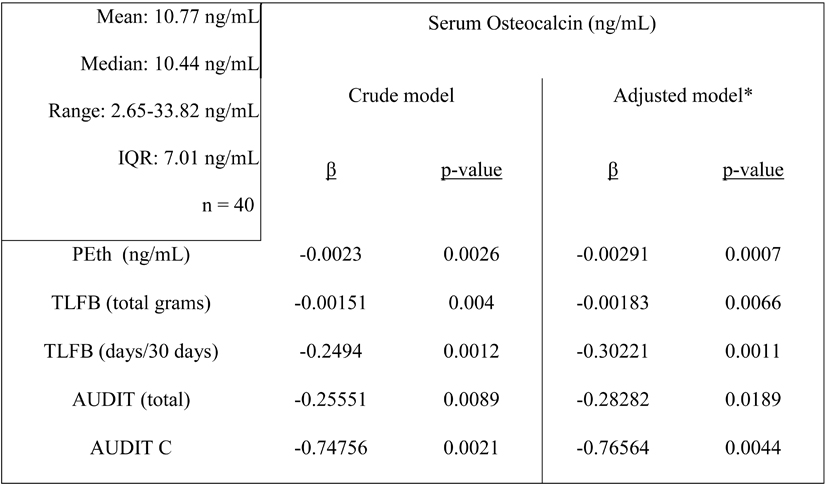 |
Descriptive statistics of serum osteocalcin listed in left panel
Model adjusted for age, gender, education, race, smoking status, viral load, CD4 count
We hypothesized that alcohol consumption would be a significant predictor of general oxidative stress (measured by serum 4-hydroxynonenal (4-HNE)) and inflammation (measured by circulating CRP). Results of the crude and adjusted analyses (as above) are presented in Table 3. We found small, but significant, positive associations between all measures of alcohol consumption and circulating 4-HNE before and after adjusting for the above covariates, suggesting that individuals in this study with higher alcohol consumption have increased systemic oxidative stress. There were no significant associations between the measures of alcohol consumption and inflammation, as measured by CRP (Table 4).
Table 3.
Multivariate linear regression of serum 4-hydroxynonenal (4-HNE) vs. alcohol consumption measures.
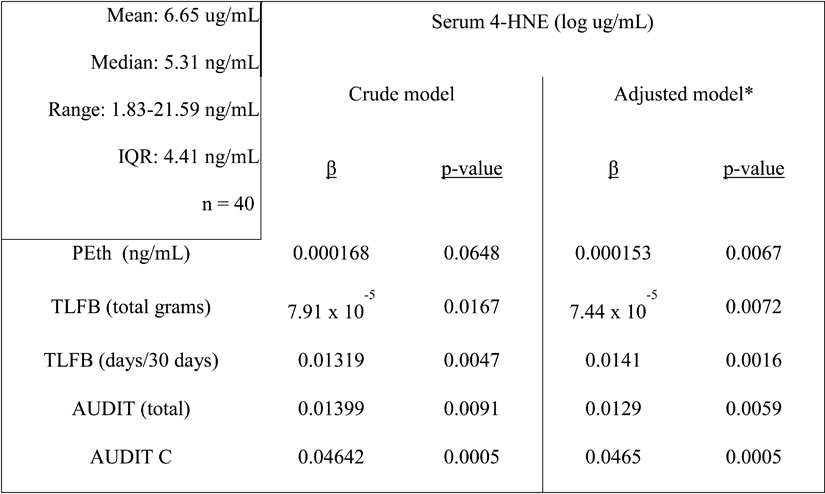 |
Descriptive statistics of serum 4-HNE listed in left panel.
Model adjusted for age, gender, education, race, smoking status, viral load, CD4 count
Table 4.
Multivariate linear regression of serum c-reactive protein vs. alcohol consumption measures.
