Abstract
Objectives:
In the present investigation, bioadhesive buccal tablets were prepared using the sustained-release polymer hydroxypropyl methylcellulose (HPMC) K100M, bioadhesive polymer neem gum, and an impervious backing layer of ethyl cellulose. Nicorandil is sensitive to the first-pass effect; therefore, a buccal-adhesive dosage form can avoid this effect.
Materials and Methods:
We used the direct compression technique to prepare the tablet formulation. A 32 full factorial design was composed in which the amounts of HPMC K100M (X1) and neem gum (X2) were chosen as the independent variables and the dependent variables were the percentage drug release at 6 h (Y1) and mucoadhesive strength in grams (Y2). Various in vitro parameters, i.e. thickness, friability, hardness, weight variation, surface pH, moisture absorption ratio, dissolution studies, and drug release kinetics, and ex vivo parameters like mucoadhesive strength and mucoadhesion time were determined for the prepared tablets. We subjected the optimized batch to a comparison with the marketed formulation and stability studies were performed.
Results:
The formulation containing a 50:50 ratio of neem gum and HPMC K100M (F5) was considered optimum. The zero-order release kinetics model best fitted the optimized batch release profile, suggesting the system would release the drug at a constant rate.
Conclusion:
The release by the optimized formulation of the drug at a sustained rate along with its bioadhesive nature showed that the buccal route can be an option for the administration of nicorandil.
Keywords: Nicorandil, neem gum, buccal tablets, factorial design
INTRODUCTION
Hypertension and angina pectoris are cardiovascular diseases for which constant monitoring is crucial. Angina pectoris is a medical condition that causes chest pain by reduced blood flow to the heart. Potassium channel openers are currently regarded as an important drug class for the treatment of such conditions. A primary medicinal agent that possesses an ability to tackle such a situation is nicorandil, a vasodilatory drug.1,2,3 It appears to be active in all types of angina pectoris and has the twin properties of nitrate and K+ adenosine triphosphate channel agonist. The major problem with orally administered nicorandil is its first-pass metabolism, which gives about 75% systemic bioavailability. Moreover, it has a short elimination half-life (1 h), which necessitates frequent administration of the drug (10 to 20 mg twice daily).4,5,6 Thus, higher fluctuation of drug concentration may give rise to undesirable side effects. In summary, there is a strong requirement for a patient-friendly sustained-release formulation of nicorandil to reduce the frequency of administration.
Such a requirement for an oral dosage form can be fulfilled by employing a buccal bioadhesive drug delivery system. It is a captivating substitute to the oral route of drug administration that overcomes the deficiencies associated with the latter mode of administration. Precisely it prevents any chance of reductant hepatic metabolism, avoiding unneeded drug degradation in the upper GIT, and also it increases the contact between drug and absorbing surface.7,8,9,10,11 Moreover, such type of delivery is considered safer since drug absorption can be concluded any time if toxicity occurs due to it by removal of the formulation from the site of application.12,13,14
Thus, in the present research, buccal bioadhesive tablets of nicorandil were prepared using the sustained-release polymer hydroxypropyl methylcellulose (HPMC) K100M,10,15 bioadhesive polymer neem gum,16 and an impermeable backing layer of ethyl cellulose. Buccal bioadhesive tablets were prepared by direct compression method employing a 32 factorial design in which the amounts of HPMC K100M (X1) and neem gum (X2) were selected as independent variables and their effects on dependent variables, i.e. percentage drug release at 6 h (Y1) and mucoadhesive strength in grams (Y2), were studied.
MATERIALS AND METHODS
Materials
Nicorandil was a gratis sample from Sun Pharma Laboratories Ltd. (East Sikkim). Neem gum was purchased from the local market and the remaining materials were purchased from Chem Dyes Corporation (Vadodara, Gujarat, India).
Methods
Drug-excipients compatibility study
Accurately weighed (3 mg) nicorandil was taken and mixed thoroughly with 100 mg of potassium bromide (dried at 40-50°C). The mixture was compressed into pellets (under 10-t pressure) using a hydraulic press followed by scanning between 4000 and 400 cm-1 using an fourier transform-infrared (FT-IR) 410 PC spectrophotometer. The obtained IR spectra of pure drug (Figure 1a) were compared with those of the reference standard (Figure 1b) taken from Indian Pharmacopoeia as well as with the IR spectra (Figure 1c) of the prepared nicorandil tablet formulation to check the drug excipient compatibility.
Figure 1.
