Abstract
Lactic acid bacteria (LAB) ferment plants, fish, meats and milk and turn them into tasty food products with increased shelf life; other LAB help digesting food and create a healthy environment in the intestine. The economic and societal importance of these relatively simple and small bacteria is immense. In this review we hope to show that their adaptations to nutrient-rich environments provides fascinating and often puzzling behaviours that give rise to many fundamental evolutionary biological questions in need of a systems biology approach. We will provide examples of such questions, compare the (metabolic) behaviour of LAB to that of other model organisms, and provide the latest insights, if available.
Keywords: Lactic acid bacteria, Lactococcus, Lactobacillus, Food fermentation, Systems biology, Biotechnology
Highlights
-
•
Lactic acid bacteria (LAB) are champions in nutrient-rich environments such as foods.
-
•
Their adaptations raise inspiring questions for systems biology.
-
•
Nutrient-rich environments introduce auxotrophies but how and why is not yet fully understood.
-
•
Ecosystems dynamics and diversity are key to application but poorly understood.
-
•
Lactate as overflow product still needs proper mechanistic and evolutionary explanations.
Introduction
With over 100 billion Euros annually [1], the economic value of foods fermented by such small bacteria – LAB have genome sizes of only 2–3 Mbp-is impressive. Yogurt, cheese and sauerkraut, but also breads, hams, and olives, or pickles, soy- and fish sauce require the metabolic activities of LAB. Many of these foods have a long history, and the associated industry is rather traditional and wary of revolutionary changes in production processes, let alone genetic modifications to improve traits. Rather, the industry takes advantage of the enormous diversity in species and strains with different functionalities, such as flavour production profiles or texturing properties. Furthermore, one may change pH and temperature a bit, or change some ingredients perhaps.
It is also not so easy to decide what to change, or how to change it: foods are chemically complex and undefined, and often the fermentation is not carried out by a single strain, but a complex mixture of LABs. Finally, analyses in sticky, solid and inhomogeneous food matrices can be tedious, and they often are. Therefore, in contrast to the industrial biotechnology field that produces biobased chemicals, largely on the basis of monocultures in relatively well-defined growth media, research in LAB has much less adopted engineering and systems approaches – although metabolic engineering activities in LAB are ongoing 2, 3, 4.
We believe this is a pity, from both sides: Systems biology has a lot to offer to the LAB field, and the LABs have a lot to offer to the systems biology field. LABs provide questions, challenges, and biological examples of adaptations to environments rich in nutrients and full of stress [5]. They can offer interesting and relevant cases to test the generality of findings in other, better studied, model organisms, Escherichia coli in particular. Systems biology on the other hand, can provide structure to, and understanding of, the complex systems comprising LAB. In this review, we will describe some features of LAB physiology that we believe are interesting from a systems biology perspective, and we will describe our current understanding and open questions. We hope this will attract more systems biologists to these fascinating microorganisms.
Metabolic adaptation to rich nutrient environments: auxotrophies and division of labour
Environments in which LAB thrive are rich in sugars and protein; fats, vitamins and nucleotides are also often available. Consequently, most LAB are auxotrophic for a large number of amino acids and vitamins. The loss of function in the presence of some specific nutrients may be caused by genetic drift or provides a selective advantage. Recent works make strong cases for the latter. It was shown experimentally in E. coli that introduction of auxotrophies and subsequent exchange of amino acids provided the auxotrophic mutant an increased growth rate over the wildtype [6]. These traits are evolvable in laboratory evolution experiments and make the mutants depend on cross-feeding [7].
The differences between LAB species and even strains from the same species, however, is large and although it was explained in general by niche adaptation for Lactococcus lactis strains [8], the diversity in amino acid metabolism and corresponding auxotrophies remains puzzling as the environments in which LAB are isolated often appear equally rich in amino acids, peptides and/or proteins. This matters for LAB applications as their catabolic products are flavour compounds [9], or bitter or health-promoting [10] peptides. The physiological role of such catabolism is not always clear: its wide spread occurrence may be a consequence of our selection for flavour production, but it may also contribute to stress and energy metabolism [11], chemical warfare, host–microbe interactions [12] or possibly geographic spreading through attracted insects.