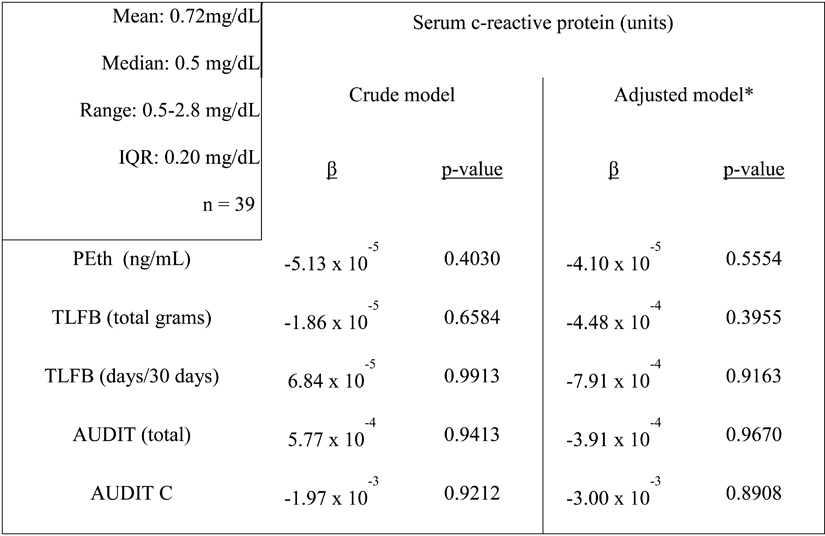 |
Descriptive statistics of serum c-reactive protein listed in left panel.
Model adjusted for age, gender, education, race, smoking status, viral load, CD4 count.
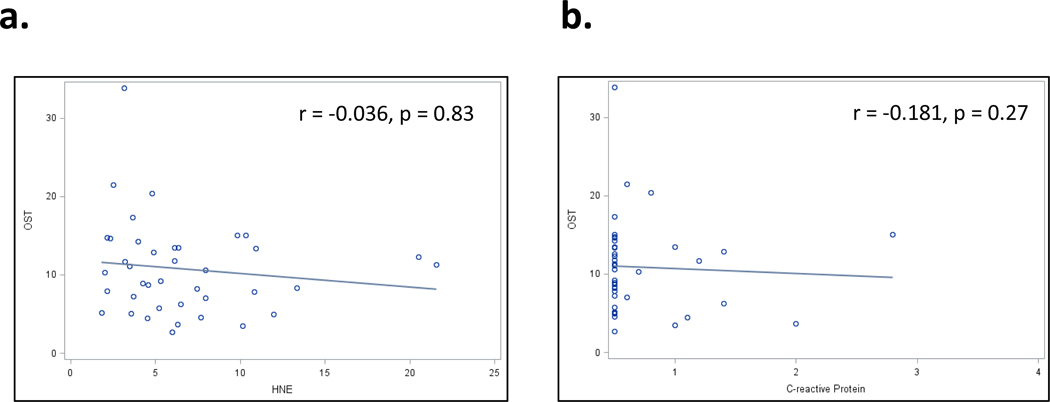
We did not find osteocalcin to be associated with 4-HNE (Figure 4a, Spearman r = −0.036, p = 0.83), suggesting that the observed suppression of osteocalcin is independent of systemic oxidative stress. As expected based on the linear regression results, there was no significant correlation between osteocalcin and CRP (Figure 4b, Spearman r = −0.181, p = 0.27).
Figure 4.
Linear correlations between serum osteocalcin (ng/mL) and (a) serum 4-hydroxynonenal (HNE) (ug/mL) and (b) c-reactive protein (mg/dL). Each data point is one subject within the 40-sample low/high PEth subsample. r: Spearman correlation, p: p-value for Spearman correlation.
Discussion
This study determined relationships between biomarkers of bone turnover and multiple assessments of alcohol consumption, including self-report (time-linked follow back, AUDIT) and a biomarker of recent consumption (PEth) (Isaksson et al., 2011), to test the hypothesis that at risk alcohol use adversely affects bone health in adult PLWH. Additionally, we performed linear regression analyses to determine associations between alcohol consumption and markers of oxidative stress and inflammation. Subdividing the subject population by extreme levels of alcohol consumption (20 subjects with PEth levels greater than 845 ng/mL and a random sample of 20 non-detectable PEth scores) revealed a pairwise significant difference in the serum bone formation marker osteocalcin, suggesting a lower rate of bone formation in subjects with greater alcohol consumption. Within this sub-cohort, osteocalcin negatively correlated to multiple assessments of both self-report and biochemically-determined alcohol consumption (PEth) after controlling for several related covariates. The range of serum osteocalcin concentrations in this subset of 40 individuals (2.65 – 33.82 ng/mL) is comparable to reported intervals in other adult populations of similar age or older (Chubb et al., 2015; Shou et al., 2017; Ardawi et al., 2010) but with a higher central tendency value (median 10.77 ng/mL) compared to previously published values in PLWH (Serrano et al., 1995, Teichmann et al., 2000).