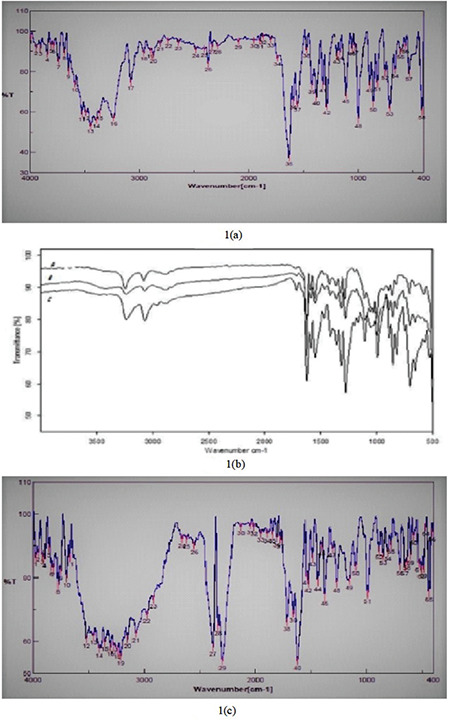
a, b, c. FT-IR spectra
FT-IR: Fourier transform-infrared
Preparation of buccal tablets
Initially, the drug was accurately measured and mixed thoroughly with mannitol on butter paper using a stainless-steel spatula. Except the lubricant, all the additives were blended for 10 min by geometric dilution. After uniform blending of the additives, lubricant was added followed by mixing for 2 min. Next, 100 mg of such blends of each formulation was pre-compressed on a 10-station rotary tablet punching machine at a low compression force, resulting in single-layered core tablets 8 mm in diameter. The prepared core tablet was placed in the center of the 12-mm lower punch and the backing layer of 100 mg of ethyl cellulose was added around and over the core tablet; the two layers were then compressed into a mucoadhesive bilayer tablet. A tablet (200 mg) was formed whose thickness was 1.6 to 1.8 mm. Table 1 shows the results of preliminary trials to evaluate the bioadhesive polymers and Table 2 depicts formulations to evaluate the sustained-release characteristics of various compositions.
Table 1. Preliminary trial for selection of bioadhesive polymer (B1-B5).
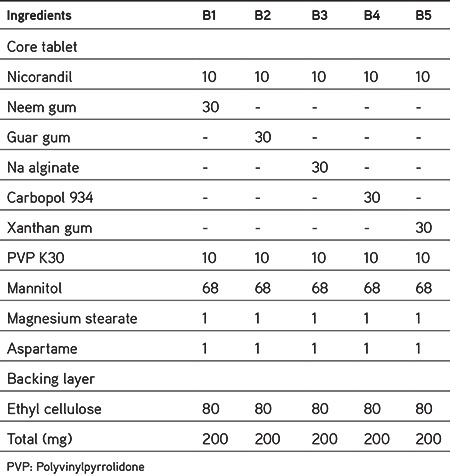
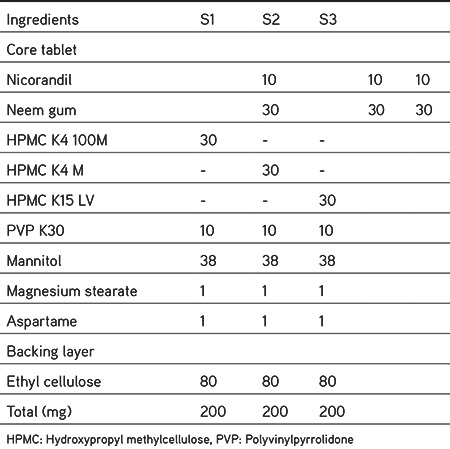
Table 2. Compositions of formulation for optimization of sustained release polymer (S1-S3).
Factorial batches
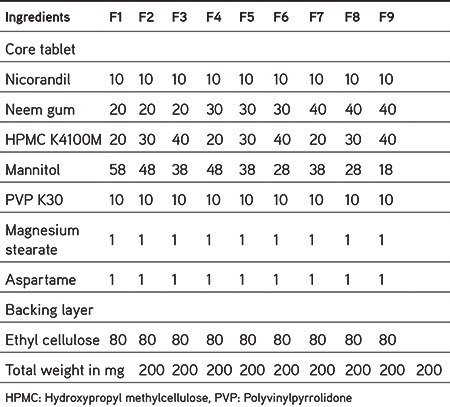
Based on the preliminary studies, a 32 full factorial design was constructed in which the amounts of HPMC K100M (X1) and neem gum (X2) were selected as independent variables and their levels were defined. The dependent variables were % drug release at 6 h (Y1) and mucoadhesive strength in grams (Y2). Table 3 gives details regarding the employed factorial design. ANOVA was performed using the software Design Expert 11.0 demo version (State-ease) and the responses were studied.
Table 3. 32 Experimental design for buccal tablet formulation.
Evaluation of buccal tablets
Thickness
A vernier caliper was used to calculate the thickness of tablets (n=10) and the mean tablet thickness was calculated.
Friability and hardness
Friability (n=20) and hardness (n=3) were measured by a Roche friabilator and a Monsanto type hardness tester, respectively.17
Weight variation
Tablets (n=20) were weighed individually and their weight variation was found by comparing these weights to the label claim.17
Drug contents
The prepared tablets (n=10) were powdered and an amount corresponding to 10 mg of nicorandil was accurately weighed. The powder was extracted with a volume of buffer solution (phosphate buffer saline, pH 6.8) and analyzed using a spectrophotometer at 262 nm after appropriate dilution.