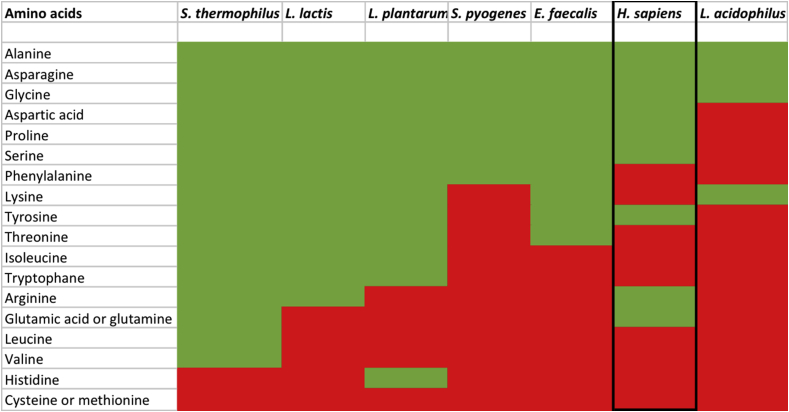
In Table 1 the experimentally-determined auxotrophies for amino acids are shown for a number of representatives of LAB species. However, defining such auxotrophies is nontrivial by dependencies on the presence of other nutrients: also in humans the dichotomy between essential and non-essential amino acids was questioned and condition-dependent essential amino acids introduced [13]. For example, if glutamine is present, glutamate is not required, but almost all LAB require either glutamine or glutamate, because they cannot synthesize its precursor α-ketoglutarate de novo, by lack of a complete TCA cycle [11]. Alternatively, the presence of some amino acids can provide feedback inhibition on the synthesis of another amino acid. This happens e.g. for aromatic and branched chain amino acids [14]. Therefore, auxotrophies predicted from genome-scale metabolic models require careful experimental validation, as recently done extensively for Enterococcus faecalis [15] and Streptococcus pyogenes [16].
Table 1.
Amino acid requirement for selected LAB and man (in black box). Green indicates that growth is possible (but often at a lower rate) in the absence of the amino acid, red means no or extremely poor growth is observed. Based on studies of specific strains of S.t. [18], L. l. [19], L.p. [14], S.p. [16], E.f. [16], H.s. [13] and L.a. [17]. Note that auxotrophies between different strains of the same species can differ.
The similarities between auxotrophies of LAB species and humans perhaps suggest an underlying cause or constraint. Methionine or cysteine are always required, as is histidine (except for Lactobacillus plantarum); Aromatic and branched chain amino acids, especially valine, are often needed for growth, or at least for fast growth [14]. Why these amino acids? Are these the most expensive to make, or require complex co-factors or vitamins, and are the genes therefore most easily lost? A comparative study in lactobacilli showed a great diversity for different amino acids in true loss of biosynthetic genes and loss of activity by mutations [17]. However, the growth of all tested lactobacilli could not be restored without glutamate, even after mutagenesis and selective plating. The collective loss by LAB of the ability to synthesize such a key amino acid as glutamate is mysterious.
From diversity in auxotrophies to microbial ecology
Quite a number of lactobacilli that express proteases and thus can rely on proteolysis, lost the ability to synthesize or interconvert many more amino acids. As a result, excess amino acids are excreted that depend on the protein composition, protease specificity and the amino acid interconversion metabolism of the protease positive strain. This in turn may provide specific amino acid niches for other bacteria. Some LAB-residents of the human gut even specialized into growth on peptides derived from gastric proteolysis [20]. And so, perhaps the specific auxotrophies we observe today are the result of co-evolution dynamics and testimonies of diversities arising by chance and necessity [21] in complex ecosystems. Systems biology may help explain the necessity [22].
Auxotrophies introduce diversity and dependencies that result in ecological interactions such as cross feeding, which was readily observed in spatiotemporal models of bacterial communities that mimic the gut [23]. The gut may be too complex and experimentally inaccessible, however, for deeper quantitative systems approaches. Many consortia of LAB (sometimes with yeasts) that turn milk into yogurt or cheese, or wheat into sour-bread, are of a more manageable complexity, between two species for yogurt and in the order of a hundred for cheese and kefir (Kiran Patil, personal communication) cultures. Moreover, many of these consortia are stable as they are formed by long histories of back-swapping, and their fitness may have been optimized 24, 25, making them interesting model systems for systems-level studies of ecosystem functioning.
