Key Points
Question
Is high-intensity interval training (HIIT) superior to moderate-intensity continuous training (MICT) for improving cardiorespiratory fitness during a 4-week hospital-based cardiac rehabilitation program and long-term with home-based training over 12 months?
Findings
In this randomized clinical trial including 93 participants, cardiorespiratory fitness significantly improved by 10% with HIIT compared with 4% with MICT after the 4-week cardiac rehabilitation program. Over 12 months, both HIIT and MICT were safe and feasible and offered similar improvements in cardiorespiratory fitness (by 10% and 7%, respectively).
Meaning
This study supports using HIIT as an alternative or adjunct form of exercise prescription in cardiac rehabilitation.
This randomized clinical trial compares high-intensity interval training with moderate-intensity continuous training for feasibility, safety, adherence, and efficacy of improving VO2 in patients with coronary artery disease.
Abstract
Importance
High-intensity interval training (HIIT) is recognized as a potent stimulus for improving cardiorespiratory fitness (volume of oxygen consumption [VO2] peak) in patients with coronary artery disease (CAD). However, the feasibility, safety, and long-term effects of HIIT in this population are unclear.
Objective
To compare HIIT with moderate-intensity continuous training (MICT) for feasibility, safety, adherence, and efficacy of improving VO2 peak in patients with CAD.
Design, Setting, and Participants
In this single-center randomized clinical trial, participants underwent 4 weeks of supervised training in a private hospital cardiac rehabilitation program, with subsequent home-based training and follow-up over 12 months. A total of 96 participants with angiographically proven CAD aged 18 to 80 years were enrolled, and 93 participants were medically cleared for participation following a cardiopulmonary exercise test. Data were collected from May 2016 to December 2018, and data were analyzed from December 2018 to August 2019.
Interventions
A 4 × 4-minute HIIT program or a 40-minute MICT program (usual care). Patients completed 3 sessions per week (2 supervised and 1 home-based session) for 4 weeks and 3 home-based sessions per week thereafter for 48 weeks.
Main Outcomes and Measures
The primary outcome was change in VO2 peak during the cardiopulmonary exercise test from baseline to 4 weeks. Further testing occurred at 3, 6, and 12 months. Secondary outcomes were feasibility, safety, adherence, cardiovascular risk factors, and quality of life.
Results
Of 93 randomized participants, 78 (84%) were male, the mean (SD) age was 65 (8) years, and 46 were randomized to HIIT and 47 to MICT. A total of 86 participants completed testing at 4 weeks for the primary outcome, including 43 in the HIIT group and 43 in the MICT group; 69 completed testing at 12 months for VO2 peak, including 32 in the HIIT group and 37 in the MICT group. After 4 weeks, HIIT improved VO2 peak by 10% compared with 4% in the MICT group (mean [SD] oxygen uptake: HIIT, 2.9 [3.4] mL/kg/min; MICT, 1.2 [3.4] mL/kg/min; P = .02). After 12 months, there were similar improvements from baseline between groups, with a 10% improvement in the HIIT group and a 7% improvement in the MICT group (mean [SD] oxygen uptake: HIIT, 2.9 [4.5] mL/kg/min; MICT, 1.8 [4.3] mL/kg/min; P = .30). Both groups had high feasibility scores and low rates of withdrawal due to serious adverse events (3 participants in the HIIT group and 1 participant in the MICT group). One event occurred following exercise (hypotension) in the HIIT group. Over 12 months, both home-based HIIT and MICT had low rates of adherence (HIIT, 18 of 34 [53%]; MICT, 15 of 37 [41%]; P = .35) compared with the supervised stage (HIIT, 39 of 44 [91%]; MICT, 39 of 43 [91%]; P > .99).
Conclusions and Relevance
In this randomized clinical trial, a 4-week HIIT program improved VO2 peak compared with MICT in patients with CAD attending cardiac rehabilitation. However, improvements in VO2 peak at 12 months were similar for both groups. HIIT was feasible and safe, with similar adherence to MICT over 12-month follow-up. These findings support inclusion of HIIT in cardiac rehabilitation programs as an adjunct or alternative modality to moderate-intensity exercise.