Surface pH
To evaluate the possibility of the prepared tablets causing irritation to the oral mucosa, surface pH studies were performed. Tablets were soaked in 12 mL of buffer solution (phosphate buffer saline, pH 6.8) and allowed to swell for 2 h at room temperature. A pH meter containing a glass electrode was utilized to find out the pH of the resultant swelled tablets by bringing the glass electrode into contact with the surface of the tablet and allowing it to equilibrate for 1 min.18
Moisture absorption ratio
Hot water was taken and the required quantity of agar (5% w/v) was added to it. The resultant solution was added to petri plates and inducted to solidify. Previously, vacuum dried nicorandil buccal tablets (n=6) were taken, weighed individually, and one was laminated with cellophane tape (impermeable backing membrane). The tablets were then placed individually in petri plates so that their other side was in contact with the agar medium, followed by incubation at 37°C for 1 h. After incubation, the buccal tablets were reweighed, and the percentage of moisture absorption was calculated using the following formula:
% Moisture absorption=[(Final weight - Initial weight) / Initial weight] × 100
Ex vivo mucoadhesive strength
Ex vivo mucoadhesive strength was determined using a modified balance method. On the day of the experiment, the authors visited a nearby slaughterhouse and collected surgically cut out goat buccal mucosa, which can be used within 2 h of slaughter, a model substrate used for the present study. To prevent it going rotten, a piece of buccal mucosa was kept in Krebs buffer and stored at 4°C for 2 h. The goat mucosa reached room temperature before further use. This model substrate was then tied to a glass slide to provide it with mechanical strength. On that membrane a tablet was gently put with manual pressure for 5 min after moistening with fluid, which led to bioadhesion. To that biologically attached tablet water was added to detach it from the model substrate, and the amount of water (in grams) needed to detach the tablet from the surface was determined as mucoadhesive strength. Such a procedure was repeated three times and the average mucoadhesive strengths were reported.19,20
Ex vivo mucoadhesion time
Freshly cut goat buccal mucosa was used for the measurement of ex vivo mucoadhesion time (n=3) according to the reported method. Fresh goat buccal mucosa was collected and maintained as described above. A glass slide was taken and excised goat buccal mucosa was tied onto it. Upon this goat buccal membrane a bioadhesive side of the tablet, previously wetted with fluid, was pasted and light force was applied with a fingertip for 30 s. The glass slide along with the pasted tablet was placed in a beaker containing 200 mL of phosphate buffer (pH 6.8) and it was kept at 37±1°C. The beaker containing the entire assembly was slowly stirred similar to the buccal cavity and the entire assembly was monitored for 12 h. The ex vivo mucoadhesion time was calculated as the time required to detach the tablet from the goat membrane that was tied to a glass slide.19,20
Drug release studies
A method previously reported by Daravath et al.21 for furosemide sustained release bilayered buccal tablets was simply followed in the present drug release studies using the US Pharmacopeia XXIII rotating paddle apparatus. An instant adhesive (cyanoacrylate adhesive) was used for pasting the backing layer of the buccal tablet on a glass slide. The slide was then placed at the bottom of the dissolution vessel containing 250 mL of phosphate buffer saline (pH 6.8), which was maintained at 37±0.5°C and rotated at 50 rpm throughout the experiment. Samples (10 mL) were withdrawn at predetermined time intervals in sink condition, followed by filtering through Whatman filter (0.45 µm) paper and analysis by ultraviolet (UV) spectrophotometer at 262 nm.
Ex vivo permeation of drug from buccal tablets
Ex vivo permeation of drug from buccal tablets was performed using a Franz diffusion cell through porcine buccal mucosa at 37±0.5°C and at 50 rpm. Fresh porcine buccal mucosa was obtained from a local slaughterhouse and used within 2 h of slaughter. The mucosal membrane was separated by removing the underlying fat and loose tissues, and washed with distilled water and then with phosphate buffer (pH 6.8) at 37°C. The fresh porcine buccal mucosa was cut into pieces and washed with phosphate buffer (pH 6.8). The membrane was collected and was stored at 4°C in Krebs buffer. This membrane was arranged between the two chambers and phosphate buffer saline (pH 6.8) was used to fill the receiver chamber. To the donor chamber was added 1 mL of phosphate buffer saline and a buccal tablet was suspended there. Aliquots of 5 mL samples were collected at predefined times. The collected samples were filtered, suitably diluted, and the amount of drug permeated was determined using a double beam UV spectrophotometer at λmax=262 nm. The flux (J) and permeability coefficient (P) were calculated using the following formulae:
J=[dQ/dt]÷ ∆CA
P=[dQ/dt]÷A
Here J is flux (mg/h cm2), P is a permeability coefficient (cm/h), dQ/dt is the slope of the steady-state portion of the curve, ΔC is the difference in concentration across the membrane, and A is the area of diffusion (cm2).22
ANOVA studies
To evaluate the effect of independent variables on the responses, ANOVA was applied to the nine formulations prepared using Design Expert software. The p values for the respective responses were also calculated to check whether the effect was statistically significant or not.
