This phase 2 randomized clinical trial compares the efficacy of eribulin plus pembrolizumab vs eribulin alone in hormone receptor–positive, ERBB2-negative metastatic breast cancer.
Key Points
Question
Does the addition of pembrolizumab to eribulin improve efficacy compared with eribulin alone in patients with hormone receptor–positive/ERBB2-negative metastatic breast cancer?
Findings
In this phase 2 randomized clinical trial that included 88 patients, the median progression-free survival was 4.1 months for patients receiving pembrolizumab and eribulin vs 4.2 months for patients receiving eribulin alone.
Meaning
The results do not support the use of pembrolizumab in combination with eribulin for patients with hormone receptor–positive/ERBB2-negative metastatic breast cancer, independent of programmed cell death ligand 1 status.
Abstract
Importance
Prior studies have shown that only a small proportion of patients with hormone receptor (HR)–positive metastatic breast cancer (MBC) experience benefit from programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors given as monotherapy. There are data suggesting that activity may be greater with combination strategies.
Objective
To compare the efficacy of eribulin plus pembrolizumab vs eribulin alone in patients with HR-positive, ERBB2 (formerly HER2)–negative MBC.
Design, Setting, and Participants
Multicenter phase 2 randomized clinical trial of patients with HR-positive, ERBB2-negative MBC who had received 2 or more lines of hormonal therapy and 0 to 2 lines of chemotherapy.
Interventions
Patients were randomized 1:1 to eribulin, 1.4 mg/m2 intravenously, on days 1 and 8 plus pembrolizumab, 200 mg/m2 intravenously, on day 1 of a 21-day cycle or eribulin alone. At time of progression, patients in the eribulin monotherapy arm could cross over and receive pembrolizumab monotherapy.
Main Outcomes and Measures
The primary end point was progression-free survival (PFS). Secondary end points were objective response rate (ORR) and overall survival (OS). Exploratory analyses assessed the association between PFS and PD-L1 status, tumor-infiltrating lymphocytes (TILs), tumor mutational burden (TMB), and genomic alterations.
Results
Eighty-eight patients started protocol therapy; the median (range) age was 57 (30-76) years, median (range) number of prior lines of chemotherapy was 1 (0-2), and median (range) number of prior lines of hormonal therapy was 2 (0-5). Median follow-up was 10.5 (95% CI, 0.4-22.8) months. Median PFS and ORR were not different between the 2 groups (PFS, 4.1 vs 4.2 months; hazard ratio, 0.80; 95% CI, 0.50-1.26; P = .33; ORR, 27% vs 34%, respectively; P = .49). Fourteen patients started crossover treatment with pembrolizumab; 1 patient experienced stable disease. All-cause adverse events occurred in all patients (grade ≥3, 65%) including 2 treatment-related deaths in the combination group, both from immune-related colitis in the setting of sepsis, attributed to both drugs. The PD-L1 22C3 assay was performed on archival tumor samples in 65 patients: 24 (37%) had PD-L1–positive tumors. Analysis indicated that PD-L1 status, TILs, TMB, and genomic alterations were not associated with PFS.
Conclusions and Relevance
In this randomized clinical trial of patients with HR-positive, ERBB2-negative MBC, the addition of pembrolizumab to eribulin did not improve PFS, ORR, or OS compared with eribulin alone in either the intention-to-treat or PD-L1–positive populations. Further efforts to explore the benefits of adding checkpoint inhibition to chemotherapy among less heavily pretreated patients are needed.
Trial Registration
ClinicalTrials.gov Identifier: NCT03051659
Introduction
One major challenge for the clinical development of immunotherapy in hormone receptor (HR)–positive breast cancer is its immunologically cold nature. Relative to other subtypes, HR-positive tumors are associated with lower rates of programmed cell death ligand 1 (PD-L1) positivity,1 lower levels of tumor-infiltrating lymphocytes (TILs),2 and lower median tumor mutational burden (TMB).3
Prior clinical trials testing programmed cell death 1 (PD-1)/PD-L1 inhibitors given as monotherapy in HR-positive metastatic breast cancer (MBC) have shown that only a small proportion of patients experience benefit.4,5 A trial combining chemotherapy with PD-1 inhibition in the preoperative setting demonstrated a near tripling in pathologic complete response rates in patients with HR-positive disease, suggesting that activity may be greater with combination strategies and/or when used in earlier-stage disease.6
Eribulin inhibits microtubule polymerization and is a commonly used chemotherapeutic agent for pretreated MBC.7 Additionally, eribulin has been found to decrease transforming growth factor β (TGF-β) signaling in vivo8,9; therefore, the combination of eribulin and pembrolizumab may exert antitumor immune effects by simultaneously inhibiting TGF-β and the PD-1/PD-L1 axis. A previously conducted phase 1/2 study of eribulin and pembrolizumab in metastatic triple-negative breast cancer (mTNBC) found promising antitumor activity and no new toxic effects regardless of prior lines of chemotherapy or PD-L1 expression.10 Given these encouraging preclinical and clinical data, we developed an investigator-initiated randomized phase 2 study of eribulin with or without pembrolizumab for patients with HR-positive, ERBB2-negative MBC.