Trial Registration
Australian New Zealand Clinical Trials Registry Identifier: ACTRN12615001292561
Introduction
Cardiac rehabilitation (CR) is an essential component in the secondary prevention of coronary artery disease (CAD), with proven reductions in cardiovascular and all-cause mortality.1 Exercise plays an important role as cardiorespiratory fitness (measured as volume of oxygen consumption [VO2] peak) exerts the largest influence on cardiovascular disease prognosis in this population.2,3 High-intensity interval training (HIIT) has shown superior improvements in VO2 peak compared with moderate-intensity continuous training (MICT) in patients with CAD.4,5 However, current international CR guidelines dictate a need for further investigation into the feasibility, safety, and long-term adherence associated with HIIT.6
The primary aim of this investigator-initiated study was to compare the efficacy of HIIT with MICT for improving VO2 peak during a 4-week supervised hospital-based CR program. Secondary aims investigated the efficacy of HIIT compared with MICT for improving VO2 peak following a supervised CR program over 12-month follow-up and whether implementation of a HIIT program was safe and feasible, promoted greater exercise adherence, modified cardiovascular risk factors, and improved quality of life.
Methods
A detailed trial protocol for the Feasibility, Safety, Adherence, and Efficacy of High Intensity Interval Training in Rehabilitation for Coronary Heart Disease (FITR Heart Study) is available in Supplement 1.7 This trial was approved by both UnitingCare Health and the University of Queensland ethics committees. All participants provided written informed consent.
Patient Selection and Allocation
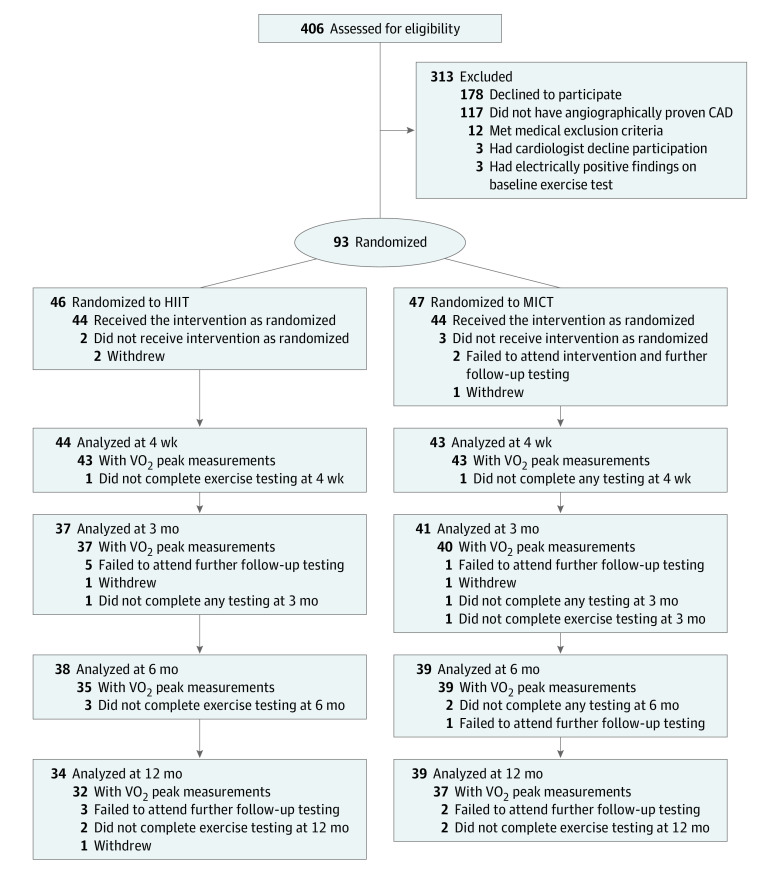
Patients were considered for inclusion in the study if they had angiographically proven CAD, were aged 18 to 80 years, and were eligible to participate in the hospital CR program. Patients were excluded if they had any absolute or relative contraindications to exercise testing.7,8 After providing consent, participants underwent baseline testing before 1:1 randomization to either HIIT or MICT (usual care) (Figure). All participants underwent a medically supervised cardiopulmonary exercise test (CPET). Patients were further excluded from the study if abnormal results identified from the baseline CPET resulted in further angiography or recommended exclusion by the patients’ treating physician.
Figure. CONSORT Flowchart of Study Enrollment, Allocation, and Follow-up.
Participants underwent 4 weeks of supervised training in a private hospital cardiac rehabilitation program, with subsequent home-based training and follow-up over 12 months. CAD indicates coronary artery disease; HIIT, high-intensity interval training; MICT, moderate-intensity continuous training; VO2, volume of oxygen consumption.