Drug release kinetics
To define the kinetics of drug release, the dissolution profile of the optimized batch (F5) was fitted to various models such as zero order, first order, Higuchi, Hixon Crowell, Korsmeyer, and Peppas.23
Stability studies
To determine the change in bioadhesive strength and in vitro release profile during storage, a 3-month short-term stability study for the optimized batch was performed at 40±2°C in a stability chamber with 75±5% relative humidity (RH). Tablets were taken out at 1-month intervals and evaluated for any change in bioadhesive strength and in vitro drug release pattern. The difference factor (f1) and similarity index (f2) were calculated to find out the similarity between the dissolution profile of batch F5 before and after storage at the level of significant (p<0.05) by using the paired t-test.
The formulae to calculate difference factor (f1) and similarity index (f2) are as follows:
Here t is 1 to n, n is the dissolution time, and Rt and Tt are the reference and test dissolution value at time t.24,25
RESULTS AND DISCUSSION
Drug excipient compatibility study
FT-IR spectroscopy was employed to study any kind of interaction between the drug and additives used in the formulation. As per the FT-IR graph, no significant shift in the positions of the wave numbers was found for the formulation (F5) when compared to that of the pure drug values, which inferred no interaction between the drug and the employed additives in the formulation (Figure 1).
Preliminary study
Initially, the study was started with the preparation of preliminary tablets using various natural polymers to check their bioadhesive properties along with tableting properties and the same were compared with synthetic polymer (Carbopol 934). Table 4 shows the evaluation results for these formulations, which indicated that batch B1, which contained neem gum, has better bioadhesive strength as well as hardness. Hence, neem gum was chosen as a bioadhesive polymer for further study. To assess the synergistic sustained-release characteristics of neem gum along with different grades of HPMC, tablet formulations (S1, S2, and S3) were prepared and checked with respect to drug release for 12 h. It was found that formulations S2 and S3 (containing HPMC K4M and HPMC K15 LV) were not able to sustain drug release for 12 h, whereas S1 (containing neem gum and HPMC K4 100M) showed sustained drug release for 12 h as depicted in Table 5. Such sustained effect would be needed for our study and hence was selected for further study.
Table 4. Evaluation of preliminary batches for selection of bioadhesive polymer.
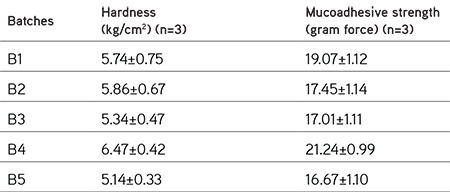
Table 5. Evaluation of preliminary batches for optimization of sustained release polymer.
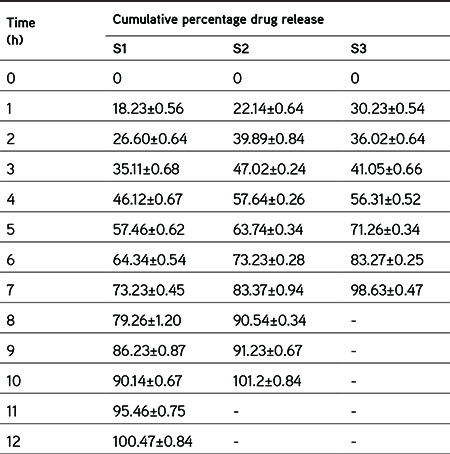
Full factorial design
Physico-chemical parameters
The prepared factorial formulations were evaluated for various physicochemical parameters. From the results, it was found that the weight variation within 7.5% deviation, hardness (4.33-5.71 kg/cm2), thickness (1.69-1.86 mm), friability LT 1%, and drug contents (98.97-101.21%) were within the specified limits.
Surface pH of all the formulations was found to be between 5.5 and 7.5 (Figure 2), which seemed within the acceptable salivary pH range (5.5-7.0). It was inferred that the tablets would not produce local irritation to the mucosal surface.
Figure 2.
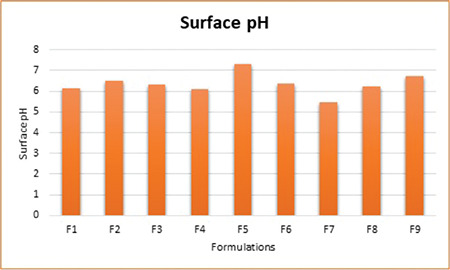
Surface pH of factorial batches
Moisture absorption ratio
The moisture absorption ratio was calculated to assess the relative moisture absorption potential of polymers as well as their strengths to maintain the integrity of the formulation after that absorption. The prepared tablets were subjected to such studies and the results are shown in Figure 3. Formulation F4 was found to have the minimum value (28%), whereas F8 had the maximum value (51%), which may be attributed to the high concentration of hydrophilic neem gum.