Methods
Study Design and Patient Population
This was a randomized, open-label, phase 2 study of eribulin with or without pembrolizumab for patients with HR-positive (estrogen receptor >1% and/or progesterone receptor >1%), ERBB2 (formerly HER2)–negative (per American Society of Clinical Oncology/College of American Pathologists guidelines) MBC treated with 0 to 2 lines of chemotherapy in the advanced-disease setting. In addition, to be eligible, patients were required to have received at least 2 prior lines of endocrine therapy in either the adjuvant or metastatic setting, unless the treating physician believed that they were not appropriate candidates for endocrine therapy. Participants with previously treated brain metastases were eligible if they had completed treatment at least 4 weeks prior to registration. Patients could not have received prior eribulin or PD-1/PD-L1 inhibitor therapy. Ninety patients were randomized (1:1) to eribulin with pembrolizumab or eribulin monotherapy. Patients randomized to the eribulin monotherapy arm had the option to receive pembrolizumab monotherapy at the time of progression. A random number generator was used to randomize patients to each arm, and patients were sequentially registered and allocated to each arm based on order of entry into the study. The study statistician generated the random allocation sequence; the Dana-Farber Cancer Institute Office of Data Quality randomized participants to the intervention, and the study staff enrolled the patients.
The institutional review board at each participating institution approved the study (protocol in Supplement 1), and written informed consent from all participants was provided before study entry. Participating centers were Dana-Farber Cancer Institute, Massachusetts General Hospital, and Beth Israel Deaconess Medical Center, all in Boston, Massachusetts. The study was monitored by the Data Safety Monitoring Board of the Dana-Farber/Harvard Cancer Center. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Procedures
All patients received eribulin mesylate, 1.4 mg/m2, on days 1 and 8 of each 21-day cycle. Patients in the combination arm also received pembrolizumab, 200 mg, on day 1 of each 21-day cycle. Participants randomized to the eribulin monotherapy arm had the option to receive pembrolizumab monotherapy, 200 mg, on day 1 of each 21-day cycle at the time of disease progression as long as they started pembrolizumab treatment within 2 months of progression and did not receive any intervening therapy. Merck & Co provided funding for the study and provided pembrolizumab. Eribulin was provided by Eisai.
Statistical Considerations
The primary end point of this study was progression-free survival (PFS), defined as the time from study randomization to disease progression per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 or death due to any cause, whichever occurred first. Patients alive without disease progression were censored at the date of last disease evaluation. The study was designed to have 83% power to distinguish a 3-month improvement in PFS from 4.1 to 7.1 months (hazard ratio, 0.58) with a 1-sided α of .1. The final analysis was planned when 70 PFS events were observed and was anticipated to occur after a constant accrual over 18 months with 6 months of additional follow-up. Further assumptions of the primary power analysis were a constant hazard of PFS and dropout such that 5% of patients were lost to follow-up at 1 year. This allowed for 1 futility analysis at 35 PFS events with early stopping log-rank statistic below 0. Median PFS months were estimated using the Kaplan-Meier estimation method and compared between treatment arms via log-rank test. Hazard ratios were obtained using Cox proportional hazard models. All tests were 2-sided with a type I error of .05.
Secondary end points included PFS per immune-related RECIST (irRECIST); objective response rate (ORR) per RECIST 1.1; ORR per irRECIST; duration of response; clinical benefit rate, defined as the proportion of patients achieving a complete response, a partial response, or stable disease for 12 or more weeks; overall survival (OS); and safety and tolerability. Secondary end points were reported using point estimates to compare arms and 95% CIs. Demographic characteristics, baseline characteristics, and safety results were summarized descriptively. All analyses were carried out via SAS, version 9.4 (SAS Institute).