One such study pointed to other causes for community diversity beyond metabolic interactions. For cheese starter cultures, the dynamics of strain frequencies during serial cultivation in milk was followed [26], and genetic heterogeneity in the culture was stabilized by frequency-dependent phage predation, coined a “kill-the-winner” mechanism – possibly a specific case of frequency-dependent predation known in ecology. This mechanism prevents one lineage to take over the population. Resistance to phages -or bacteriocins, naturally produced antibiotics-introduce another layer of interactions that is extremely relevant for food production and evoke fundamental scientific questions, from molecular mechanistic [27] to evolutionary [28]. The function of Crispr as a phage immune response was discovered in a LAB: Streptococcus thermophilus [29].
Metabolic adaptation to rich nutrient environments: cheating and overflow
Proteases appear useful in protein-rich environments: indeed, providing a L. lactis plant isolate with casein (milk protein) protease increased fitness substantially. Yet, subsequent laboratory evolution experiments in milk showed a loss of the protease and a decrease in fitness [30]. The trait is evolutionary unstable because of cheaters that profit from the peptides released by the efforts of others. The loss of protease activity can be prevented by cultivation at low densities. Extracellular sucrose hydrolysis by invertase expression in yeast showed a similar instability [31]. These examples demonstrate the need to consider the strategies of competitors, and to seriously consider “the tragedy of the commons”, i.e. the idea that common goods drive individuals to behaviours that are good for themselves, but not for the population as a whole [32].
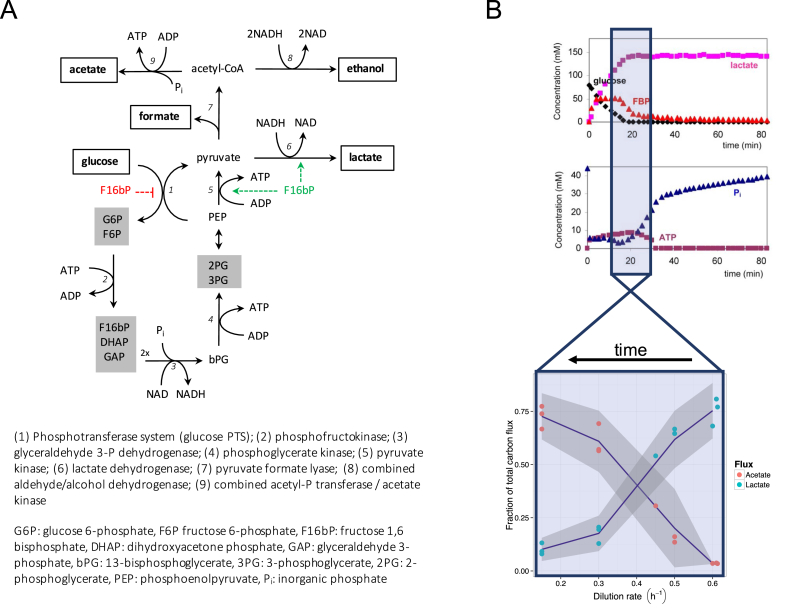
This brings us to the metabolic activity that gave LAB their name: the fermentation of sugars to lactic acid. For many LAB, lactate is the only major catabolic byproduct, but for many others, alternative fermentation routes exist. Of these “mixed acid fermentation” whereby a mixture of acetate, formate and ethanol is produced is the most prevalent case (Figure 1A). Lactate fermentation extracts only 2 ATP per glucose, whereas mixed acid fermentation does slightly better with 3 ATP per glucose. L. lactis shows the high-ATP yield mixed acid fermentation strategy at low growth rates, and switches to lactate formation as the growth rate increases [33]. This is reminiscent of the Crabtree effect in yeast [35], or of overflow metabolism E coli [36]. Thus, production of lactate, associated with fast and wasteful growth, seems the result of a tragedy of the commons. Indeed, when we privatized the common sugar by propagation in water-in-oil emulsions, we could select L. lactis mutants that switched to mixed acid fermentation [37]. The mechanism by which mutants achieved this switch, contains an interesting general lesson: we found mutations in the glucose transport system that we hypothesize create an internal state that mimics a low-glucose environment. Such internal states can subsequently drive metabolic regulation, either through allosteric interactions or gene expression (see Refs. 38, ∗∗39 for recent examples in E. coli, or [40] in L. lactis).
Figure 1.