Figure 3.
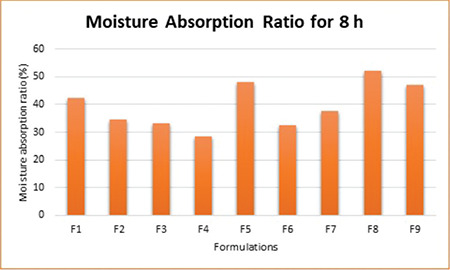
Moisture absorption ratio for 8 h of factorial batches
Ex vivo mucoadhesive strengths and time
The ex vivo mucoadhesive strengths and time of the tablets were determined for all formulations using goat buccal mucosa. The mucoadhesive strengths and time were found to be increased with increased concentrations of polymers. The best bioadhesive strength was found for F9 (21.28 g) and the lowest for F1 (17.25 g). The mucoadhesion was attributed to the formation of a hydrogen bond between polymers due to swelling and mucin of the mucus membrane. F9 was prepared with higher concentrations of neem gum and HPMC, which might have resulted in high swelling and ultimately higher values of mucoadhesion. Figure 4 shows the results obtained from the test. This test indicated the mucoadhesive potential of polymers used in formulations.
Figure 4.
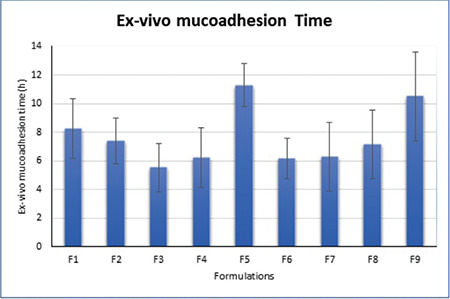
Ex vivo mucoadhesion time of factorial batches
In vitro drug release studies
The prepared factorial tablets were subjected to in vitro dissolution studies for 12 h to check the effect of the various concentrations of neem gum with HPMC K100M and the results are given in Figure 5. The dissolution pattern was found to be F1<F7<F2<F4<F8<F3<F6<F5<F9. Based on the criteria selected according to the theoretical drug release profile of nicorandil at 1 h (12%), 5 h (50%), and 8 h (80%), F5 is considered to be promising as it had drug release of 11.25% at 1 h, 51.81% at 5 h, and 79.20% at 8 h. Additionally, it sustained the drug release for 12 h, which was attributed to the synergistic effect that occurred due to the presence of HPMC K100M as well as neem gum. To ensure the drug release kinetics from the optimized buccal tablet, the dissolution profile was fitted to different release kinetic models: zero-order, first-order, Hixson-Crowell, Higuchi, and Weibull’s equations. The regression analysis was performed for batch F5 and residual values were used to analyze the best fit of the experimental data to the predicted models (r2>0.99 and minimum residual mean square and model parameters). The results are shown in Figure 6. As can be seen, the zero-order model was suited best to the dissolution data for F5, which suggested that the rate of drug release was perpetual over the course of time independent of the drug concentration.
Figure 5.
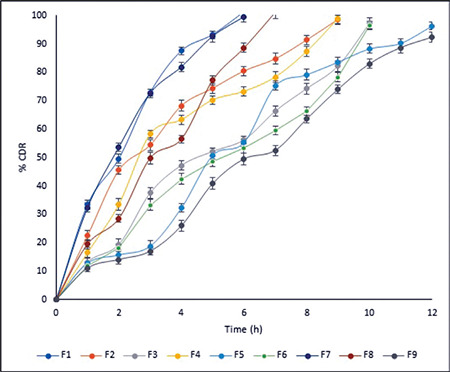
Cumulative percentage drug release profiles
Figure 6.
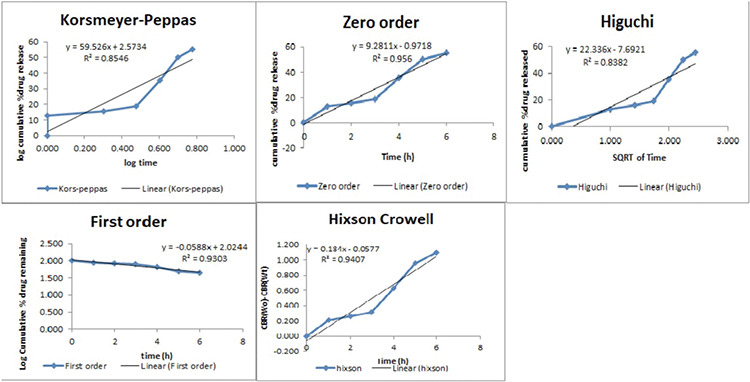
Release kinetics results of the optimized formulation (F5)
Ex vivo permeation studies
Ex vivo permeation studies (n=3) were performed for the optimized buccal tablet (F5). The slope, flux, and permeability coefficient for various formulations were 0.623, 0.889±0.12, and 0.241±0.07, respectively. Cumulative percentage of drug permeated from the prepared formulation is shown in Figure 7. The results of the permeation study affirmed that the drug was liberated controllably from the tablet and impregnated steadily through the porcine buccal membrane and could possibly be infiltrated through the human buccal membrane as well.