Exploratory Objectives
We explored the association of PD-L1 status, TILs, and TMB with outcomes in the whole population. A total of 65 patients (74%) had PD-L1 testing assessed centrally by QualTek using the 22C3 antibody, and results were reported as a modified proportion score (MPS), defined as the proportion of cells, including both tumor and mononuclear inflammatory cells (MICs), located within tumor nests that stain for PD-L1. Positivity for PD-L1 was defined as 1% or greater using the MPS score. The MPS is similar to the combined positive score but not quite synonymous. The MPS scoring scheme does not include the MICs within tumor-induced/tumor-associated stroma as part of the percentage. Rather, reactive MICs within the tumor-induced/tumor-associated stroma are considered for the stromal interface parameter as yes or no for presence or absence only.11 The MPS was used in this study because it was the standard assay for central testing on Merck investigator-initiated trials at the time the trial was conducted.
A total of 58 patients (66%) had a hematoxylin-eosin–stained section available to assess stromal TILs according to the International TIL Working Group guidelines.12 Stromal TILs were quantified as the percentage of stroma within the invasive area covered by mononuclear cells over the total intratumoral stromal area (0%, 1%, 5%, 10%, 15%, 20%, or >20% in 10% increments). All mononuclear cells, including lymphocytes and plasma cells, were scored, and granulocytes and polymorphonuclear leucocytes were excluded. The TIL level was evaluated as a continuous measure and as 2 ordinal levels (≤10% and >10%). The TIL analysis was performed on archival tumor samples—75% from primary tumors or local recurrences and 25% from metastatic tumors.
All patients had neutrophil-lymphocyte ratio (NLR) determined. High NLR was defined as greater than 4 and was used as a discrete variable in the analysis.
Fifty-two cases (59%) had comprehensive genomic profiling using our in-house next-generation sequencing panel (OncoPanel) and had TMB assessed from these results. OncoPanel is performed in a Clinical Laboratory Improvement Amendments–certified laboratory environment and uses targeted exome sequencing to detect copy number alterations, single nucleotide variants, and translocations across the full coding regions and selected intronic regions of a predefined subset of cancer-related genes by using tumor-derived DNA.13,14 The TMB is calculated by determining the number of nonsynonymous somatic mutations that occur per megabase of exonic sequence data across all genes on the panel. High TMB was defined as 10 or more mutations/Mb and was used as a discrete variable in the analysis. OncoPanel was performed on archival tumor samples—54% from primary tumors or local recurrences and 45% from metastatic tumors.
Results
Patient Characteristics
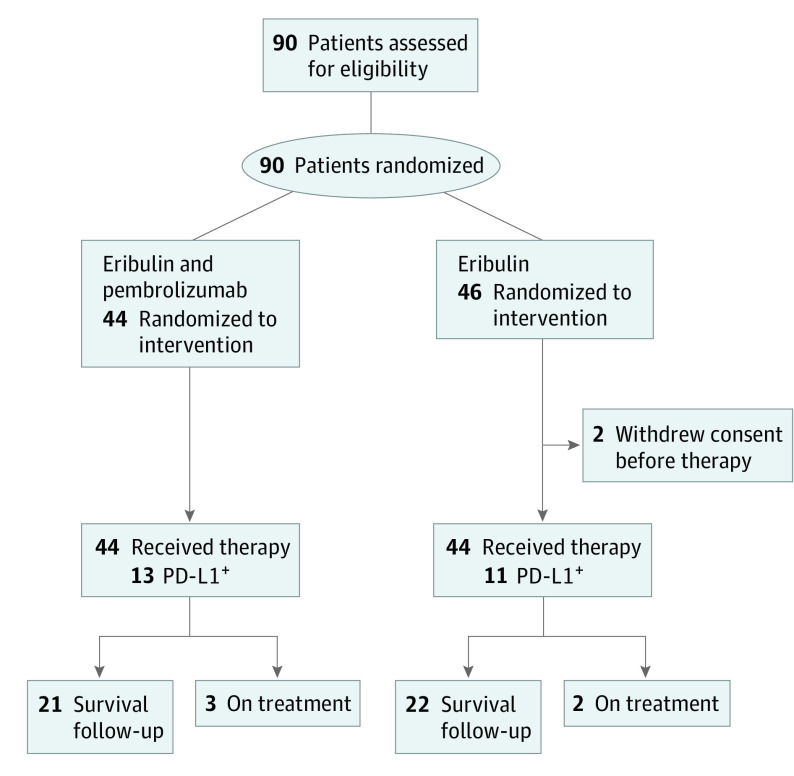
From April 6, 2017, through August 28, 2018, a total of 90 eligible patients were enrolled in this study; 44 patients were randomized to therapy with eribulin and pembrolizumab and 46 to eribulin alone. Two patients randomized to eribulin withdrew consent prior to receipt of treatment. Thus, 88 patients initiated the protocol therapy. The median duration of follow-up at the time of data cutoff was 10.5 (95% CI, 0.4-22.8) months, and 5 patients remained on study treatment (Figure 1).