Fermentation pathways in L. lactis to lactate or mixed acid fermentation. (A) Overview of the biochemical reactions, with external metabolites in white boxes, lumped metabolite pools in grey boxes, and positive (green) and negative (red) regulation by F16bP. (B) Is the metabolic regulation of acetate versus lactate observed in the chemostat (lower figure, based on [33]) a series of still pictures from a normally fast transition to glucose depletion (upper figure, based on [34])? During a brief period of glucose depletion, growth rate decreases rapidly, and a shift of lactate towards acetate production takes place.
In E. coli, it was recently shown that differences in “proteome-efficiency” can explain overflow metabolism [36]. Indeed, the perspective of (optimal 25, 41) allocation of limited resources has appeared as a powerful determinant of regulatory strategies 42, 43. The central idea is that protein is costly [44] and ATP-efficient pathways cost more resources to implement, which at higher growth rates becomes too large a burden and drives cells to switch to cheaper and less ATP-efficient pathways ∗∗36, 42. We tested this idea by a careful characterisation of the metabolic switch in L. lactis, and found that the proteome hardly changed with growth rate [33]. Glycolytic enzyme levels remained largely constant despite a more than threefold increase in flux. The apparent overcapacity of glycolytic enzymes at the lower growth rates, also observed for Bacillus subtilis [45] and yeast [46], is difficult to reconcile with optimal proteome management. We performed prolonged chemostat cultivation studies at different growth rates, and found that expression of membrane-associated processes was specifically affected, indicating that under these conditions uptake of nutrient, rather than its further intracellular processing, limits growth [Claire Price, Filipe Branco dos Santos, unpublished]. Genome-scale metabolism and expression modeling in E. coli also suggested a distinction between nutrient (or uptake) limitation at low growth rates, and proteome limitation at high growth rates [47].
What remains puzzling, however, is that in the absence of an apparent proteome limitation, L. lactis still switches to lactic acid formation as growth rate increases [33]. This strongly suggests a dominant metabolic regulation, but why? Structural kinetic modeling of L. lactis central metabolism hinted at a negative control of the acetate branch on the glycolytic flux, through its impact on NADH levels [48]. How this subsequently affects growth rate and fitness is unclear, however, but glycolytic flux as biological objective does fit with an old observation that the glycolytic flux in L. lactis appears always on, independent from ATP demand [49]. Alternatively, we should also keep in mind that resource allocation and the associated bacterial growth laws are steady state relationships. At constant conditions, metabolic regulation is not needed and costly, which points to a role under dynamic conditions. Recent studies have shown how important it is to properly respond to extracellular dynamics [50], and how cellular heterogeneity in such responses can create subpopulations of responders and non-responders, in L. lactis [51], but also E. coli [52] and Saccharomyces cerevisiae [53]. If LAB have adapted to conditions of feast and famine, with relatively fast and frequent transitions between them, metabolic regulation to rapidly switch fluxes may make sense. One idea (Frank Bruggeman, personal communication), is that the titration of growth rate through the dilution rate in the chemostat may provide artificial steady-state still pictures of what normally is a brief transition for the organism (Figure 1B).
The molecular mechanism by which metabolic regulation achieves the switch between mixed acid and lactate fermentation, is also not yet resolved, despite much research in the (distant) past. Complexity arises from the involvement of moiety conservations and couplings between enzyme activities through the cofactors NADH/NAD and ATP/ADP [54]. But similar perhaps to what has been suggested for yeast [55] and E. coli [56], regulation by fructose 1,6-bisphosphate seems important for the switch. F16bP activates lactate dehydrogenase of L. lactis, E. faecalis and S. pyogenes, but not that of L. plantarum [57]; suggestively, only the latter organism does not switch to acetate production at low dilution rate [58]. This activation by F16bP is different from that on pyruvate kinase, observed from LAB to man: in L. lactis the current suggestion from computational studies is that feedforward activation ensures robustness to feast-famine dynamics [48], possibly through maintaining adequate phosphoenol pyruvate levels [50], the substrate required for sugar uptake and phosphorylation.
Conclusion
We have given a brief overview of a number of what we suggest are adaptive behaviours of lactic acid bacteria to their rich nutrient environments: a fascinating mix of chemical warfare, cheating, robustness and rat races. We focused on current open scientific questions and our latests insights, and on the commonalities and differences with other model organisms that are more often studied in the systems biology field. The models and advanced molecular and functional genomics tools are available [59].