Figure 7.
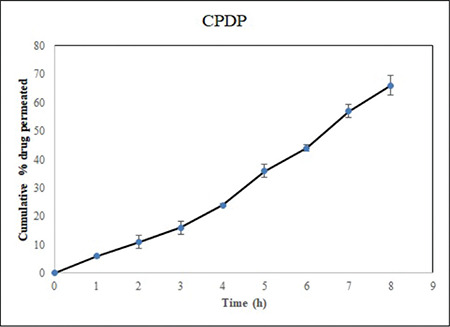
Cumulative percentage drug permeation of F5
Statistical analysis
ANOVA studies
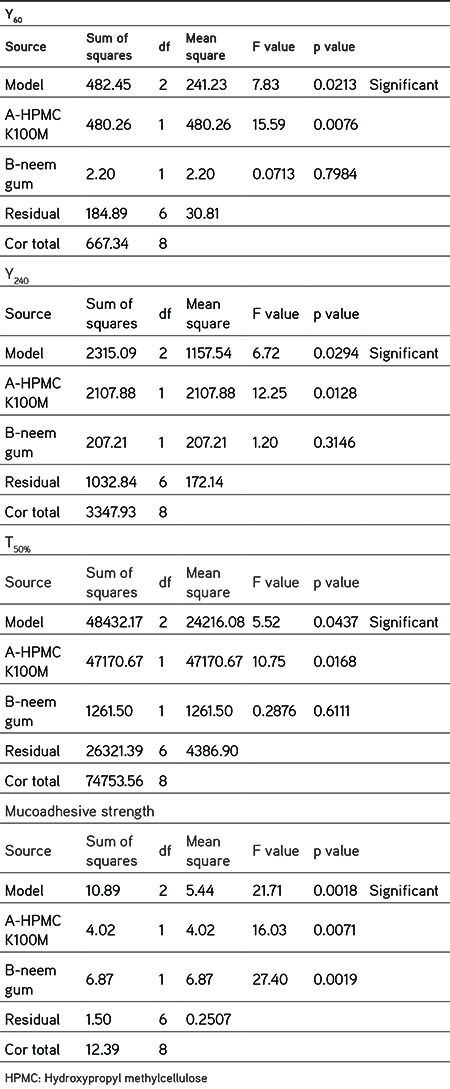
A total of nine formulations were advised by the 32 factorial design for two independent variables: the amount of neem gum (X1, mg) and HPMC K100M (X2, mg). The effect of these factors on Y60 (release in 60 min), Y240 (release in 240 min), T50% (time in min required for 50% release), and mucoadhesive strength in gram force (MS) was examined as response parameters in the study. Summaries of the variables and observed responses are given in Tables 6 and 7. The software Design Expert 7.0 calculated suitable model equations after fitting these data. According to the ANOVA results, all the models were significant (p<0.05). Model simplification was carried out by eliminating nonsignificant terms (p>0.05) in the equations, giving the model equation relating
Table 6. Response values for buccal tablet formulations as per experimental design.
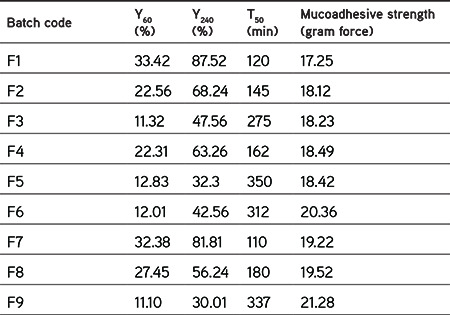
Table 7. ANOVA for linear model.
Y60=20.60-8.95X1+0.6050X2 Equation no (1)
Y240=56.61-18.74X1-5.88X2 Equation no (2)
T50%=221.22+88.67X1+14.50X2 Equation no (3)
MS=18.99+0.8183X1+1.07X2 Equation no (4)
The data obviously demonstrated that the response values are strongly dependent on the independent variables chosen. From the equations (1-4), it was established that the independent variables (X1 and X2) have significant effects on the chosen responses. The effects of factors (X1 and X2) on responses were demonstrated by plotting 3D surface plots and contour plots as shown in Figure 8. It was found that responses may be changed by a convenient choice of the levels of X1 and X2. The results of dependent variables were selected to check the suitability of the prepared tablets as a mucoadhesive sustained-release formulation. Based on the theoretical requirement to prepare a sustained-release tablet formulation of nicorandil, Y60 should be 11.25%, Y240 should be 35%, and T50% should be 300 min. F5 was found to have results similar to the theoretically calculated requirements. Hence, it is considered to be the optimized formulation and can be explored further in future research.