A strength of this study is that it measured alcohol consumption in several dimensions. Self-reported alcohol consumption can be biased (Stockwell et al., 2004; Devaux and Sassi, 2016). However, measurement of PEth as a biomarker of recent alcohol consumption correlated well with the self-reported consumption estimates, and PEth is considered a valid measure to protect against consumption underestimation (Stewart et al., 2014; Bajunirwe et al., 2014; Littlefield et al., 2017, Schrock et al., 2017). Further assessments of the full cohort as the study progresses will add validity to these consumption estimates via repeat measurements.
The strongest predictor of the association between osteocalcin and consumption was AUDIT-C, a 3-question subset of the AUDIT scale that specifically addresses consumption measures (Bush et al., 1998). In our sample, changes in osteocalcin from the lowest quartile to the highest correspond to a 3-point increase per AUDIT-C question, which may correspond to a difference between safe and hazardous drinking. That this association should be the most striking is not surprising in light of the association with PEth (Schrock et al., 2017), as levels of osteocalcin (and other biochemical markers) are more likely altered by consumption patterns rather than social consequences of drinking as examined in other dimensions of the AUDIT scale.
These results agree with prior findings that levels of the bone-specific protein osteocalcin in serum are dose-dependently and negatively associated with alcohol use (Laitinen et al., 1991b; Santori et al., 2008; Gonzales-Reimers et al., 2011). Levels increase following periods of abstinence from alcohol (Laitinen et al., 1991b; Nyquist et al., 1996, Marrone et al., 2012), suggesting a reversible effect that is responsive to intervention measures. Bone structural changes as measured by dual x-ray absorptiometry are the best measures of bone mineral density. We did not perform these measures in our study. However, decreased osteocalcin has been reported to be predictive of lower BMD (Tian et al., 2017). Outside of skeletal health, osteocalcin has been subject of increased interest for its putative role as a circulating signaling factor that increases insulin production and sensitivity (Ducy, 2011; Lee et al., 2007). Given that PLWH have higher rates of metabolic complications (Willig et al., 2016), our results warrant consideration of osteocalcin as a marker linking alcohol use and greater susceptibility to metabolic outcomes among PLWH.
Additional studies have found alterations to bone formation markers and BMD in PLWH who consume alcohol. Saitz et al., (2018), using self-reported drinking, found decreases in serum bone formation marker P1NP, but no association to changes in BMD in HIV-infected individuals, the majority of which had low BMD at baseline. In the same cohort, Ventura et al., (2017) reported a cross-sectional, negative association between BMD and recent alcohol consumption, with the strongest association during the post-diagnosis/pre-ART phase of the HIV care continuum. We did not examine alcohol consumption during different phases (i.e. pre-HIV, pre-ART, post-ART). However, it is important to note that the majority of our study population is virally suppressed (83.8% <200 copies/mL with 75.6 <50 copies/mL) with a high adherence to ART. ART has been shown to be an independent stressor to bone health (Bruera et al., 2003). Further analyses into precise consumption patterns, particularly into the second phase of HIV care, will help illuminate associations between alcohol consumption and bone turnover markers that are modified by ART.
Despite observing significant differences in osteocalcin, this study did not identify an effect on the bone turnover marker CTX-1. These findings are in line with those reported by Saitz et al. (2018). Results in the literature are equivocal regarding the effect of alcohol on net bone turnover markers in humans (Nyquist et al., 1996; Sripanyakorn et al., 2009; Marrone et al., 2012; Alvisa-Negrin et al., 2009). In the cohort subset herein, subjects are older and predominately male. Accelerated bone turnover (i.e. increases in both osteocalcin and CTX-1) may be more pronounced in post-menopausal women (Garnero et al., 1996). Our finding that serum calcium is not affected by recent alcohol use is in line with other studies that found no change to circulating calcium levels with alcohol, despite decreased osteocalcin (Laitinen et al., 1991b; Nyquist et al., 1996), suggesting perhaps tighter control of calcium homeostasis compared to secretion of bone turnover markers.