Acknowledgements
We thank prof J Ellers for useful comments. LAB-related work in our group is supported by STW grant 13858, TI Food and Nutrition project 16MF01, EraSysApp project SysMilk: NWO 832.14.001 and the ITN MicroWine: Horizon2020 grant 643031.
This review comes from a themed issue on Systems biology of model organisms (2017)
Edited by Jens Nielsen and Kiran Raosaheb Patil
References
- 1.de Vos W.M. Systems solutions by lactic acid bacteria: from paradigms to practice. Microb Cell Factories. 2011;10:S2. doi: 10.1186/1475-2859-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauer M., Russmayer H., Grabherr R., Peterbauer C.K., Marx H. The efficient clade: lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017 doi: 10.1016/j.tibtech.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Petersen K.V., Liu J., Chen J., Martinussen J., Jensen P.R., Solem C. Metabolic characterization and transformation of the non-dairy Lactococcus lactis strain KF147, for production of ethanol from xylose. Biotechnol J. 2017 doi: 10.1002/biot.201700171. [DOI] [PubMed] [Google Scholar]
- 4.Song A.A.-L., In L.L.A., Lim S.H.E., Rahim R.A. A review on Lactococcus lactis: from food to factory. Microb Cell Factories. 2017;16:55. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadimitriou K., Alegría Á., Bron P.A., de Angelis M., Gobbetti M., Kleerebezem M., Lemos J.A., Linares D.M., Ross P., Stanton C. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev. 2016;80:837–890. doi: 10.1128/MMBR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pande S., Merker H., Bohl K., Reichelt M., Schuster S., de Figueiredo L.F., Kaleta C., Kost C. Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J. 2014;8:953–962. doi: 10.1038/ismej.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza G., Kost C. Experimental evolution of metabolic dependency in bacteria. PLoS Genet. 2016;12:e1006364. doi: 10.1371/journal.pgen.1006364. [DOI] [PMC free article] [PubMed] [Google Scholar]; Simple but effective set of laboratory evolution experiments in the presence and absence of exogenous amino acids show that auxotrophies and cross feeding are selected for.
- 8.Kelleher P., Bottacini F., Mahony J., Kilcawley K.N., van Sinderen D. Comparative and functional genomics of the Lactococcus lactis taxon; insights into evolution and niche adaptation. BMC Genom. 2017;18:267. doi: 10.1186/s12864-017-3650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M., Nauta A., Francke C., Siezen R.J. Comparative genomics of enzymes in flavor-forming pathways from amino acids in lactic acid bacteria. Appl Environ Microbiol. 2008;74:4590–4600. doi: 10.1128/AEM.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai A.K., Sanjukta S., Jeyaram K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit Rev Food Sci Nutr. 2017;57:2789–2800. doi: 10.1080/10408398.2015.1068736. [DOI] [PubMed] [Google Scholar]
- 11.Fernández M., Zúñiga M. Amino acid catabolic pathways of lactic acid bacteria. Crit Rev Microbiol. 2006;32:155–183. doi: 10.1080/10408410600880643. [DOI] [PubMed] [Google Scholar]
- 12.Goffin P., van de Bunt B., Giovane M., Leveau J.H.J., Höppener-Ogawa S., Teusink B., Hugenholtz J. Understanding the physiology of Lactobacillus plantarum at zero growth. Mol Syst Biol. 2010;6:413. doi: 10.1038/msb.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeds P.J. Dispensable and indispensable amino acids for humans. J Nutr. 2000;130:1835S–1840S. doi: 10.1093/jn/130.7.1835S. [DOI] [PubMed] [Google Scholar]
- 14.Teusink B., van Enckevort F.H.J., Francke C., Wiersma A., Wegkamp A., Smid E.J., Siezen R.J. In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: comparing predictions of nutrient requirements with those from growth experiments. Appl Environ Microbiol. 2005;71:7253–7262. doi: 10.1128/AEM.71.11.7253-7262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veith N., Solheim M., van Grinsven K.W.A., Olivier B.G., Levering J., Grosseholz R., Hugenholtz J., Holo H., Nes I., Teusink B. Using a genome-scale metabolic model of Enterococcus faecalis V583 to assess amino acid uptake and its impact on central metabolism. Appl Environ Microbiol. 2015;81:1622–1633. doi: 10.1128/AEM.03279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levering J., Fiedler T., Sieg A., van Grinsven K.W.A., Hering S., Veith N., Olivier B.G., Klett L., Hugenholtz J., Teusink B. Genome-scale reconstruction of the Streptococcus pyogenes M49 metabolic network reveals growth requirements and indicates potential drug targets. J Biotechnol. 