Figure 8.
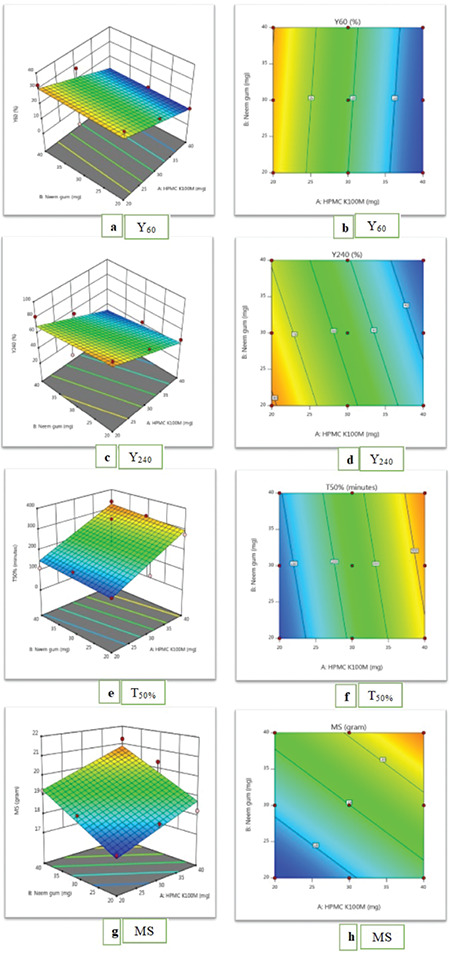
Surface plots (a, c, e, g) and contour plots (b, d, f, h)
MS: Gram force
Stability studies
To assess the physicochemical nature of the optimized formulation (F5) with respect to dissolution characteristics and mucoadhesive strength, it was wrapped in aluminum foil and kept in the stability chamber with well-controlled conditions of temperature (40±2°C) and humidity (75±5% RH). After storage, dissolution parameters and mucoadhesive strength were determined and the results are depicted in Table 8. The precise way to find similarities between dissolution curves is to find out the similarity factor f2 and the difference factor f1. According to the food and drug administration, f1 values less than 15 and f2 values greater than 50 should establish an agreement between the dissolution curves, demonstrating an average disparity of no more than 10% at the sample time points. According to this guideline, the dissolution curves corresponding to F5 before storage were similar to those obtained with the same formulation after storage. No significant changes were found according to the results, which indicated that the prepared tablet formulation is stable.
Table 8. Evaluation of stability studies of formulation (F5) for 3 months (mean ± SD, n=3).

CONCLUSION
To prevent the first-pass metabolism and provide sustained drug release, buccal drug delivery of nicorandil is considered to be one of the best surrogate routes of administration. Additionally, it will lead to patient compliance as well by reducing the frequency of administration. To attain this, a factorial approach was used with a combination of HPMC K100M and neem gum to prepare sustained-release buccal tablets of nicorandil that resulted in a sustained formulation, which can be used in a once a day tablet.
Acknowledgments
The authors are thankful to Sun Pharma Laboratories Ltd., East Sikkim, for providing nicorandil as a gratis sample to carry out this research work.
Footnotes
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of this article.
References
- 1.Ahmed AB, Nath LK. Design and development of controlled release floating matrix tablet of Nicorandil using hydrophilic cellulose and pHindependent acrylic polymer: in-vitro and in-vivo evaluations. Expert Opin Drug Deliv. 2016;13:315–324. doi: 10.1517/17425247.2016.1118047. [DOI] [PubMed] [Google Scholar]
- 2.Falase B, Easaw J, Youhana A. The role of nicorandil in the treatment of myocardial ischaemia. Expert Opin Pharmacother. 2001;2:845–856. doi: 10.1517/14656566.2.5.845. [DOI] [PubMed] [Google Scholar]
- 3.Singh B, Garg T, Goyal AK, Rath G. Development, optimization, and characterization of polymeric electrospun nanofiber: a new attempt in sublingual delivery of nicorandil for the management of angina pectoris. Artif Cells Nanomed Biotechnol. 2016;44:1498–1507. doi: 10.3109/21691401.2015.1052472. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaiah YS, Al-Saidan SM, Chandrasekhar DV, Satyanarayana V. Controlled in vivo release of nicorandil from a carvone-based transdermal therapeutic system in human volunteers. Drug Deliv. 2006;13:69–77. doi: 10.1080/10717540500309107. [DOI] [PubMed] [Google Scholar]
- 5.Prajapati G, Patel R. Design and in vitro evaluation of novel nicorandil sustained release matrix tablets based on combination of hydrophillic and hydrophobic matrix systems. International Journal of Pharmaceutical Sciences Review and Research. 2010;1:33–38. [Google Scholar]
- 6.Tamilvanan S, Babu VR, Nappinai A, Sivaramakrishnan G. In vitro and in vivo evaluation of hydrophilic and hydrophobic polymers-based nicorandil-loaded peroral tablet compared with its once-daily commercial sustained-release tablet. Drug Development and Industrial Pharmacy. 2011;37:436–445. doi: 10.3109/03639045.2010.521161. [DOI] [PubMed] [Google Scholar]