Outcome Measures
The primary outcome (VO2 peak) was measured by CPET at baseline and 4 weeks. Further testing occurred at 3, 6, and 12 months. Safety was assessed continuously throughout the study period. Adherence to the exercise protocol was assessed as 70% attendance or higher at the recommended number of exercise sessions when training at the prescribed exercise intensity during the exercise sessions (eMethods in Supplement 2). Data were also obtained for anthropometric measures, fasting blood markers, supine blood pressure, habitual dietary intake, physical activity (by accelerometry), and participant questionnaires related to feasibility, quality of life, and exercise enjoyment.7
Exercise Protocols
The HIIT protocol involved 4 × 4-minute high-intensity intervals corresponding to a rating of perceived exertion (RPE) of 15 to 18 on the Borg 6 to 20 scale,9 interspersed with 3-minute active recovery intervals (RPE of 11 to 13). The MICT protocol involved usual care exercise of 40-minute moderate-intensity exercise at an RPE of 11 to 13 (eMethods in Supplement 2). Participants were instructed to complete 3 sessions of their allocated training per week (2 supervised and 1 home-based session) during the 4-week CR program and then to continue home-based training (at least 3 sessions per week of their allocated training) for a further 11 months.
Statistical Analysis
The sample size calculation conducted for the primary outcome, the comparison of groups for change in VO2 peak over a 4-week supervised program, determined 80 participants (40 per group) would be sufficient to detect a 1–metabolic equivalent difference (3.5 mL/kg/min) between groups with an SD of 4.75 mL/kg/min and a power of 0.9 at an α of .05.7 Intention-to-treat analyses using linear mixed modeling were performed to investigate the time and group interaction effects for the supervised study period (baseline to 4 weeks) and 12-month period (all time points). Baseline characteristics and exercise adherence data were compared using t tests for continuous variables and Fisher exact test for categorical data. Prespecified per-protocol analyses were conducted including only participants meeting the criteria for exercise adherence.7 Sensitivity analyses were conducted to account for medication changes. Statistical analyses were performed using SPSS Statistics version 25 (IBM). Significance was set at a P value less than .05, and all P values were 2-tailed.
Results
Participant Characteristics
A total of 96 participants were recruited between May 2016 and November 2017. The Figure outlines allocation to the HIIT and MICT groups after exclusions. A total of 3 of 96 participants (3%) were medically excluded following baseline CPET, with 1 of 96 participants (1%) requiring further coronary intervention. Of 93 randomized participants, 78 (84%) were male, the mean (SD) age was 65 (8) years, and 46 were randomized to HIIT and 47 to MICT. Dropout rates between HIIT (12 of 46 [26%]) and MICT (8 of 47 [17%]) were not different over 12-month follow-up (P = .32). A total of 86 participants completed testing at 4 weeks for the primary outcome, including 43 in the HIIT group and 43 in the MICT group; 69 completed testing at 12 months for VO2 peak, including 32 in the HIIT group and 37 in the MICT group. Baseline characteristics are outlined in Table 1. For medication adjustments, see eTable 1 in Supplement 2.
Table 1. Participant Characteristics at Baseline.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| HIIT (n = 46) | MICT (n = 47) | ||
| Male | 39 (85) | 39 (83) | >.99 |
| Age, mean (SD), y | 65 (7) | 65 (8) | .98 |
| Body mass index, mean (SD)a | 28.2 (4.2) | 28.5 (4.2) | .67 |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 128 (15) | 130 (14) | .62 |
| Diastolic | 75 (10) | 74 (9) | .72 |
| Resting heart rate, mean (SD), beats/min | 57 (10) | 57 (8) | .92 |
| Reason for hospital admission | |||
| Acute coronary syndrome | 13 (28) | 16 (34) | .66 |
| ST-elevation myocardial infarction | 1 (2) | 9 (19) | .02 |
| Non–ST-elevation myocardial infarction | 8 (17) | 6 (13) | .57 |
| Unstable angina pectoris | 4 (9) | 1 (2) | .20 |
| Diagnostic angiography only | 33 (72) | 31 (66) | .66 |
| Coronary intervention | |||
| Coronary artery bypass grafting | 15 (33) | 11 (23) | .36 |
| Percutaneous coronary intervention | 23 (50) | 23 (49) | >.99 |
| Medical therapy only | 8 (17) | 13 (28) | .32 |
| Comorbidities | |||
| Diabetes | 2 (4) | 7 (15) | .16 |
| Current smoking | 1 (2) | 2 (4) | >.99 |
| Left ventricular dysfunctionb | 3 (7) | 5 (11) | .72 |
| Chronic atrial fibrillation | 1 (2) | 1 (2) | >.99 |
| Medications | |||
| β-Blocker | 18 (39) | 20 (43) | .83 |
| Angiotensin-converting enzyme inhibitor | 9 (20) | 17 (36) | .11 |
| Angiotensin II receptor blocker | 16 (35) | 16 (34) | >.99 |
| Calcium channel blocker | 3 (7) | 6 (13) | .49 |
| Diuretic | 7 (15) | 7 (15) | >.99 |
| Antiarrhythmic | 2 (4) | 2 (4) | >.99 |
| Anticoagulant | 4 (9) | 1 (2) | .20 |
| Statin | 45 (98) | 44 (94) | .62 |
| Aspirin | 44 (96) | 43 (92) | .68 |
| Other antiplatelet | 25 (54) | 27 (57) | .84 |
Abbreviations: HIIT, high-intensity interval training; MICT, moderate-intensity continuous training.
