Key Points
Question
Is airborne transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a potential means of spreading coronavirus disease 2019 (COVID-19)?
Findings
In this cohort study of 128 individuals who rode 1 of 2 buses and attended a worship event in Eastern China, those who rode a bus with air recirculation and with a patient with COVID-19 had an increased risk of SARS-CoV-2 infection compared with those who rode a different bus. Airborne transmission may partially explain the increased risk of SARS-CoV-2 infection among these bus riders.
Meaning
These results suggest that future efforts at prevention and control must consider the potential for airborne spread of SARS-CoV-2, which is a highly transmissible pathogen in closed environments with air recirculation.
Abstract
Importance
Evidence of whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), can be transmitted as an aerosol (ie, airborne) has substantial public health implications.
Objective
To investigate potential transmission routes of SARS-CoV-2 infection with epidemiologic evidence from a COVID-19 outbreak.
Design, Setting, and Participants
This cohort study examined a community COVID-19 outbreak in Zhejiang province. On January 19, 2020, 128 individuals took 2 buses (60 [46.9%] from bus 1 and 68 [53.1%] from bus 2) on a 100-minute round trip to attend a 150-minute worship event. The source patient was a passenger on bus 2. We compared risks of SARS-CoV-2 infection among at-risk individuals taking bus 1 (n = 60) and bus 2 (n = 67 [source patient excluded]) and among all other individuals (n = 172) attending the worship event. We also divided seats on the exposed bus into high-risk and low-risk zones according to the distance from the source patient and compared COVID-19 risks in each zone. In both buses, central air conditioners were in indoor recirculation mode.
Main Outcomes and Measures
SARS-CoV-2 infection was confirmed by reverse transcription polymerase chain reaction or by viral genome sequencing results. Attack rates for SARS-CoV-2 infection were calculated for different groups, and the spatial distribution of individuals who developed infection on bus 2 was obtained.
Results
Of the 128 participants, 15 (11.7%) were men, 113 (88.3%) were women, and the mean age was 58.6 years. On bus 2, 24 of the 68 individuals (35.3% [including the index patient]) received a diagnosis of COVID-19 after the event. Meanwhile, none of the 60 individuals in bus 1 were infected. Among the other 172 individuals at the worship event, 7 (4.1%) subsequently received a COVID-19 diagnosis. Individuals in bus 2 had a 34.3% (95% CI, 24.1%-46.3%) higher risk of getting COVID-19 compared with those in bus 1 and were 11.4 (95% CI, 5.1-25.4) times more likely to have COVID-19 compared with all other individuals attending the worship event. Within bus 2, individuals in high-risk zones had moderately, but nonsignificantly, higher risk for COVID-19 compared with those in the low-risk zones. The absence of a significantly increased risk in the part of the bus closer to the index case suggested that airborne spread of the virus may at least partially explain the markedly high attack rate observed.
Conclusions and Relevance
In this cohort study and case investigation of a community outbreak of COVID-19 in Zhejiang province, individuals who rode a bus to a worship event with a patient with COVID-19 had a higher risk of SARS-CoV-2 infection than individuals who rode another bus to the same event. Airborne spread of SARS-CoV-2 seems likely to have contributed to the high attack rate in the exposed bus. Future efforts at prevention and control must consider the potential for airborne spread of the virus.
This cohort study examines the potential for airborne spread of COVID-19 through investigation of an outbreak among bus riders in Eastern China.
Introduction
Human infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in late 2019 in Wuhan city of Hubei province in China.1,2 The World Health Organization declared the coronavirus disease 2019 (COVID-19) outbreak a public health emergency of international concern on January 30, 2020. The ongoing epidemic has since affected more than 150 countries and territories. As of August 5, 2020, more than 18 million cases have been confirmed and more than 650 000 people have died.3
Greater efforts are needed to contain and combat the virus; however, much remains unknown about SARS-CoV-2 transmission, limiting our ability to implement effective interventions. Several studies have demonstrated transmission through close contact and respiratory droplets produced when an infected person coughs or sneezes.4,5,6 Whether SARS-CoV-2 can be transmitted as an aerosol (ie, airborne) through inhalation of virus suspended in the air is unknown. Previous studies have suggested possible airborne transmission of other virulent coronaviruses, such as the severe acute respiratory syndrome coronavirus and the Middle East respiratory syndrome coronavirus.7,8,9,10,11 Recent reports suggest that closed environments may facilitate secondary transmission of SARS-CoV-2.12,13 An experimental study demonstrated that SARS-CoV-2 can remain viable in aerosols for 3 hours or longer,14 and experimental evidence of transmission of SARS-CoV-2 between ferrets via the air was also established.15,16 Therefore, evidence supporting the potential for an airborne transmission route of SARS-CoV-2 is emerging. However, epidemiologic evidence from actual community transmission in human cohorts is lacking. To investigate the potential airborne transmission route, we present the investigation of an outbreak of COVID-19 among lay Buddhists worshiping in a temple in Zhejiang province.