Figure 1. CONSORT Diagram.
PD-L1 indicates programmed cell death ligand 1.
Baseline characteristics by arm are summarized in Table 1 and eTable 1 in Supplement 2. The median (range) age was 57 (30-76) years. Patients had received a median (range) of 1 (0-2) line of chemotherapy, with 34 patients (39%) treated on study for their first line of chemotherapy, and a median (range) of 2 (0-5) lines of endocrine therapy in the metastatic setting. Approximately 67 patients (76%) had previously received a cyclin-dependent kinase (CDK) 4/6 inhibitor. Most patients had visceral disease, with approximately 63 (72%) having liver metastases. Of the 65 patients who had PD-L1 status available, 24 (37%) had PD-L1–positive tumors (69% tested on primary tumors; 31% tested on metastatic tumors). Of the 58 patients who had TIL counts available, most (81%) had low levels of TILs (≤10%). A total of 46 patients (52%) had an NLR greater than 4. Among the 52 patients with genomic information available, the median TMB was 6 mutations/Mb, and 7 patients (13%) presented with a high TMB. PIK3CA and TP53 were the 2 most common genetic variations in 16 (31%) and 15 (29%) patients, respectively (eFigure 1 in Supplement 2).
Table 1. Patient Characteristics at Baseline.
| Characteristic | No. (%) | |
|---|---|---|
| Eribulin plus pembrolizumab (n = 44) | Eribulin (n = 44) | |
| Age, median (range), y | 58 (30-76) | 57 (37-76) |
| Female | 44 (100) | 43 (98) |
| Male | 0 | 1 (2) |
| Race | ||
| White | 40 (911) | 41 (93) |
| Asian | 2 (5) | 0 |
| Black/African American | 0 | 2 (5) |
| Other/multiple | 2 (5) | 1 (2) |
| ECOG PS | ||
| 0 | 35 (80) | 36 (82) |
| 1 | 9 (21) | 7 (16) |
| 2 | 0 | 1 (2) |
| Lines of chemotherapy for metastatic disease | ||
| Median (range) | 1 (0-2) | 1 (0-2) |
| 0 | 20 (46) | 14 (33) |
| 1 | 15 (34) | 19 (43) |
| 2 | 9 (21) | 11 (25) |
| Prior metastatic chemotherapy | ||
| Taxane | 6 (14) | 5 (11) |
| Capecitabine | 17 (39) | 26 (59) |
| Lines of endocrine therapy for metastatic disease | ||
| Median (range) | 2 (0-4) | 2 (0-5) |
| 0 | 9 (21) | 8 (18) |
| 1 | 11 (25) | 10 (23) |
| 2 | 13 (30) | 10 (23) |
| ≥3 | 11 (25) | 16 (36) |
| Prior CDK4/6 inhibitor | ||
| Yes | 34 (77) | 33 (75) |
| No | 10 (23) | 11 (25) |
| Sites of disease | ||
| Lung | 9 (20) | 17 (39) |
| Liver | 32 (73) | 31 (70) |
| Bone | 36 (82) | 31 (70) |
| Lymph nodes | 19 (43) | 14 (32) |
| CNS | 0 | 1 (2) |
| Bone only | 2 (5) | 3 (7) |
| ER 1%-10% and PR 1%-10% | 2 (5) | 2 (5) |
Abbreviations: CDK, cyclin-dependent kinase; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; PR, progesterone receptor.
Efficacy
The median PFS was 4.1 (95% CI, 3.5-6.2) months in the combination arm and 4.2 (95% CI, 3.7-6.1) months in the eribulin arm (hazard ratio, 0.80; 95% CI, 0.50-1.26; P = .33; Figure 2A), demonstrating no significant difference between the arms. In the PD-L1–positive population (n = 24), the median PFS was 4.2 (95% CI, 1.8-8.4) months in the combination arm and 4.3 (95% CI, 2.8-7.3) months in the eribulin arm (hazard ratio, 0.84; 95% CI, 0.35-2.00; P = .69; Figure 2B). The median PFS according to irRECIST in the combination arm was 5.8 (95% CI, 3.8-8.5) months.