Increased resorption markers are associated with inflammation and immune cell dysregulation in PLWH (Gazzola et al., 2013; Titanji et al., 2018). Our results did not show an increase in the inflammatory marker CRP in subjects with the highest PEth values. Our finding that alcohol consumption was positively associated with oxidative stress (via increased 4-HNE) agrees with previously reported mechanisms of alcohol (Ronis et al., 2005; Alund et al., 2017), However, these changes were modest and not correlated to osteocalcin. That these two factors do not correlate in this study suggests independent mechanisms of alcohol-induced systemic oxidative stress and suppression of bone formation. Our measures would not be able to detect bone-localized oxidative stress and this could be the underlying cause of the suppressed osteocalcin secretion by osteoblasts. Suppressed osteocalcin is consistent with NADPH-oxidase dependent ROS production observed in bone from rodents exposed to ethanol in vivo, and in mesenchymal stem cells and osteoblasts exposed to ethanol in vitro (Chen et al., 2011, Alund et al., 2017, Watt et al., 2018).
Several limitations to our study bear noting. The present analysis is cross-sectional and thus associations are not necessarily causative. Our ongoing longitudinal study will collect prospective data that will help elucidate the interaction between HIV, ART, and alcohol use with bone health. As noted, several of measures of alcohol consumption are self-reported (AUDIT, TLFB). Although PEth (which is most effective as a very-recent measure of consumption) can help validate these responses, by selecting subjects with high recent drinking levels we may be selecting participants with greater recall bias toward higher AUDIT responses. Subjects were not excluded based on concurrent recreational drug use, which has been considered a confounder in similar analyses (Saitz et al., 2018; Ventura et al., 2017). Thus, additional factors related to adverse health effects (with the exception of smoking) have not been fully characterized in this analysis. The study population as a whole is HIV positive and majority low income, presenting complications in comparing these results to the general US population. On the other hand, African-Americans are currently the most affected HIV-infected population in the United States (CDC, 2018), a demographic reflected in our NOAH cohort (Welsh et al., 2019).
This study helps clarify a complex picture of the relationship between HIV and bone health as modified by alcohol consumption, demonstrating a decrease in the bone formation marker osteocalcin that is associated with high levels of drinking but not increased oxidative stress. Additional self-reported measures describing recent and month-long drinking habits supported this relationship. Studies of HIV’s impact on late endpoints of bone pathologies are lacking due to the relatively recent advent of improved ART and prolonged lifespan of individuals with HIV. However, in uninfected populations, fracture late in life is heavily associated with excess mortality and lower quality of life, and individuals suffering a fracture typically require surgical intervention and acquire severe risk for a second fracture (Haentjens et al., 2010; Johansson et al., 2017; Willig et al., 2001). Given the combinatorial risks of both HIV and alcohol use towards adverse bone outcomes, early biomarker research in longitudinal studies such as NOAH will serve as valuable predictors of risk and determinants of intervention, particularly in underserved populations that make up the majority of HIV cases in the United States.
Supplementary Material
Table 5.