Methods
Data Collection
Data on demographics, travel history, and social and family activities were collected by a standard questionnaire and additional phone or in-person interviews through epidemiologic investigation carried out by local Centers for Disease Control and Prevention staff between January 27 and February 23, 2020, and reported to the China Information System for Disease Control and Prevention (CISDCP). The standard case report form and details regarding the initiation of the outbreak investigation are provided in the eAppendix in the Supplement. The research protocol was approved by the institutional review board at the Zhejiang Provincial Centers for Disease Control and Prevention and all human participants gave written informed consent.
Sample Collection and Diagnosis of COVID-19
Throat swabs (oropharynx and nasopharynx) were collected for all individuals involved in the outbreak and their close contacts identified through follow-up contact tracing. All samples were tested by reverse transcription polymerase chain reaction or by viral genome sequencing. Screened individuals were categorized into noncases, suspected cases, and confirmed cases of COVID-19. Criteria for COVID-19 case definitions and disease severity are provided in the eAppendix in the Supplement.
Statistical Analyses
Attack rates were estimated as the number of diagnosed COVID-19 cases divided by the total number of people at risk, excluding the source patient of the outbreak. We compared the risk of COVID-19 between individuals taking the exposed bus (bus 2) and individuals taking the unexposed bus (bus 1) as well as the COVID-19 risk between individuals in bus 2 and all other individuals attending the worship event, excluding bus 2. In addition, we divided seats in bus 2 into high-risk and low-risk zones according to the definition of close contact with COVID-19 in travel-associated settings, an area within 2 meters17 (classification 1) or 2 rows18 (classification 2) of the source patient. On bus 2, the distance between 2 rows was measured at 0.75 m, which converts 2 meters to 3 rows. Therefore, the high-risk zone includes seats in the same row and within 2 or 3 rows (rows 6-10 or rows 5-11) of the index patient (seated in row 8); low-risk zones include seats in other rows (Figure17,18). The COVID-19 risks in the 2 types of zones were compared. All comparisons used χ2 or Fisher exact tests. Both risk ratios (RRs) and risk differences and corresponding 95% confidence intervals were calculated. For an exposure-disease category with no observations, we added a value of 0.5 to all cells.19 A Spearman rank correlation test was performed to test the correlation between the disease severity of those who developed infection and the distance to the index patient on bus 2 (eAppendix in the Supplement). Analyses were conducted using SAS, version 9.4 (SAS Institute), and statistical significance was set at P < .05.
Figure. Schematic Diagram of Bus 2, the Bus Carrying the Coronavirus Disease 2019 (COVID-19) Initial Patient (IP).