- 7.Boddupalli BM, Mohammed ZN, Nath RA, Banji D. Mucoadhesive drug delivery system: An overview. J Adv Pharm Technol Res. 2010;1:381–387. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinna Reddy P, Chaitanya KS, Madhusudan Rao Y. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19:385–403. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogstraate JAJ, Wertz PW, Wertz PW. Drug delivery via the buccal mucosa. Pharmaceutical Science Technology Today. 1998;1:309–316. [Google Scholar]
- 10.Mohamed FAA, Roberts M, Seton L, Ford JL, Levina M, Rajabi-Siahboomi AR. Production of extended release mini-tablets using directly compressible grades of HPMC. Drug Dev Ind Pharm. 2013;39:1690–1697. doi: 10.3109/03639045.2012.730524. [DOI] [PubMed] [Google Scholar]
- 11.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. II. Formulation and in-vitro/in-vivo evaluation of buccal mucoadhesive tablets containing water-soluble drugs. Drug Dev Ind Pharm. 2004;30:995–1004. doi: 10.1081/ddc-200037226. [DOI] [PubMed] [Google Scholar]
- 12.Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharm Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 13.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 14.Yamsani VV, Gannu R, Kolli C, Rao ME, Yamsani MR. Development and in vitro evaluation of buccoadhesive carvedilol tablets. Acta Pharm. 2007;57:185–197. doi: 10.2478/v10007-007-0015-7. [DOI] [PubMed] [Google Scholar]
- 15.Vueba ML, Batista de Carvalho LA, Veiga F, Sousa JJ, Pina ME. Influence of cellulose ether mixtures on ibuprofen release: MC25, HPC and HPMC K100M. Pharm Dev Technol. 2006;11:213–228. doi: 10.1080/10837450600561349. [DOI] [PubMed] [Google Scholar]
- 16.Krishna LNV, Kulkarni PK, Dixit M, Lavanya D, Raavi PK. Brief introduction of natural gums, mucilages and their applications in novel drug delivery systems-a review. IJDFR. 2011;2:54–71. [Google Scholar]
- 17.Saha RN, Sajeev C, Sahoo J. A comparative study of controlled release matrix tablets of diclofenac sodium, ciprofloxacin hydrochloride, and theophylline. Drug Deliv. 2001;8:149–154. doi: 10.1080/107175401316906919. [DOI] [PubMed] [Google Scholar]
- 18.Mohamad SA, Abdelkader H, Elrehany M, Mansour HF. Vitamin B12 buccoadhesive tablets: auspicious non-invasive substitute for intra muscular injection: formulation, in vitro and in vivo appraisal [published correction appears in Drug Dev Ind Pharm. 2019;45:x] Drug Dev Ind Pharm. 2019;45:244–251. doi: 10.1080/03639045.2018.1529787. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Garg S, Khar RK. Measurement of bioadhesive strength of mucoadhesive buccal tablets: Design of an in-vitro assembly. Indian Drugs. 1993;30:152–155. [Google Scholar]
- 20.Patel DM, Shah PM, Patel CN. Formulation and evaluation of bioadhesive buccal drug delivery of repaglinide tablets. Asian Journal of Pharmaceutics. 2014;6:171–179. [Google Scholar]
- 21.Daravath B, Swathi D, Rao BB. Formulation Development and Evaluation of Sustained Release Bioadhesive Bilayered Buccal Tablets of Furosemide. Analytical Chemistry Letters. 2017;7:215–277. [Google Scholar]
- 22.Biswal B, Karna N, Bhavsar B. Formulation and Evaluation of Repaglinide Buccal Tablet: Ex Vivo Bioadhesion Study and Ex Vivo Permeability Study. Journal of Applied Pharmaceutical Science. 2014;4:96–103. [Google Scholar]
- 23.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 24.Malaterre V, Ogorka J, Loggia N, Gurny R. Evaluation of the tablet core factors influencing the release kinetics and the loadability of push-pull osmotic systems. Drug Dev Ind Pharm. 2009;35:433–439. doi: 10.1080/03639040802425230. [DOI] [PubMed] [Google Scholar]
- 25.Simionato LD, Petrone L, Baldut M, Bonafede SL, Segall AI. Comparison between the dissolution profiles of nine meloxicam tablet brands commercially available in Buenos Aires, Argentina. Saudi Pharm J. 2018;26:578–584. doi: 10.1016/j.jsps.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