Study highlights
| Study Highlights |
|---|
|
Acknowledgements
We thank the NOAH Study participants for their willingness to participate. We acknowledge the essential contributions by study staff and referring clinicians. They are key to the success of the study. The authors recognize the editorial support received from Rebecca Gonzales and the contributions of study personnel: Angela Amedee, Bryant Autin, Chloe Ball, Sadie Beckett, Jonathan Boudin, Leslie Brennan, Kenya Brooks, Jessica Cucinello, Scott Edwards, Kimberly Edwards, Pauline Fink, Virginia Garrison, Betsy Giaimo, Nicholas Gilpin, Paula Gregory, Meryl Hahne, Jasmine Hall, Brittney Harbin, Kasi Islam, Mark Juanet, Stephen Kantrow, Julie Ketchens, Alexandra Kharalampiev, Tracey Knaus, Danielle Levitt-Budnar, Hui-Yi Lin, Vincent Maffei, Soham Mahato, Charlotte Marshall, Rhonda Martinez, Heather McGarrah, Patrick McTernan, Donald Mercante, Erin Meyaski, Mary Meyaski-Schluter, Patricia Mott, Steve Nelson, Ikenna Nnamani, Lauren O’Rear, Oluwaseun Oguntomole, Christopher Parsons, Francesca Peruzzi, Connie Porretta, Stefany Primeaux, Lauren Richey, Erika Rosen, Derrick Samuelson, Wendemi Sawadogo, Robert Siggins, Liz Simon, Jane Schexnayder, Tianjiao Shen, Aneisha Simon, Aubrey Spriggs, Katherine Theall, Quinette Thomas, Curtis Vande Stouwe, Maeve Wallace, Alice Yeh, and Arnold Zea.
Support
NIAAA R37 AA018282 (MJJR)
NIAAA F32 AA024680 (JW)
NIAAA P60 AA009803 (PM)
References
- Alund AW, Mercer KE, Pulliam CF, Suva LJ, Chen J-R, Badger TM, Ronis MJJ (2017) Partial Protection by Dietary Antioxidants Against Ethanol-Induced Osteopenia and Changes in Bone Morphology in Female Mice. Alcohol Clin Exp Res 41:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvisa-Negrín J, González-Reimers E, Santolaria-Fernández F, García-Valdecasas-Campelo E, Valls MRA, Pelazas-González R, Durán-Castellón MC, de los Ángeles Gómez-Rodríguez M (2009) Osteopenia in alcoholics: Effect of alcohol abstinence. Alcohol Alcohol 44:468–475. [DOI] [PubMed] [Google Scholar]
- Ardawi MM, Maimani AA, Bahksh TA, Rouzi AA, Qari MH, Raddadi RM (2010) Reference intervals of biochemical bone turnover markers for Saudi Arabian women: a cross-sectional study. Bone 47:804–14. [DOI] [PubMed] [Google Scholar]
- Bajunirwe F, Haberer JE, Boum Y, Hunt P, Mocello R, Martin JN, Bangsberg DR, Hahn JA (2014) Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in Southwestern Uganda. PLoS One 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Malik R, Arnsten JH (2008) Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 121:406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J (2003) Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS 17:1917–23. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 158:1789–95. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2018). HIV Surveillance Report, 2017; vol 29. Available at http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed May 4, 2019.
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ (2010) A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/β-catenin signaling. J Bone Miner Res 25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM and Ronis MJJ (2011) Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J. Pharmacol. Exp. Ther. 336: 734–742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb SAP, Byrnes E, Manning L, Beilby JP, Ebeling PR, Vasikaran SD, Golledge J, Flicker L, Yeap BB (2015) Reference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men study. J Clin Endocrinol Metab 100:90–9. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC, Veterans Aging Cohort 3-Site Study (2003) How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr 33:521–5. [DOI] [PubMed] [Google Scholar]
- Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP (2007) HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses 23:1521–30. [DOI] [PubMed] [Google Scholar]
- Devaux M, Sassi F (2016) Social disparities in hazardous alcohol use: self-report bias may lead to incorrect estimates. Eur J Public Health 26:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SE, Kanter JR, Grinspoon S (2006) Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 91:2938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54:1291–7. [DOI] [PubMed] [Google Scholar]
- Fakruddin JM, Laurence J (2003) HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem 278:48251–8. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M (2002) The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol 63:179–86. [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–49. [DOI] [PubMed] [Google Scholar]