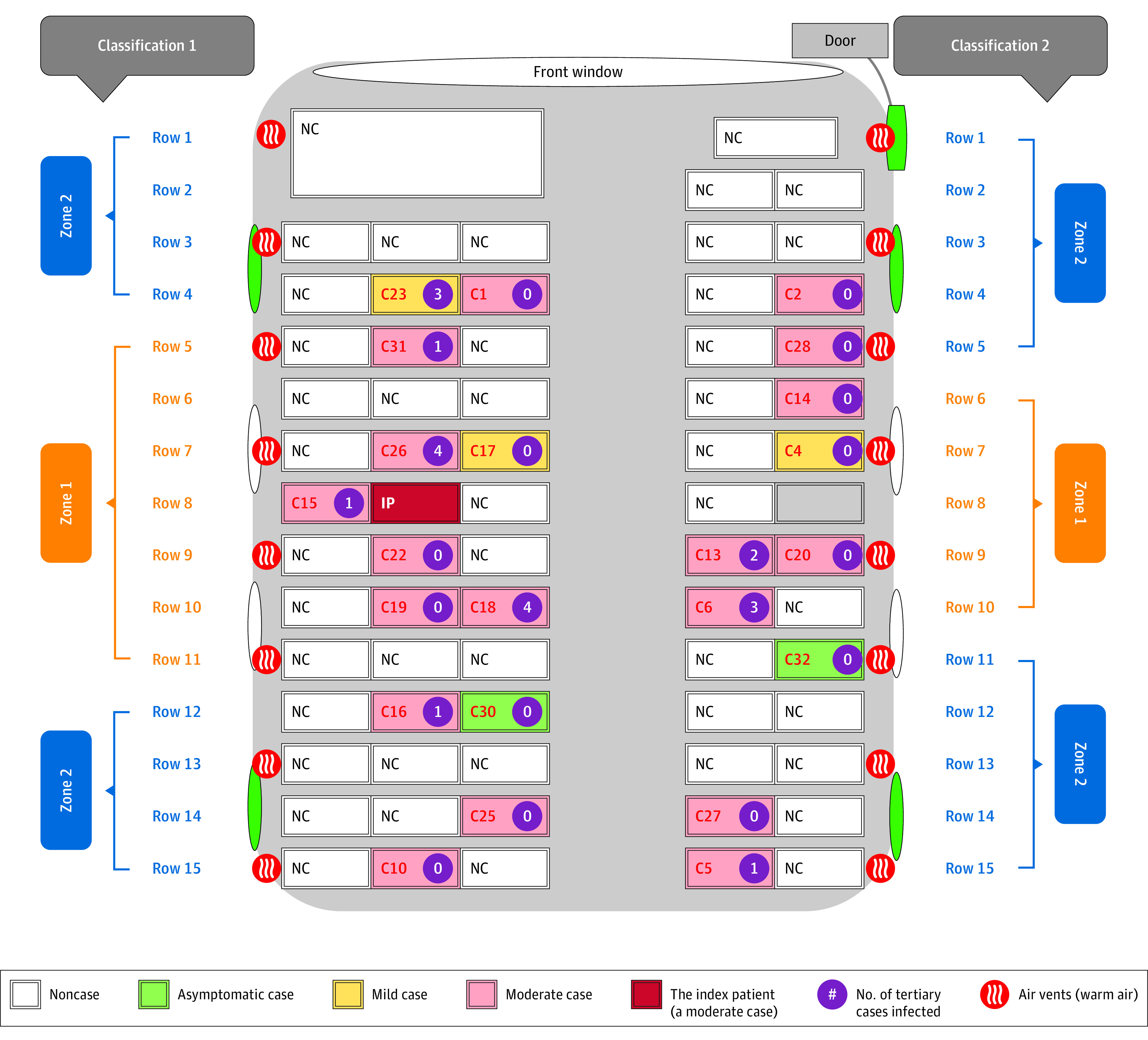
Classification 117 and 2.18 Two different approaches to define high-risk and low-risk COVID-19 zones are indicated: zone 1 (high-risk zone) and zone 2 (low-risk zones). Severity levels of cases were indicated. Windows are indicated with ovals, and there are 4 green side windows and that could be opened for fresh air. C indicates case; NC, noncase.
Results
Evidence from an outbreak suggesting airborne transmission of SARS-CoV-2 is presented. Other materials associated with the transmission dynamics of the outbreak are included in the eAppendix in the Supplement.