2016;232:25–37. doi: 10.1016/j.jbiotec.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Morishita T., Deguchi Y., Yajima M., Sakurai T., Yura T. Multiple nutritional requirements of lactobacilli: genetic lesions affecting amino acid biosynthetic pathways. J Bacteriol. 1981;148:64–71. doi: 10.1128/jb.148.1.64-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastink M.I., Teusink B., Hols P., Visser S., de Vos W.M., Hugenholtz J. Genome-scale model of Streptococcus thermophilus LMG18311 for metabolic comparison of lactic acid bacteria. Appl Environ Microbiol. 2009;75:3627–3633. doi: 10.1128/AEM.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flahaut N.A.L., Wiersma A., van de Bunt B., Martens D.E., Schaap P.J., Sijtsma L., Dos Santos V.A.M., de Vos W.M. Genome-scale metabolic model for Lactococcus lactis MG1363 and its application to the analysis of flavor formation. Appl Microbiol Biotechnol. 2013;97:8729–8739. doi: 10.1007/s00253-013-5140-2. [DOI] [PubMed] [Google Scholar]
- 20.Arakawa K., Matsunaga K., Takihiro S., Moritoki A., Ryuto S., Kawai Y., Masuda T., Miyamoto T. Lactobacillus gasseri requires peptides, not proteins or free amino acids, for growth in milk. J Dairy Sci. 2015;98:1593–1603. doi: 10.3168/jds.2014-8860. [DOI] [PubMed] [Google Scholar]
- 21.Pál C., Papp B., Lercher M.J., Csermely P., Oliver S.G., Hurst L.D. Chance and necessity in the evolution of minimal metabolic networks. Nature. 2006;440:667–670. doi: 10.1038/nature04568. [DOI] [PubMed] [Google Scholar]
- 22.Zelezniak A., Andrejev S., Ponomarova O., Mende D.R., Bork P., Patil K.R. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc Natl Acad Sci U S A. 2015;112:6449–6454. doi: 10.1073/pnas.1421834112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MJA van Hoek, Merks R.M.H. Emergence of microbial diversity due to cross-feeding interactions in a spatial model of gut microbial metabolism. BMC Syst Biol. 2017;11:56. doi: 10.1186/s12918-017-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottstein W., Olivier B.G., Bruggeman F.J., Teusink B. Constraint-based stoichiometric modelling from single organisms to microbial communities. J R Soc Interface. 2016:13. doi: 10.1098/rsif.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towbin B.D., Korem Y., Bren A., Doron S., Sorek R., Alon U. Optimality and sub-optimality in a bacterial growth law. Nat Commun. 2017;8:14123. doi: 10.1038/ncomms14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkus O., de Jager V.C.L., Spus M., van Alen-Boerrigter I.J., van Rijswijck I.M.H., Hazelwood L., Janssen P.W.M., van Hijum S.A.F.T., Kleerebezem M., Smid E.J. Multifactorial diversity sustains microbial community stability. ISME J. 2013;7:2126–2136. doi: 10.1038/ismej.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]; Population dynamics study of a cheese starter culture (a mixture of LAB strains added to milk to start the cheese making process) that identifies phage sensitivity as a major driver in maintaining genetic diversity.
- 27.Mahony J., McDonnell B., Casey E., van Sinderen D. Phage-host interactions of cheese-making lactic acid bacteria. Annu Rev Food Sci Technol. 2016;7:267–285. doi: 10.1146/annurev-food-041715-033322. [DOI] [PubMed] [Google Scholar]
- 28.Ferenci T. Trade-off mechanisms shaping the diversity of bacteria. Trends Microbiol. 2016;24:209–223. doi: 10.1016/j.tim.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 30.Bachmann H., Molenaar D., Kleerebezem M., van Hylckama Vlieg J.E.T. High local substrate availability stabilizes a cooperative trait. ISME J. 2011;5:929–932. doi: 10.1038/ismej.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gore J., Youk H., van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Goel A., Eckhardt T.H., Puri P., de Jong A., Branco Dos Santos F., Giera M., Fusetti F., de Vos W.M., Kok J., Poolman B. Protein costs do not explain evolution of metabolic strategies and regulation of ribosomal content: does protein investment explain an anaerobic bacterial Crabtree effect? Mol Microbiol. 2015;97:77–92. doi: 10.1111/mmi.13012. [DOI] [PubMed] [Google Scholar]; Careful quantitative study of the shift from mixed acid to lactate fermentation in the chemostat, showing at the level of mRNA, protein and enzyme activity that flux increases over threefold without appreciable changes in corresponding protein level.