Calculated as weight in kilograms divided by height in meters squared.
Left ventricular dysfunction was defined either quantitatively (ejection fraction less than 50%) or qualitatively from the patient’s most recent echocardiography or left heart ventriculography during angiography procedure.
Cardiorespiratory Fitness
Following the 4-week supervised program, VO2 peak increased by 10% with HIIT and 4% with MICT (mean [SD] oxygen uptake: HIIT, 2.9 [3.4] mL/kg/min; MICT, 1.2 [3.4] mL/kg/min; mean difference [MD], 1.7 mL/kg/min; P = .02) (Table 2). This was similar for VO2 peak normalized for lean body mass (mean [SD] oxygen uptake: HIIT, 4.1 [4.9] mL/kg/min [10% improvement]; MICT, 1.0 [5.0] mL/kg/min [2% improvement]; MD, 3.1 mL/kg/min; P = .004) (Table 2). After 12-month follow-up, participants in the HIIT and MICT groups showed similar improvement in VO2 peak from baseline, with a 10% improvement in the HIIT group and a 7% improvement in the MICT group (mean [SD] oxygen uptake: HIIT, 2.9 [4.5] mL/kg/min; MICT, 1.8 [4.3] mL/kg/min; MD, 1.1 mL/kg/min; P = .30).
Table 2. Efficacy Results for Cardiorespiratory Fitness, Exercise Testing Variables, Cardiorespiratory Risk Factors, and Quality of Life.
| Outcome measure | No. | Supervised training (stage 1) | P value, time × group | Home-based training (stages 2 and 3), mean within-group difference (95% CI) | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | Change in 4 wk, mean within-group difference (95% CI) | Change in 3 mo | Change in 6 mo | Change in 12 mo | Time | Time × group | ||||||||
| HIIT | MICT | HIIT | MICT | HIIT | MICT | HIIT | MICT | HIIT | MICT | |||||
| Cardiorespiratory fitness and exercise testing | ||||||||||||||
| Peak oxygen uptake, mL/kg/min, total body weight | 93 | 27.7 (6.1) | 27.4 (7.4) | 2.9 (1.9 to 3.9)a | 1.2 (0.2 to 2.2)a | .02 | 2.6 (0.8 to 4.4)a | 2.2 (0.5 to 4.0)a | 3.1 (1.3 to 4.9)a | 1.7 (0 to 3.5) | 2.9 (1.0 to 4.8)a | 1.8 (0 to 3.6)a | <.001 | .30 |
| Peak oxygen uptake, mL/kg/min, lean body mass | 93 | 41.3 (7.1) | 42.0 (9.4) | 4.1 (2.7 to 5.5)a | 1.0 (−0.4 to 2.5) | .004 | 3.1 (0.5 to 5.7)a | 2.0 (−0.6 to 4.5) | 3.7 (1.0 to 6.3)a | 1.2 (−1.3 to 3.8) | 4.1 (1.4 to 6.9)a | 1.8 (−0.9 to 4.4) | <.001 | .14 |
| Peak oxygen uptake, L/min | 93 | 2.33 (0.61) | 2.39 (0.73) | 0.23 (0.15 to 0.32)a | 0.09 (0.01 to 0.18)a | .02 | 0.18 (0.03 to 0.33)a | 0.17 (0.02 to 0.32)a | 0.21 (0.06 to 0.37)a | 0.10 (−0.05 to 0.25) | 0.20 (0.04 to 0.36)a | 0.11 (0.04 to 0.26) | <.001 | .26 |
| Peak respiratory exchange ratio | 93 | 1.14 (0.09) | 1.14 (0.09) | −0.01 (−0.04 to 0.02) | −0.01 (−0.04 to 0.02) | .94 | 0.01 (−0.03 to 0.05) | −0.01 (−0.05 to 0.03) | −0.01 (−0.05 to 0.03) | 0.01 (−0.03 to 0.05) | 0 (−0.05 to 0.04) | 0.01 (−0.03 to 0.05) | .41 | .37 |