Outbreak of COVID-19 Among Lay Buddhists Worshiping in the Temple
The COVID-19 outbreak started on January 19, 2020, among 293 lay Buddhists, 2 bus drivers, and 5 monks attending an outdoor worship event held in a temple in Ningbo city of Zhejiang province. Ningbo city, located approximately 700 km (900 km through the highway) to the east of Wuhan city, is one of the most populated cities in Zhejiang province, with a total population of more than 8 million in 2010. It has an area of 9816.23 km2. Before January 19, 2020, no confirmed COVID-19 cases were reported in Ningbo city. Lay Buddhists live in the broader community rather than living within a religious order. Of all the lay Buddhists, 126 traveled to the temple in 2 buses, with 59 participants (46.8%) in bus 1 and the other 67 (53.2%) in bus 2. Each bus also had a driver, and all passengers on the 2 buses were from the same district of the city. The 2 buses were similar in design, with an air conditioning system on a heating and recirculating mode (vents were below the windows) and 4 openable windows (2 on each side); neither had an attached toilet. All other individuals traveled to the temple through other methods of transportation. The 2 busses came from 1 district in Ningbo city (Haishu District) to the temple, which is located in another district of Ningbo city (Yinzhou District). The travel duration to and from the temple on the bus was 50 minutes each way (100 minutes total). Passengers, including the index patient, remained seated in their own seats during the bus rides and did not change seats on the way back. The weather was sunny with a gentle breeze during the day (33.8 °F-50 °F). The worship event lasted 150 minutes, beginning at 10:00 am and ending at 12:30 pm. The event included a luncheon, with 10 attendees sitting at each round table in a spacious room with no recirculating central air conditioning systems on. The lunch lasted 15 to 30 minutes. Passengers from bus 2 did not sit together and were randomly mixed at lunch. All 293 lay Buddhists (including those who traveled on the 2 buses and the others) and the 5 monks presented at the worship place and they were mixed into large crowds. None of the event participants wore mask or any prevention during the rides and worshiping on January 19, 2020, as there was no public awareness of COVID-19 in the city at that time.
Among all patients who received a COVID-19 diagnosis during this outbreak, the presumed index patient, a lay Buddhist in her 60s, was the only person exposed to residents from Wuhan. On January 17, the individual had dinner at the same table with a group of 10 individuals, among whom 4 had travel histories to Hubei province. According to the CISDCP, the index patient was also the first to develop clinical symptoms. As such, the index patient was presumed to be the source of transmission in this outbreak. According to the field investigation report, the index patient reported being asymptomatic during the bus trip and to have started to experience cough, chills, and myalgias on the evening after returning from the temple. However, during follow-up investigations conducted by field investigators in February 2020, the index patient reported that she had had a mild cough the night before the trip (personal communication, Dongliang Zhang, Ningbo CDC, September 4, 2020). The field investigators determined that there was not enough information to indicate that this mild symptom was COVID-19 related, and they did not update the original investigation report. The symptom onset date for the index patient is still recorded as January 19 in the CISDCP database.
The day after the bus trip and event, the patient felt better after bathing in a hot tub. However, the patient’s spouse and child started to have fever and cough on January 22, 2020, and the entire family went to a hospital seeking treatment. During the hospital visit, the index patient had a normal body temperature. On January 25, 2020, the index patient’s daughter received a diagnosis of suspected COVID-19, and consequently the entire family was admitted to a hospital for quarantine, where a computed tomography scan showed exudative inflammation in the lungs of the index patient. All 3 family members had confirmed COVID-19 positive results from reverse transcription polymerase chain reaction assays on January 28, 2020. The index patient’s spouse and daughter did not participate in the worship event on January 19, 2020. Other secondary patients also started to develop symptoms within a relatively short period after the worship event. A detailed record for the length of time to first symptoms for all secondary cases and beyond (the secondary cases went on to transmit the disease to others) is included in eFigure 1 in the Supplement, and the corresponding transmission dynamics are presented in eFigure 2 in the Supplement. Many of these individuals were close contacts of the secondary cases, but they did not participate in the worship event.
Analyses Suggesting That the Transmission Largely Occurred on Bus 2
Bus 2 carried 68 individuals (67 lay Buddhist passengers and a driver), of whom 24 passengers (35.3% [including the index patient]) developed infection and received a COVID-19 diagnosis after the event. None of the 60 individuals (59 lay Buddhist passengers and a driver) on bus 1 received a subsequent diagnosis of COVID-19. In addition, among the other 172 individuals (167 individuals [97.1%] who traveled to the worship event through other methods of transportation and 5 monks [2.9%]) at the worship event, 7 (4.1%) received a subsequent diagnosis of COVID-19, and all of them described being in close contact with the index patient during the event. Overall, 30 of the 299 individuals (10.0%) at risk during the event developed COVID-19 (excluding the index patient). Compared with individuals in the nonexposed bus (bus 1), those in the exposed bus (bus 2) were 42.2 (95% CI, 2.6-679.3) times more likely to develop COVID-19 (Table), and the risk difference was 34.3% (95% CI, 23.0%-45.7%). Compared with all other individuals attending the worship event, passengers in bus 2 had an 11.4 (95% CI, 5.1-25.4) times higher chance of developing COVID-19 (Table).