- 34.Neves A.R., Ventura R., Mansour N., Shearman C., Gasson M.J., Maycock C., Ramos A., Santos H. Is the glycolytic flux in Lactococcus lactis primarily controlled by the redox charge? Kinetics of NAD(+) and NADH pools determined in vivo by 13C NMR. J Biol Chem. 2002;277:28088–28098. doi: 10.1074/jbc.M202573200. [DOI] [PubMed] [Google Scholar]
- 35.van Dijken J.P., Weusthuis R.A., Pronk J.T. Kinetics of growth and sugar consumption in yeasts. Ant Van Leeuwenhoek. 1993;63:343–352. doi: 10.1007/BF00871229. [DOI] [PubMed] [Google Scholar]
- Basan M., Hui S., Okano H., Zhang Z., Shen Y., Williamson J.R., Hwa T. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature. 2015;528:99–104. doi: 10.1038/nature15765. [DOI] [PMC free article] [PubMed] [Google Scholar]; A set of elegant quantitative perturbation studies are interpreted with phenomenological bacterial growth laws to show that respiration costs more protein per ATP than overflow metabolism, explaining the latter phenomenon as a resource allocation strategy.
- 37.Bachmann H., Fischlechner M., Rabbers I., Barfa N., Branco Dos Santos F., Molenaar D., Teusink B. Availability of public goods shapes the evolution of competing metabolic strategies. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1308523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You C., Okano H., Hui S., Zhang Z., Kim M., Gunderson C.W., Wang Y.-P., Lenz P., Yan D., Hwa T. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature. 2013;500:301–306. doi: 10.1038/nature12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanowski K., Gerosa L., Brunner S.F., Christodoulou D., Nikolaev Y.V., Sauer U. Few regulatory metabolites coordinate expression of central metabolic genes in Escherichia coli. Mol Syst Biol. 2017;13:903. doi: 10.15252/msb.20167402. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper combines promoter activity and metabolomics to conclude that the coordination of gene expression seems relievingly simple. For a theoretical underpinning, see bioRxiv 115428; doi: https://doi.org/10.1101/115428.
- 40.Hove-Jensen B., Andersen K.R., Kilstrup M., Martinussen J., Switzer R.L., Willemoës M. Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization, and metabolic significance. Microbiol Mol Biol Rev MMBR. 2017:81. doi: 10.1128/MMBR.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosdriesz E., Molenaar D., Teusink B., Bruggeman F.J. How fast-growing bacteria robustly tune their ribosome concentration to approximate growth-rate maximization. FEBS J. 2015 doi: 10.1111/febs.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molenaar D., van Berlo R., de Ridder D., Teusink B. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol. 2009;5:323. doi: 10.1038/msb.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott M., Gunderson C.W., Mateescu E.M., Zhang Z., Hwa T. Interdependence of cell growth and gene expression: origins and consequences. Science. 2010;330:1099–1102. doi: 10.1126/science.1192588. [DOI] [PubMed] [Google Scholar]
- Kafri M., Metzl-Raz E., Jona G., Barkai N. The cost of protein production. Cell Rep. 2016;14:22–31. doi: 10.1016/j.celrep.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegant study in yeast with reporter constructs to address whether costs of making a protein are in transcription or translation; this, it turned out, depends on the conditions.
- 45.Chubukov V., Uhr M., Le Chat L., Kleijn R.J., Jules M., Link H., Aymerich S., Stelling J., Sauer U. Transcriptional regulation is insufficient to explain substrate-induced flux changes in Bacillus subtilis. Mol Syst Biol. 2013;9:709. doi: 10.1038/msb.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solis-Escalante D., Kuijpers N.G.A., Barrajon-Simancas N., van den Broek M., Pronk J.T., Daran J.-M., Daran-Lapujade P. A minimal set of glycolytic genes reveals strong redundancies in Saccharomyces cerevisiae central metabolism. Eukaryot Cell. 2015;14:804–816. doi: 10.1128/EC.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E.J., Lerman J.A., Chang R.L., Hyduke D.R., Palsson B.Ø. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol Syst Biol. 2013;9:693. doi: 10.1038/msb.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]; The capabilities of a ME model of E. coli were demonstrated, showing for the first time how augmenting metabolic networks with protein costs can enhance their biological interpretation and capabilities, including overflow metabolism.