| Peak heart rate, beats/min | 93 | 151 (17) | 150 (20) | 1 (−2 to 5) | −2 (−5 to 2) | .26 | 4 (−2 to 10) | 3 (−3 to 9) | 3 (−3 to 9) | 3 (−3 to 9) | 4 (−2 to 10) | 7 (1 to 13)a | <.001 | .29 |
| Peak oxygen pulse, mL/beat | 93 | 15.5 (3.8) | 15.9 (4.0) | 1.5 (0.9 to 2.2)a | 0.8 (0.2 to 1.5)a | .14 | 0.8 (−0.3 to 1.9) | 0.7 (−0.4 to 1.8) | 1.0 (−0.1 to 2.2) | 0.2 (−0.9 to 1.3) | 0.9 (−0.3 to 2.1) | 0 (−1.1 to 1.4) | <.001 | .34 |
| Maximal oxygen uptake efficiency slope | 93 | 2.5 (0.7) | 2.4 (0.6) | 0.2 (0.1 to 0.4)a | 0.2 (0.1 to 0.3)a | .79 | 0.2 (0 to 0.4)a | 0.2 (0 to 0.4) | 0.2 (0 to 0.4)a | 0.1 (−0.1 to 0.3) | 0.1 (−0.1 to 0.4) | 0.1 (−0.1 to 0.3) | <.001 | .57 |
| Abdominal obesity | ||||||||||||||
| Body mass, kg | 93 | 84 (15) | 87 (16) | −0.4 (−0.8 to 0.1) | −0.5 (−0.9 to 0)a | .79 | −1.2 (−2.3 to −0.1)a | −1.2 (−2.3 to −0.2)a | −1.2 (−2.3 to −0.1)a | −2.0 (−3.1 to −0.9)a | −1.1 (−2.2 to 0.1) | −1.6 (−2.7 to −0.5)a | <.001 | .54 |
| Body mass indexb | 93 | 28.2 (4.2) | 28.6 (4.2) | −0.1 (−0.3 to 0.2) | −0.2 (−0.5 to 0) | .30 | −0.4 (−0.8 to 0) | −0.5 (−0.9 to −0.1)a | −0.4 (−0.8 to 0) | −0.8 (−1.2 to −0.4)a | −0.4 (−0.8 to 0.1) | −0.7 (−1.1 to −0.3)a | <.001 | .35 |
| Waist circumference, cm | 93 | 98.9 (12.3) | 99.7 (11.9) | −1.8 (−2.7 to −0.8)a | −1.5 (−2.4 to −0.5)a | .66 | −2.4 (−4.0 to −0.8)a | −2.2 (−3.7 to −0.7)a | −2.7 (−4.3 to −1.1)a | −3.5 (−4.1 to −2.0)a | −2.6 (−4.2 to −1.0)a | −3.5 (−5.1 to −2.0)a | <.001 | .34 |
| Waist-to-hip ratio | 93 | 0.94 (0.09) | 0.94 (0.08) | −0.01 (−0.02 to 0)a | −0.01 (−0.02 to 0)a | .92 | −0.02 (−0.03 to 0)a | −0.01 (−0.03 to 0)a | −0.02 (−0.03 to 0)a | −0.03 (−0.04 to 0)a | −0.02 (−0.03 to 0)a | −0.02 (−0.04 to 0)a | <.001 | .44 |
| Waist-to-height ratio | 93 | 0.57 (0.07) | 0.57 (0.06) | −0.01 (−0.02 to 0)a | −0.01 (−0.01 to 0)a | .82 | −0.01 (−0.02 to −0.01)a | −0.01 (−0.02 to 0)a | −0.02 (−0.03 to −0.01)a | −0.02 (−0.03 to −0.01)a | −0.02 (−0.02 to −0.01)a | −0.02 (−0.03 to −0.01)a | <.001 | .40 |
| Visceral adipose tissue measured by DEXA, cm2 | 93 | 190 (75) | 198 (74) | −9 (−14 to −4)a | −9 (−14 to −4)a | .99 | −13 (−24 to −3)a | −17 (−27 to −6)a | −14 (−25 to −3)a | −18 (−29 to −8)a | −7 (−18 to 4) | −16 (−26 to −5)a | <.001 | .52 |
| Lipid profile | ||||||||||||||
| Total cholesterol, mg/dL | 93 | 147 (31) | 147 (31) | −4 (−12 to 4) | 0 (−8 to 8) | .36 | 0 (−12 to 8) | 0 (−8 to 12) | 4 (−15 to 8) | 0 (−8 to 12) | 0 (−12 to 12) | 12 (4 to 23)a | .01 | .28 |
| LDL cholesterol, mg/dL | 93 | 77 (27) | 73 (23) | −4 (−12 to 4) | 0 (−8 to 8) | .28 | −4 (−12 to 8) | 0 (−8 to 8) | −4 (−12 to 4) | 0 (−8 to 12) | −4 (−12 to 4) | 4 (−4 to 15) | .70 | .30 |
| HDL cholesterol, mg/dL | 93 | 50 (12) | 50 (15) | 0 (0 to 4) | 0 (0 to 4) | .88 | 4 (0 to 8)a | 4 (0 to 4) | 4 (0 to 4) | 4 (0 to 4) | 4 (0 to 4)a | 4 (4 to 8)a | <.001 | .47 |