Table. COVID-19 Risk Assessment of Different Sections of the Exposed Bus and Between the Exposed Bus and Unexposed Controlsa.
| Characteristic | Total | No. with COVID-19 | % (95% CI) | Relative risk (95% CI) | P value | Relative risk (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Attack rate | Risk difference | ||||||||
| Exposed bus and other attendees of the worship event, excluding the index patient | |||||||||
| Bus 1 | 60 | 0 | 0 (0 to 6.0) | 0 [Reference] | NA | 1 [Reference] | NA | NA | NA |
| All individuals except bus 2 | 232 | 7 | 3.0 (1.3 to 6.2) | NA | 0 [Reference] | NA | 1 [Reference] | ||
| Bus 2 | 67 | 23 | 34.3 (24.1 to 46.3) | 34.3 (23.0 to 45.7) | 31.3 (19.7 to 42.9) | 42.2 (2.6 to 679.3) | <.01 | 11.4 (5.1 to 25.4) | <.01 |
| Overall | 299 | 30 | 10.0 (7.1 to 14.0) | NA | |||||
| Different sections of the exposed bus, excluding the index patient | |||||||||
| Classification 117 | |||||||||
| Low-risk zones (rows 1-4, 12-15) | 34 | 9 | 26.5 (14.4 to 43.3) | 0 [Reference] | NA | 1 [Reference] | NA | NA | NA |
| High-risk zone (rows 5-11) | 33 | 14 | 42.4 (27.2 to 59.2) | 16.0 (−6.5 to 38.4) | 1.6 (0.8 to 3.2) | .17 | |||
| Classification 218 | |||||||||
| Low-risk zones (rows 1-5, 11-15) | 44 | 12 | 27.3 (16.2 to 42.0) | 0 [Reference] | NA | 1 [Reference] | NA | NA | NA |
| High-risk zone (rows 6-10) | 23 | 11 | 47.8 (29.2 to 67.0) | 20.6 (−3.7 to 44.8) | 1.8 (0.9 to 3.3) | .09 | |||
Abbreviations: COVID-19, coronavirus disease 2019; NA, not applicable.
For exposure-disease categories with 0 counts, we added a value of 0.5 to all cells to calculate risk ratio.
Analyses Suggesting Potential Airborne Transmission in Bus 2
We were able to identify seats for each passenger in the exposed bus (Figure). The bus had 15 rows of seats. Starting from the third row, each row had 3 seats on 1 side of the aisle and 2 seats on the other side of the aisle. The index patient sat in the middle seat on the 3-seat side of the eighth row. Other than the passengers sitting close to the index patient, the seats of other cases were scattered in the bus. Passengers in the high-risk zones had moderately but nonsignificantly higher risk of getting COVID-19 than those in the low-risk zones using either classification 1 (RR, 1.6; 95% CI, 0.8-3.2) or classification 2 (RR, 1.8; 95% CI, 0.9-3.3) (Table). On the 3-seat side of the bus, except for the passenger sitting next to the index patient, none of the passengers sitting in seats close to the bus window developed infection. In addition, the driver and passengers sitting close to the bus door also did not develop infection, and only 1 passenger sitting by an openable window developed infection. The index patient developed moderate symptoms (Figure). Among passengers who eventually developed COVID-19 on bus 2, 2 were asymptomatic, 3 had mild symptoms, and the remaining 17 had moderate symptoms. The disease severity of the secondary patients was not associated with their proximity to the index patient on the bus (Spearman correlation coefficient, 0; P = .99). In a further contact investigation of the 23 patients with COVID-19 on bus 2, the numbers of tertiary cases transmitted by each of them were reported (Figure).