- 48.Murabito E., Verma M., Bekker M., Bellomo D., Westerhoff H.V., Teusink B., Steuer R. Monte-Carlo modeling of the central carbon metabolism of Lactococcus lactis: insights into metabolic regulation. PLoS One. 2014;9:e106453. doi: 10.1371/journal.pone.0106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koebmann B.J., Solem C., Pedersen M.B., Nilsson D., Jensen P.R. Expression of genes encoding F(1)-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl Environ Microbiol. 2002;68:4274–4282. doi: 10.1128/AEM.68.9.4274-4282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolatshahi S., Fonseca L.L., Voit E.O. New insights into the complex regulation of the glycolytic pathway in Lactococcus lactis. II. Inference of the precisely timed control system regulating glycolysis. Mol Biosyst. 2015;12:37–47. doi: 10.1039/c5mb00726g. [DOI] [PubMed] [Google Scholar]
- 51.Solopova A., van Gestel J., Weissing F.J., Bachmann H., Teusink B., Kok J., Kuipers O.P. Bet-hedging during bacterial diauxic shift. Proc Natl Acad Sci U S A. 2014;111:7427–7432. doi: 10.1073/pnas.1320063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotte O., Volkmer B., Radzikowski J.L., Heinemann M. Phenotypic bistability in Escherichia coli's central carbon metabolism. Mol Syst Biol. 2014;10:736. doi: 10.15252/msb.20135022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Heerden J.H., Wortel M.T., Bruggeman F.J., Heijnen J.J., Bollen Y.J.M., Planqué R., Hulshof J., O'Toole T.G., Wahl S.A., Teusink B. Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science. 2014;343:1245114. doi: 10.1126/science.1245114. [DOI] [PubMed] [Google Scholar]
- 54.Christensen C.D., Hofmeyr J.-H.S., Rohwer J.M. Tracing regulatory routes in metabolism using generalised supply-demand analysis. BMC Syst Biol. 2015;9:89. doi: 10.1186/s12918-015-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Heerden J.H., Bruggeman F.J., Teusink B. Multi-tasking of biosynthetic and energetic functions of glycolysis explained by supply and demand logic. BioEssays News Rev Mol Cell Dev Biol. 2015;37:34–45. doi: 10.1002/bies.201400108. [DOI] [PubMed] [Google Scholar]
- 56.Kochanowski K., Volkmer B., Gerosa L., Haverkorn van Rijsewijk B.R., Schmidt A., Heinemann M. Functioning of a metabolic flux sensor in Escherichia coli. Proc Natl Acad Sci U S A. 2013;110:1130–1135. doi: 10.1073/pnas.1202582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman-Salit A., Hering S., Messiha H.L., Veith N., Cojocaru V., Sieg A., Westerhoff H.V., Kreikemeyer B., Wade R.C., Fiedler T. Regulation of the activity of lactate dehydrogenases from four lactic acid bacteria. J Biol Chem. 2013;288:21295–21306. doi: 10.1074/jbc.M113.458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teusink B., Wiersma A., Molenaar D., Francke C., de Vos W.M., Siezen R.J., Smid E.J. Analysis of growth of Lactobacillus plantarum WCFS1 on a complex medium using a genome-scale metabolic model. J Biol Chem. 2006;281:40041–40048. doi: 10.1074/jbc.M606263200. [DOI] [PubMed] [Google Scholar]
- Grosseholz R., Ching-Chiek K., Veith N., Fiedler T., Strauss M., Olivier B.G., Collins B.C., Schubert O.T., Bergman F.T., Kreikemeyer B. Integrating highly quantitative proteomics and genome-scale metabolic modeling to study pH adaptation in the human pathogen Enterococcus faecalis. NPJ Syst Biol Appl. 2016 doi: 10.1038/npjsba.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive time-resolved proteomics data after a pH challenge that was integrated in a genome-scale metabolic model.