| Triglycerides, mg/dL | 93 | 124 (133) | 106 (53) | −18 (−35 to 9) | −9 (−27 to 9) | .60 | −18 (−35 to 9) | −9 (−27 to −9) | −9 (−35 to 9) | 0 (−18 to 18) | −9 (−35 to 18) | 9 (−9 to 27) | .06 | .57 |
| Glucose tolerance | ||||||||||||||
| Fasting glucose, mg/dL | 93 | 106 (29) | 110 (20) | −4 (−11 to 4) | −4 (−7 to 7) | .35 | −2 (−11 to 5) | 2 (−5 to 9) | −2 (−9 to 5) | 4 (−4 to 11) | −2 (−11 to 5) | 4 (−4 to 13) | .66 | .47 |
| Insulin resistance measured by HOMA | 93 | 2.7 (2.1) | 2.5 (1.8) | −0.2 (−0.7 to 0.3) | −0.2 (−0.7 to 0.3) | .96 | −0.1 (−0.9 to 0.6) | −0.1 (−0.7 to 0.6) | −0.3 (−1.0 to 0.4) | −0.1 (−0.8 to 0.7) | 0.1 (−0.6 to 0.9) | 0 (−0.7 to 0.7) | .61 | .90 |
| Blood pressure and heart rate | ||||||||||||||
| Peripheral systolic blood pressure, mm Hg | 93 | 128 (15) | 130 (14) | 2 (−1 to 5) | −3 (−7 to 0) | .03 | 0 (−5 to 6) | −3 (−8 to 2) | 1 (−5 to 6) | −3 (−9 to 2) | 2 (−4 to 7) | 1 (−4 to 7) | .60 | .14 |
| Peripheral diastolic blood pressure, mm Hg | 93 | 75 (10) | 74 (9) | 1 (−1 to 3) | −2 (−3 to 0) | .04 | 0 (−3 to 3) | −1 (−4 to 2) | −1 (−4 to 2) | −2 (−4 to 1) | 0 (−3 to 3) | 1 (−2 to 4) | .10 | .08 |
| Resting heart rate, beats/min | 93 | 57 (10) | 57 (8) | −3 (−4 to −1)a | −1 (−3 to 1) | .19 | −3 (−6 to 0)a | −2 (−5 to 1) | −4 (−7 to −1)a | −1 (−4 to 2) | −3 (−6 to 0)a | −2 (−5 to 1) | .001 | .18 |
| Quality of life and exercise enjoyment | ||||||||||||||
| McNew Global score | 93 | 5.9 (0.8) | 6.0 (0.6) | 0.5 (0.4 to 0.7)a | 0.4 (0.2 to 0.5)a | .17 | 0.5 (0.3 to 0.7)a | 0.4 (0.2 to 0.6)a | 0.5 (0.3 to 0.7)a | 0.4 (0.2 to 0.6)a | 0.5 (0.3 to 0.7)a | 0.4 (0.2 to 0.6)a | <.001 | .46 |
| McNew Physical score | 92 | 5.8 (1.0) | 5.9 (0.8) | 0.7 (0.5 to 0.9)a | 0.5 (0.2 to 0.7)a | .17 | 0.7 (0.4 to 1.0)a | 0.5 (0.3 to 0.8)a | 0.7 (0.5 to 1.0)a | 0.5 (0.3 to 0.8)a | 0.7 (0.4 to 1.0)a | 0.6 (0.4 to 0.9)a | <.001 | .31 |
| McNew Emotional score | 93 | 6.0 (0.6) | 6.0 (0.6) | 0.4 (0.2 to 0.5)a | 0.2 (0.1 to 0.4)a | .17 | 0.3 (0.1 to 0.5)a | 0.2 (0 to 0.4) | 0.2 (0 to 0.5)a | 0.2 (0 to 0.4) | 0.3 (0 to 0.5)a | 0.1 (−0.1 to 0.3) | <.001 | .55 |
| McNew Social score | 92 | 5.7 (1.0) | 6.0 (0.9) | 0.9 (0.6 to 1.1)a | 0.6 (0.3 to 0.8)a | .12 | 0.9 (0.6 to 1.1)a | 0.6 (0.4 to 0.9)a | 0.9 (0.6 to 1.2)a | 0.6 (0.3 to 0.9)a | 0.9 (0.6 to 1.2)a | 0.7 (0.4 to 0.9)a | <.001 | .14 |
| Exercise enjoyment, % | 92 | 74 (17) | 78 (16) | 3 (−2 to 8) | 3 (−2 to 8) | .96 | 6 (−1 to 13) | −2 (−9 to 4) | 1 (−6 to 8) | −3 (−10 to 3) | −2 (−9 to 6) | −5 (−11 to 2) | .02 | .15 |
Abbreviations: DEXA, dual-energy x-ray absorptiometry; HDL, high-density lipoprotein; HIIT, high-intensity interval training; HOMA, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; MICT, moderate-intensity continuous training.