Discussion
Previous investigations have reported respiratory droplets, either through close contact or the touching of inanimate objects (ie, fomites), as the major transmission route for COVID-19. As a result, washing hands using soap under running water for 20 seconds and masking mouths and noses when coughing or sneezing is widely suggested for disease prevention.17 Through detailed epidemiologic analysis, airborne transmission within a bus with recycled air seems likely to have contributed to a COVID-19 outbreak in eastern China. A natural occurrence involved in the outbreak helped to identify where most the transmission occurred. A review study on transmission of infectious diseases assessed the quality of evidence from various sources and considered “epidemiologic evidence of transmission through air over long distances” as very strong evidence of aerosol transmission.8 Our study provides such evidence and adds to other sources of existing evidence showing experimental infection in animal models through the aerosol route, as well as viable pathogens detected in air at ambient conditions for hours in laboratory media.14,15,16
During the aforementioned outbreak, the index patient was the only person exposed to individuals from Wuhan and the first who attended the event to receive a diagnosis of COVID-19, suggesting a high probability that they were the source of the outbreak. The 2 buses mimicked a quasiexperiment and the second unexposed bus, which left and arrived at the temple at similar times with similar individuals, provided a credible control group. Both buses had an air conditioning system on a recirculating mode, which may have facilitated the spread of the virus in the exposed bus. Attack rates on the exposed and unexposed buses were distinct (34.3% vs 0%), suggesting that the exposure and the environment in which the exposure took place contributed to this outbreak. Additionally, passengers sitting closer to the index patient on the exposed bus did not have statistically higher risks of COVID-19 as those sitting further away. If COVID-19 transmission occurred solely through close contact or respiratory droplets during this outbreak, the risk of COVID-19 would likely be associated with distance from the index patient, and high-risk zones on the bus would have more infected cases. The index patient on bus 2, likely a super spreader of the outbreak, only developed recognized symptoms (cough, chills, and myalgias) on the evening after returning from the temple and was either asymptomatic or minimally symptomatic during the bus rides, suggesting that individuals with infection may be able to shed virus by breathing and cause secondary cases before they are clearly symptomatic, echoing the findings from earlier presymptomatic reports.20,21
Our findings suggesting that airborne transmission of COVID-19 aligns with past reports of a severe acute respiratory syndrome outbreak on a plane and a recent COVID-19 outbreak in a restaurant.10,22 Severe acute respiratory syndrome and COVID-19 are caused by coronaviruses and all 3 outbreaks occurred in relatively enclosed spaces with airconditioned systems. The high attack rate in bus 2 is also consistent with an outbreak of influenza aboard a commercial airliner in which an inoperative ventilation system resulted in a high infection rate among passengers involved in a jet delay.23 Meanwhile, transmission at the worship event between the bus rides only led to few infections, and all of those reported close contact with the index patient. The worship event occurred largely outdoors. The findings echo a recent study in which aerosolized traces of viral RNA were found in poorly ventilated spaces of 2 hospitals.24 Specifically, their study found the highest aerosol particles concentration in a toilet that lacked ventilation, and we provide epidemiological evidence of a superspreading event resulted from the potential high aerosol particles concentration on a bus. These data suggest that forced, circulating air might play an important role in airborne spread of the virus, and gatherings in enclosed settings with minimal air ventilation should be limited.
Strengths and Limitations
Our study has several strengths. First, the outbreak in this report had a clear index and source patient and we were able to collect detailed information on the environment in which the outbreak occurred and exposure opportunities. Second, the bus outbreak mimicked a quasiexperiment in which 2 buses, 1 with an individual with disease and 1 without, carried similar passengers at similar times, providing a credible unexposed control group. The same outbreak also included an indoor (bus ride) and outdoor component (the worship event) of similar lengths, allowing a comparison between those settings. All participants were mixed into large crowds at the worship event, but most of the infected cases were from bus 2. The result suggested that the transmission largely occurred in the exposed bus, where a much higher attack rate in a closed environment with recirculating air was observed. The potential role of mechanical air circulation for COVID-19 spread is also supported by a recent study that observed virus-contaminated air exhaust outlets.25 Further, numerous nonairborne transmission options were considered through detailed epidemiologic investigations of the passengers in the exposed bus and their seating information, and the overall possibility was determined low. Therefore, it is hard to explain the lack of difference in the attack rate between individuals sitting close to the index patient and those clearly separated by distance without possible airborne transmission. For instance, cases C5 and C10 seated in the last row were more than 5 m from the index patient on the bus and neither reported direct contact nor sharing of spaces with the index patients during the event, yet they both developed infection. Lastly, the outbreak consisting of a social outdoor event with public transportation is a common daily event, providing potentially greater generalizability to our results.