Figure 2. Kaplan-Meier Analysis of Progression-Free Survival (PFS) and Overall Survival (OS).
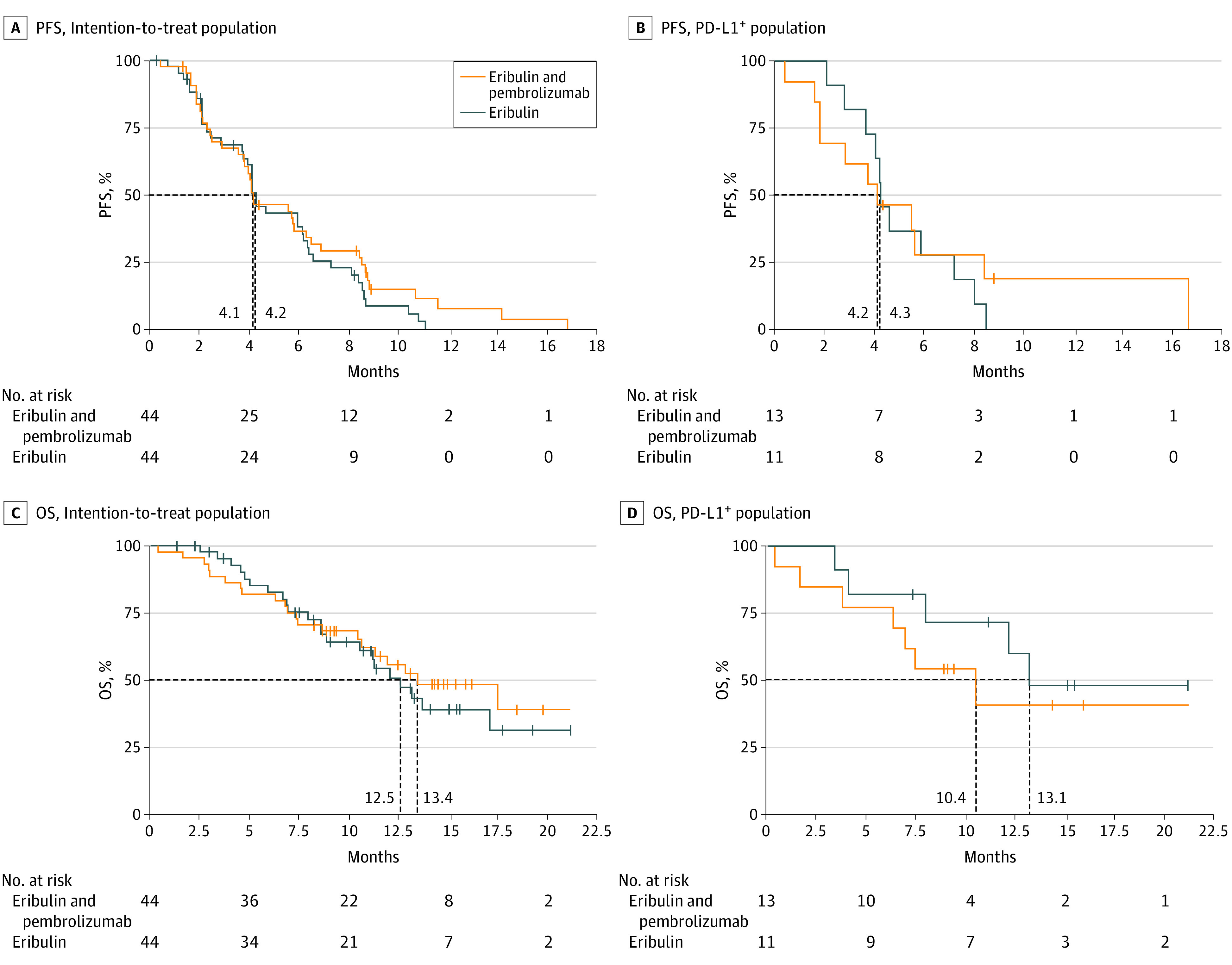
The dashed lines indicate the median PFS for each arm. PD-L1 indicates programmed cell death ligand 1.
There was no statistically significant difference in the ORR between the 2 arms. The ORR was 27% (95% CI, 14.9%-42.8%) for patients receiving eribulin with pembrolizumab and 34% (95% CI, 20.5%-49.9%) for patients receiving eribulin alone. Moreover, there were no complete responses in either arm and no significant difference in duration of response (Table 2).