SI conversion factors: To convert total cholesterol to millimoles per liter, multiply by 0.0259; LDL cholesterol to millimoles per liter, multiply by 0.0259; HDL cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; fasting glucose to millimoles per liter, multiply by 0.0555.
Significant difference from baseline.
Calculated as weight in kilograms divided by height in meters squared.
Safety
There were 9 serious adverse events reported during the study period, including 6 in the HIIT group and 3 in the MICT group (eTable 2 in Supplement 2). None of these were deemed by the treating physician to be a result of exercise training.
Exercise Adherence
Average training RPE was higher for HIIT compared with MICT (mean [SD] RPE: HIIT, 16.3 [1.3]; MICT, 12.4 [0.6]; P < .001), as was average training heart rate as a percentage of peak heart rate (mean (SD) percentage: HIIT, 87% [6]; MICT, 71% [8]; P < .001) (eTable 3 in Supplement 2). In stage 2 (home-based training), average training RPE was maintained at similar levels despite reduced supervision. Exercise adherence was high during the initial supervised stage (HIIT, 39 of 44 [91%]; MICT, 39 of 43 [91%]; P > .99) and reduced over the 12-month study period (HIIT, 18 of 34 [53%]; MICT, 15 of 37 [41%]; P = .35), with no differences between groups (eTable 4 in Supplement 2). After 6 months of home-based training, we observed a reduction in the number of participants training at the prescribed intensity, with 15 of 39 participants in the MICT group (38%) exercising at a higher intensity on their own accord and 9 of 37 participants in the HIIT group (24%) exercising at a lower intensity (eTable 4 in Supplement 2). Based on adherence to attendance and intensity, per-protocol analysis showed that HIIT was superior to MICT for improving VO2 peak at 12 months (HIIT, 5.2 [4.1] mL/kg/min [18% improvement]; MICT, 2.2 [4.1] mL/kg/min [8% improvement]; MD, 3.0 mL/kg/min; P = .02) (eTable 5 in Supplement 2).
Feasibility
Both HIIT and MICT reported high feasibility of the exercise protocols throughout the study period (eTable 6 in Supplement 2). The frequency and reasons stated for being unable to complete the exercise protocol were similar between groups, as well as unpleasant symptoms and injuries reported in relation to the exercise protocols.
Additional Outcomes
Following supervised training, there was a decrease in blood pressure after MICT compared with HIIT for both systolic pressure (mean [SD] blood pressure: HIIT, 2 [11] mm Hg; MICT, −3 [12] mm Hg; MD, 5 mm Hg; P = .03) and diastolic pressure (mean [SD] blood pressure: HIIT, 1 [6] mm Hg; MICT, −2 [6] mm Hg; MD, 3 mm Hg; P = .04) (Table 2). In contrast, similar significant reductions in blood pressure were observed with both HIIT and MICT in patients with hypertension at baseline (eTable 7 in Supplement 2). There were no group differences for any other cardiovascular risk factors (Table 2) or measures related to diet and physical activity (eTable 8 in Supplement 2). All quality of life domains improved over the study period, with no differences between groups (Table 2).
Discussion
This investigator-initiated study found that a 4-week supervised HIIT program improved cardiorespiratory fitness more than MICT without adversely affecting patient safety. However, the superior effect of HIIT was not maintained long term, with similar improvements to MICT at 12 months. Implementation of the HIIT protocol using RPE for exercise intensity was feasible. This should have broad applicability for traditional CR and home-based programs.
The greater efficacy of HIIT for improving VO2 peak compared with MICT during supervised training (MD, 1.7 mL/kg/min) is similar to previous meta-analyses reporting group differences of 1.5 to 1.6 mL/kg/min.4,5 This is clinically meaningful, as each 1–mL/kg/min improvement in VO2 peak during a CR program has been associated with a 6% reduction in hospital readmissions and 13% reduction in all-cause mortality.10 At 12 months, both groups showed similar improvement in VO2 peak; however, the MD between HIIT and MICT of 1.1 mL/kg/min could be considered clinically meaningful.