Our study also has limitations. During the worship event outbreak, alternative sources of infection cannot be ruled out completely. However, there were no confirmed cases in Ningbo city before January 19, 2020 (when the worship event occurred). The first confirmed case of COVID-19 in Ningbo city was reported on January 20, 2020, and the city had a total of 69 confirmed cases by the end of January. Apparently, the outbreak occurred during the early stage of a modest level of disease spread in Ningbo city. Considering that Ningbo is a city with a total population of more than 8 million, the possibility of having more than 1 source patient at the worship event on January 19 would be quite low. Meanwhile, most secondary cases started to show symptoms after January 21, 2020, more than 2 days after the worship event. The current literatures suggest that presymptomatic transmission often occurs within a 2-day or 3-day window before symptom onset (ie, viral shedding may begin 2 to 3 days before the appearance of the first symptoms).21 In addition, no one else on bus 2 had recent travel history to Wuhan or reported being in contact with someone from Wuhan. Considering these factors, the possibility of having another person on the bus who was able to spread the virus at the time of the bus ride was relatively low, if not completely impossible. It is also possible that the index patient in this outbreak had mild unrecognized symptoms during the bus ride. Our sample size of infected cases within the exposed bus was somewhat limited, which could have contributed to the nonsignificant results regarding the association of distance from the index patient with infection risk. While the high attack rate and the distribution of cases on bus 2 is consistent with airborne transmission, there is no way to rule out a common surface, such as a pole, because of possible insufficient recall. However, given that there were participants with infection sitting in the last row, airborne transmission is likely to be a partial transmission route.
Conclusions
We investigated a COVID-19 outbreak in Zhejiang province and found that airborne transmission likely contributed to the high attack rate seen. The investigations suggest that, in closed environments with air recirculation, SARS-CoV-2 is a highly transmissible pathogen. Our finding of potential airborne transmission has important public health significance, and future efforts at prevention and control should consider the potential for airborne spread of COVID-19.
eAppendix. COVID-19 Case Report Form, Initiation of the Outbreak Investigation, Definition of COVID-19 Cases and Severity, Details of Spearman Rank Test, and Transmission Dynamics
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Coronavirus disease 2019 (COVID-19) situation report—197. Accessed August 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200804-covid-19-sitrep-197.pdf?sfvrsn=94f7a01d_2
- 4.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. Lancet. 2020;395(10223):514-523. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu P, Zhu J, Zhang Z, Han Y, Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757-1761. doi: 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872-874. doi: 10.1056/NEJMc2001272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57(5):501-508. doi: 10.1097/JOM.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 9.Wong TW, Lee CK, Tam W, et al. ; Outbreak Study Group . Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10(2):269-276. doi: 10.3201/eid1002.030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen SJ, Chang HL, Cheung TY, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416-2422. doi: 10.1056/NEJMoa031349 [DOI] [PubMed] [Google Scholar]
- 11.Yu IT, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731-1739. doi: 10.1056/NEJMoa032867 [DOI] [PubMed] [Google Scholar]
- 12.Nishiura H, Oshitani H, Kobayashi T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv. Preprint posted online March 3, 2020. doi: 10.1101/2020.02.28.20029272 [DOI] [Google Scholar]
- 13.Hodcroft EB. Preliminary case report on the SARS-CoV-2 cluster in the UK, France, and Spain. Swiss Med Wkly. 2020;150(9-10). doi: 10.4414/smw.2020.20212 [DOI] [PubMed] [Google Scholar]
- 14.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567. doi: 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YI, Kim SG, Kim SM, et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704-709.e2. doi: 10.1016/j.chom.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard M, Kok A, de Meulder D, et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11(1):3496. doi: 10.1038/s41467-020-17367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO recommended measures for persons undertaking international travel from areas affected by severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec. 2003;78(14):97-99. [PubMed] [Google Scholar]
- 18.Hertzberg VS, Weiss H. On the 2-row rule for infectious disease transmission on aircraft. Ann Glob Health. 2016;82(5):819-823. doi: 10.1016/j.aogh.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagano M, Gauvreau K.. Principles of Biostatistics. CRC Press; 2018. [Google Scholar]
- 20.Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081-2090. doi: 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628-1631. doi: 10.3201/eid2607.200764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1-6. doi: 10.1093/oxfordjournals.aje.a112781 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557-560. doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 25.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;232(16):1610-1612. doi: 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. COVID-19 Case Report Form, Initiation of the Outbreak Investigation, Definition of COVID-19 Cases and Severity, Details of Spearman Rank Test, and Transmission Dynamics