- Gazzola L, Bellistri GM, Tincati C, Ierardi V, Savoldi A, del Sole A, Tagliabue L, d’Arminio Monforte A, Marchetti G (2013) Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Reimers E, Alvisa-Negrín J, Santolaria-Fernández F, Martín-González MC, Hernández-Betancor I, Fernández-Rodríguez CM, Viña-Rodríguez J, González-Díaz A (2011) Vitamin D and nutritional status are related to bone fractures in alcoholics. Alcohol Alcohol 46:148–155. [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Tsai JN, Wein MN (2017) Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin Chem 63:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S (2010) Meta-analysis: Excess mortality after hip fracture among older women and men. Ann Intern Med 152:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen K, Neutzsky-Wulff AV., Bonewald LF, Karsdal MA (2009) Local communication on and within bone controls bone remodeling. Bone 44:1026–1033. [DOI] [PubMed] [Google Scholar]
- Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA (2014) Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS 28:1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson A, Walther L, Hansson T, Andersson A, Alling C (2011) Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal 3:195–200. [DOI] [PubMed] [Google Scholar]
- Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen K, Lamberg-Allardt C, Tunninen R, Karonen SL, Tähtelä R, Ylikahri R, Välimäki M (1991a) Transient hypoparathyroidism during acute alcohol intoxication. N Engl J Med 324:721–7. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Lamberg-Allardt C, Tunninen R, Karonen SL, Ylikahri R, Välimäki M (1991b) Effects of 3 weeks’ moderate alcohol intake on bone and mineral metabolism in normal men. Bone Miner 13:139–51. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Lamberg-Allardt C, Tunninen R, Harkönen M, Valimaki M (1992) Bone mineral density and abstention-induced changes in bone and mineral metabolism in noncirrhotic male alcoholics. Am J Med 93:642–650. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Brown JL, DiClemente RJ, Safonova P, Sales JM, Rose ES, Belyakov N, Rassokhin V V (2017) Phosphatidylethanol (PEth) as a Biomarker of Alcohol Consumption in HIV-Infected Young Russian Women: Comparison to Self-Report Assessments of Alcohol Use. AIDS Behav 21:1938–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone JA, Maddalozzo GF, Branscum AJ, Hardin K, Cialdella-Kam L, Philbrick KA, Breggia AC, Rosen CJ, Turner RT, Iwaniec UT (2012) Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause 19:974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KE, Sims CR, Yang CS, Wynne RA, Moutos C, Hogue WR, Lumpkin CK, Suva LJ, Chen J-R, Badger TM, Ronis MJJ (2014) Loss of functional NADPH oxidase 2 protects against alcohol-induced bone resorption in female p47phox−/− mice. Alcohol Clin Exp Res 38:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Míguez MJ, Burbano-Levy X, Carmona T, Quiros C, Thompson M, Lewis JE, Asthana D, Rodríguez A, Valiathan R, Malow R (2012) Hypocalcaemia, alcohol drinking and viroimmune responses in ART recipients. Alcohol 46:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist F, Ljunghall S, Berglund M, Obrant K (1996) Biochemical markers of bone metabolism after short and long time ethanol withdrawal in alcoholics. Bone 19:51–4. [DOI] [PubMed] [Google Scholar]
- Ofotokun I, Titanji K, Vikulina T, Roser-Page S, Yamaguchi M, Zayzafoon M, Williams IR, Weitzmann MN (2015) Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 6:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM (2004) AZT enhances osteoclastogenesis and bone loss. AIDS Res Hum Retroviruses 20:608–20. [DOI] [PubMed] [Google Scholar]
- Paton NIJ, Macallan DC, Griffin GE, Pazianas M (1997) Bone mineral density in patients with human immunodeficiency virus infection. Calcif Tissue Int 61:30–32. [DOI] [PubMed] [Google Scholar]
- Ronis MJJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM (2005) Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med 39:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Mesic A, Ventura AS, Winter MR, Heeren TC, Sullivan MM, Walley AY, Patts GJ, Meli SM, Holick MF, Kim TW, Bryant KJ, Samet JH (2018) Alcohol Consumption and Bone Mineral Density in People with HIV and Substance Use Disorder: A Prospective Cohort Study. Alcohol Clin Exp Res 42:1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D’Erasmo E, Romagnoli E, Mascia ML, Cipriani C, Prastaro A, Carnevale V, Minisola S (2008) Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest 31:321–6. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Schröck A, Wurst FM, Thon N, Weinmann W (2017) Assessing phosphatidylethanol (PEth) levels reflecting different drinking habits in comparison to the alcohol use disorders identification test - C (AUDIT-C). Drug Alcohol Depend 178:80–86. [DOI] [PubMed] [Google Scholar]