Table 2. Secondary Efficacy End Points.
| Response (RECIST 1.1) | No. (%) | |
|---|---|---|
| Eribulin plus pembrolizumab (n = 44) | Eribulin (n = 44) | |
| Overall population | ||
| PR | 12 (27) | 15 (34) |
| SD | 19 (43) | 16 (36) |
| SD > 24 wk | 9 (20) | 7 (16) |
| CBR (PR + SD > 24 wk) | 21 (48) | 22 (50) |
| DOR, median (range), mo | 1.5 (0-13.6) | 2.1 (0.2-4.6) |
| PD-L1–positive patients | ||
| PR | 3 (23) | 5 (45) |
| SD | 6 (46) | 5 (45) |
| SD > 24 wk | 2 (15) | 2 (18) |
| CBR (PR + SD > 24 wk) | 5 (39) | 7 (67) |
| DOR, median (range), mo | 0.6 (0-1.0) | 2.1 (1.0-4.6) |
Abbreviations: CBR, clinical benefit rate; DOR, duration of response; PD-L1, programmed cell death ligand 1; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
The OS data are still immature, with 45 patients (51%) in survival follow-up. With the available survival data, there was no statistically significant difference in OS between arms: the median OS was 13.4 (95% CI, 10.4-not available [NA]) months for patients receiving eribulin and pembrolizumab and 12.5 (95% CI, 8.6-NA) months for those receiving eribulin alone (hazard ratio, 0.87; 95% CI, 0.48-1.59; P = .65; Figure 2C). In the PD-L1–positive population, the median OS was 10.4 (95% CI, 3.8-NA) months in the combination arm and 13.1 (95% CI, 4.1-NA) months in the eribulin arm (hazard ratio, 1.59; 95% CI, 0.50-5.06; P = .43; Figure 2D).
Exploratory subset analyses, including clinical characteristics, line of therapy, PD-L1 status, TIL, and NLR, also did not show any specific population with a statistically significant improvement in PFS with the addition of pembrolizumab (eFigure 2 in Supplement 2). For patients with high TMB (n = 7), there was a numerical trend toward greater benefit in the immunotherapy arm (eFigure 2 in Supplement 2).
Genomic alterations were similarly not associated with PFS (eFigure 1 in Supplement 2). The few nonsynonymous variations and more frequent copy number changes in 5 immunotherapy-related genes were also not associated with PFS. Copy deletions of the 3 genes residing on the 9p24.1 locus, PDL1, PDL2, and JAK2, appeared to be more common in patients with shorter PFS, but this association was not significant (P = .38 by log-rank test, no adjustment for multiple comparisons).
Fourteen patients elected to receive crossover treatment with pembrolizumab monotherapy after progression while taking eribulin. At the time of crossover, 11 patients underwent a new biopsy per protocol, and PD-L1 status was assessed. Overall, the median PFS was 1.7 months (eFigure 3 in Supplement 2), with no patient achieving partial response and just 1 patient with stable disease lasting for 4.3 months. Patients with PD-L1–positive (n = 6) and PD-L1–negative (n = 5) tumors had a median PFS of 2.0 (95% CI, 1.2-2.4) months and 0.9 (95% CI, 0-1.9) months, respectively.
Safety
All patients who started treatment on study were evaluable for safety outcomes. Adverse events (AEs), regardless of attribution, occurred in 100% of patients in both arms, and the rate of grade 3 or 4 AEs was 68% in the combination arm and 61% in the eribulin arm (eTable 2 in Supplement 2).
The most common AEs with eribulin plus pembrolizumab in both arms were fatigue (82% vs 71%, respectively), alopecia (55% vs 41%), peripheral neuropathy (53% vs 55%), nausea (50% vs 55%), neutropenia (55% vs 66%), and liver enzyme elevation (39% vs 41%). The most common grade 3 or greater events were neutropenia (37% in both arms), febrile neutropenia (9% vs 14%), and liver enzyme elevation (14% vs 7%) (eTable 3 in Supplement 2).
The most common AEs suggestive of a potential immune-related cause among patients receiving the experimental combination were elevated liver enzymes (39%), rash (30%), and hypothyroidism (14%). Fatal AEs occurred in 2 patients in the combination arm; both developed immune-related colitis, neutropenia, and sepsis (eTable 3 in Supplement 2).
Discussion
This randomized phase 2 study is, to our knowledge, the first to evaluate the efficacy of adding an anti–PD-1 agent to chemotherapy compared with chemotherapy alone in patients with HR-positive, ERBB2-negative MBC. The combination of pembrolizumab and eribulin was not associated with an improvement in any efficacy outcome, including PFS, ORR, or OS, compared with eribulin alone in this population. Additionally, exploratory analyses found no association of PD-L1, TILs, TMB, or genomic alterations with PFS. While there were no unexpected AEs in the combination arm, there were 2 treatment-related deaths that were attributed to both agents. None of the 14 patients who received pembrolizumab monotherapy following progression while taking eribulin experienced objective responses, and only 1 patient had stable disease.
One strategy to potentially augment immune response in HR-positive breast tumors has been to add a chemotherapy partner to immunotherapy.15 In mTNBC, this approach has become standard for patients with PD-L1–positive tumors16; however, in HR-positive, ERBB2-negative breast cancer, the benefit of adding immunotherapy to chemotherapy remains unknown. A recent study showed that triple-negative tumors with elevated TGF-β–dependent signatures are associated with immunosuppressive signals and have immune microenvironments characterized by T-cell exclusion.17 Additionally, preclinical work has demonstrated that eribulin decreases TGF-β signaling in breast cancer models.8,9 Based on these data, we hypothesized that pembrolizumab may be synergistic with eribulin. However, our findings did not reveal any benefit from the combination in this population. It is worth noting that the ORR seen in the control arm of this study is higher than previously reported for eribulin in this setting. In a phase 3 study that compared the efficacy of eribulin with capecitabine (Study 301) for patients with ERBB2-negative advanced breast cancer with 0 to 2 prior lines of therapy,7 the ORR was 11% in the eribulin arm, while in this current study, the ORR was 34%.
In other malignant neoplasms and in mTNBC, it has been recognized that activity of immunotherapy appears to be greater in less pretreated patients.18,19 During tumor evolution, the tumor microenvironment becomes progressively more immunosuppressed. Although there are less data available for metastatic HR-positive breast cancer, several groups have shown that, compared with primary tumors, mTNBC is less immunogenic, phenotypically characterized by low TILs and a marked reduction of interferon γ signature.20,21 Thus, an important factor to be considered for the lack of benefit seen from the addition of pembrolizumab in this trial is that 61% of patients had been previously exposed to chemotherapy in the metastatic setting and 73% had received prior CDK4/6 inhibitors. In fact, the I-SPY2 trial22 showed that the addition of pembrolizumab to preoperative chemotherapy in early-stage HR-positive breast cancer nearly tripled the pathologic complete response rate when compared with chemotherapy alone.
Exploratory analyses also did not show any association of PD-L1 status, TILs, or TMB with PFS in either arm. While there are data that PD-L1 positivity with a combined positive score of 10 or greater using the 22C3 antibody predicted benefit of the addition of pembrolizumab to chemotherapy among patients with mTNBC,23 this study was conducted prior to the availability of these results and used MPS testing with 22C3. There was a trend toward improved PFS with the addition of pembrolizumab in patients with a high TMB; however, only 59% of patients had TMB tested, and only 7 had a high TMB. Thus, our study lacked power to conclude whether TMB is predictive of benefit to immunotherapy in this population. Additional efforts should be made to definitively evaluate this issue in HR-positive, ERBB2-negative breast cancer. In this study, we also did not find any association of TILs with outcome in either arm. Similarly, our genomic analysis did not reveal specific genomic alterations associated with improved PFS, with the important caveat that this analysis was limited by small numbers and a sequencing panel with only a limited number of genes, and the majority of the sequencing data were derived from primary tumor samples.
No new AEs were identified; however, the frequency of fatigue, alopecia, and hepatitis was higher in the combination arm. Notably, 2 patients receiving eribulin with pembrolizumab died of complications from immune-related colitis and sepsis. A prior study of eribulin with pembrolizumab conducted in more than 100 patients with mTNBC showed promising results with the same combination, and the AE profiles were similar, except that no fatal AEs occurred.10
Limitations
Despite the randomized design and well-balanced population in this study, there are some limitations. First, missing tumor samples prevented us from performing the exploratory analyses related to TILs, PD-L1 status, and TMB in the entire population. Second, most samples available for the biomarker exploratory analysis came from primary tumors, and it has been recognized that the genomic landscape of recurrent MBC is distinct from that of primary tumors.24 Third, given the small sample size of patients with PD-L1–positive tumors, there was insufficient power to evaluate whether PD-L1 positivity was predictive of benefit to the experimental regimen.