- Serrano S, Mariñoso ML, Soriano JC, Rubiés-Prat J, Aubia J, Coll J, Bosch J, Del Rio L, Vila J, Goday A (1995) Bone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric study. Bone 16:185–91. [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Chen JR, Haley R, Perrien DS, Skinner RA, Lumpkin CK, Badger TM, Ronis MJJ (2008) Chronic ethanol consumption inhibits postlactational anabolic bone rebuilding in female rats. J Bone Miner Res 23:338–49. [DOI] [PubMed] [Google Scholar]
- Sharma A, Shi Q, Hoover DR, Anastos K, Tien PC, Young MA, Cohen MH, Golub ET, Gustafson D, Yin MT (2015) Increased Fracture Incidence in Middle-Aged HIV-Infected and HIV-Uninfected Women: Updated Results From the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 70:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Broun EC, Arpadi SM, Yin MT (2013) Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS 27:1949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Z, Jin X, Bian P, Li X, Chen J (2017) Reference intervals of β-C-terminal telopeptide of type I collagen, procollagen type I N-terminal propeptide and osteocalcin for very elderly Chinese men. Geriatr Gerontol Int 17:773–778. [DOI] [PubMed] [Google Scholar]
- Sobell LC and Sobell MB (1992) Timeline Follow-back: A technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods (Allen J & Litten RZ eds), pp. 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Sripanyakorn S, Jugdaohsingh R, Mander A, Davidson SL, Thompson RP, Powell JJ (2009) Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J Bone Miner Res 24:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A (2014) Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res 38:1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell T, Donath S, Cooper-Stanbury M, Chikritzhs T, Catalano P, Mateo C (2004) Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction 99:1024–33. [DOI] [PubMed] [Google Scholar]
- Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker Project (2017) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28:2541–2556. [DOI] [PubMed] [Google Scholar]
- Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE (2000) Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS 14:F63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann J, Stephan E, Discher T, Lange U, Federlin K, Stracke H, Friese G, Lohmeyer J, Bretzel RG (2000) Changes in calciotropic hormones and biochemical markers of bone metabolism in patients with human immunodeficiency virus infection. Metabolism 49:1134–1139. [DOI] [PubMed] [Google Scholar]
- Tian L, Yang R, Wei L, Liu J, Yang Y, Shao F, Ma W, Li T, Wang Y, Guo T (2017) Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore) 96:e8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titanji K, Vunnava A, Foster A, Sheth AN, Lennox JL, Knezevic A, Shenvi N, Easley KA, Ofotokun I, Weitzmann MN (2018) T-cell receptor activator of nuclear factor-κB ligand/osteoprotegerin imbalance is associated with HIV-induced bone loss in patients with higher CD4+ T-cell counts. AIDS 32:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Brown TT, Lee H, Grinspoon SK (2008) Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 93:3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura AS, Winter MR, Heeren TC, Sullivan MM, Walley AY, Holick MF, Patts GJ, Meli SM, Samet JH, Saitz R (2017) Lifetime and recent alcohol use and bone mineral density in adults with HIV infection and substance dependence. Medicine (Baltimore) 96:e6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt J, Alund AW, Pulliam CF, Mercer KE, Suva LS, Chen J-R and Ronis MJJ (2018). NOX4 deletion in mice exacerbates the effect of ethanol on trabecular bone loss and osteoblast colony formation. J. Pharmacol. Exp. Ther. 366: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DA, Ferguson T, Theall KP, Simon L, Amedee A, Siggins RW, Nelson S, Brashear M, Mercante D, Molina PE (2019) The New Orleans Alcohol Use in HIV Study: Launching a Translational Investigation of the Interaction of Alcohol Use with Biological and Socioenvironmental Risk Factors for Multimorbidity in People Living with HIV. Alcohol Clin Exp Res 43:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH (2016) Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res 40:2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig AL, Overton ET (2016) Metabolic Complications and Glucose Metabolism in HIV Infection: A Review of the Evidence. Curr HIV/AIDS Rep 13:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig R, Keinänen-Kiukaaniemi S, Jalovaara P (2001) Mortality and quality of life after trochanteric hip fracture. Public Health 115:323–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.