Conclusions
Among patients with HR-positive, ERBB2-negative MBC, the combination of eribulin and pembrolizumab was not associated with an improvement in PFS, ORR, or OS compared with eribulin alone in either the intention-to-treat or PD-L1–positive populations. It remains unclear if the lack of benefit in this trial is due to the disease subtype, pretreated population, inclusion of patients with PD-L1–negative tumors, or choice of chemotherapy backbone. Further efforts to explore the benefits of adding checkpoint inhibition to chemotherapy in HR-positive, ERBB2-negative disease are needed, specifically trials powered to assess efficacy in patients with PD-L1–positive tumors and focused on less heavily pretreated patients.
Trial Protocol
eTable 1. Patient characteristics at baseline, continued.
eTable 2. Most common adverse events during the study.
eTable 3. Adverse events suggestive of potential immune-related etiology.
eFigure 1. Tumor genomic alterations ordered by progression-free survival (PFS).
eFigure 2. Forest-plot analyses of progression-free survival (PFS) in key subgroups.
eFigure 3. Kaplan–Meier analysis of progression-free survival (PFS) in patients of Arm B who received pembrolizumab following progression to eribulin.
Data Sharing Statement
References
- 1.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361-370. doi: 10.1158/2326-6066.CIR-13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40-50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 3.Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241-250. doi: 10.1016/j.breast.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 4.Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804-2811. doi: 10.1158/1078-0432.CCR-17-3452 [DOI] [PubMed] [Google Scholar]
- 5.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167(3):671-686. doi: 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanda R, Liu MC, Yau C, Asare S, Hylton N, Van't Veer L. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2 [abstract]. J Clin Oncol. 2017;35(15)(suppl):506. doi: 10.1200/JCO.2017.35.15_suppl.50628029304 [DOI] [Google Scholar]
- 7.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594-601. doi: 10.1200/JCO.2013.52.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda S, Saeki T, Takeuchi H, et al. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer. 2016;114(11):1212-1218. doi: 10.1038/bjc.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497-1505. doi: 10.1038/bjc.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolaney SM, Kalinsky K, Kaklamani VG, et al. Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer [abstract PD6-13]. Cancer Res. 2018;78(4)(suppl). doi: 10.1158/1538-7445.SABCS17-PD6-13 [DOI] [Google Scholar]
- 11.Dolled-Filhart M, Locke D, Murphy T, et al. Development of a prototype immunohistochemistry assay to measure programmed death ligand-1 expression in tumor tissue. Arch Pathol Lab Med. 2016;140(11):1259-1266. doi: 10.5858/arpa.2015-0544-OA [DOI] [PubMed] [Google Scholar]
- 12.Salgado R, Denkert C, Demaria S, et al. ; International TILs Working Group 2014 . The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259-271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna GJ, Lizotte P, Cavanaugh M, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3(4):e98811. doi: 10.1172/jci.insight.98811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AACR Project GENIE Consortium AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818-831. doi: 10.1158/2159-8290.CD-17-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams S, Gatti-Mays ME, Kalinsky K, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2020;5(8):1205-1214. doi: 10.1001/jamaoncol.2018.7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 17.Gruosso T, Gigoux M, Manem VSK, et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. 2019;129(4):1785-1800. doi: 10.1172/JCI96313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404. doi: 10.1093/annonc/mdy517 [DOI] [PubMed] [Google Scholar]
- 19.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405-411. doi: 10.1093/annonc/mdy518 [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson KE, Yost SE, Chang CW, et al. Comprehensive profiling of poor-risk paired primary and recurrent triple-negative breast cancers reveals immune phenotype shifts. Clin Cancer Res. 2020;26(3):657-668. doi: 10.1158/1078-0432.CCR-19-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232-2239. doi: 10.1093/annonc/mdy399 [DOI] [PubMed] [Google Scholar]
- 22.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676-684. doi: 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer [abstract]. J Clin Oncol. 2020;38(15)(suppl):1000. [DOI] [PubMed] [Google Scholar]
- 24.Bertucci F, Ng CKY, Patsouris A, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569(7757):560-564. doi: 10.1038/s41586-019-1056-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Patient characteristics at baseline, continued.
eTable 2. Most common adverse events during the study.
eTable 3. Adverse events suggestive of potential immune-related etiology.
eFigure 1. Tumor genomic alterations ordered by progression-free survival (PFS).
eFigure 2. Forest-plot analyses of progression-free survival (PFS) in key subgroups.
eFigure 3. Kaplan–Meier analysis of progression-free survival (PFS) in patients of Arm B who received pembrolizumab following progression to eribulin.
Data Sharing Statement