The greater reduction in systolic and diastolic blood pressure after short-term MICT compared with HIIT is in contrast to a recent meta-analysis reporting similar mean reductions in systolic (6 mm Hg) and diastolic (4 mm Hg) pressures for HIIT and MICT.11 In patients with hypertension at baseline,12 both HIIT and MICT reduced systolic and diastolic blood pressure. This is similar to the findings by Sosner et al,13 where HIIT only reduced blood pressure in those with initially elevated levels.
There were no deaths or cardiovascular events directly caused by the exercise interventions during the study period. One serious adverse event in the HIIT group occurred in relation to exercise training (postexercise hypotension); however, the treating physician diagnosed the cause as diuretic-induced dehydration. These findings are consistent with previous trials,14,15,16,17 which consistently demonstrate a favorable safety profile of HIIT programs. In the current study, medical exclusion following baseline CPET (3%) and further coronary intervention (1%) were very low. However, these safety data should still be interpreted in the context of the small size of the study and the requirement that all patients have CPET prior to enrollment, which is not routinely done for all patients referred for CR. To maximize safety in clinical populations, we have developed clinician guidelines for appropriate screening and monitoring for HIIT implementation.18
A number of single-center trials6,9,19 have demonstrated a 2-fold increase in VO2 peak with HIIT compared with MICT. In contrast, the multicenter Study on Aerobic Interval Exercise Training in Coronary Artery Disease Patients (SAINTEX-CAD) study17 found no differences between HIIT and MICT over 6 weeks and 12 weeks. There are a number of differences between the SAINTEX-CAD study and our trial. Principally, during supervised training, the SAINTEX-CAD trial did not restrain moderate continuous training participants to exercise at lower exercise intensities, with the notion that if higher intensities of continuous training can be sustained, workloads and heart rate zones should be modified for the greatest improvement.17 During unsupervised training, we also found a large proportion of participants in the MICT group (38%) exercising at a higher intensity (RPE of 15 or greater), indicating that prescribing exercise at moderate intensity is potentially not challenging enough for some patients. As a result of participants not training at the prescribed intensity after 6 months, the per-protocol analysis at 12 months showed a different result to the intention-to-treat analysis. Instead, HIIT demonstrated a superior effect on VO2 peak compared with MICT, with a mean group difference of 3.0 mL/kg/min. These results suggest that a superior benefit of HIIT may only persist for those who maintain 3 HIIT sessions per week following supervised CR.
Limitations
Our study had limitations. Patients were recruited from a single center, and there were low rates of female patients and patients with left ventricular dysfunction, type 2 diabetes, and a history of tobacco smoking. As the primary intervention was conducted in a CR setting, optimization of drug therapy was at the discretion of the treating physician. While RPE-based prescription of exercise intensity is well accepted in CR and broadens protocol applicability, we acknowledge RPE ranges can result in a wide range of training intensities.20 Furthermore, despite patients receiving education from CR clinicians on how to progress their exercise protocols, there is limited published evidence that patients targeting an RPE range will inherently increase their workload over time. There were a number of patients who failed to maintain adherence to the prescribed exercise program (although rates were equal in both groups), and some participants in the MICT group exercised more frequently and at higher intensities than prescribed, increasing the likelihood of type 2 error. Additionally, the provision of heart rate monitors only for participants in the HIIT group during the initial 3 months could have enhanced exercise adherence, motivation, and achievement of intended heart rate targets during the initial stages of home-based exercise.
Conclusions
This study demonstrates that HIIT is superior to MICT for improving cardiorespiratory fitness during a 4-week hospital-based CR program in patients with CAD but offers similar improvement to MICT at 12 months. The HIIT protocol was safe, feasible, and successfully implemented in a home-based environment with similar adherence to MICT over 12 months. Further improvement in cardiorespiratory fitness after 12 months in patients undertaking HIIT was limited to those with good exercise adherence. These findings support the inclusion of HIIT in CR programs as an alternative or an adjunct to standard moderate-intensity exercise, allowing for prescription based on patient goals, preferences, and capabilities.
Trial protocol.
eMethods.
eTable 1. Medication adjustments throughout the study period.
eTable 2. Serious adverse events.
eTable 3. Exercise intensity during stages 1 and 2.
eTable 4. Adherence to exercise training protocols.
eTable 5. Per-protocol analyses.
eTable 6. Participant feasibility.
eTable 7. Sensitivity analyses.
eTable 8. Habitual physical activity and dietary intake.
Data sharing statement.
References
- 1.Martin BJ, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126(6):677-687. doi: 10.1161/CIRCULATIONAHA.111.066738 [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666-671. doi: 10.1161/01.CIR.0000024413.15949.ED [DOI] [PubMed] [Google Scholar]
- 3.Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156(2):292-300. doi: 10.1016/j.ahj.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 4.Elliott AD, Rajopadhyaya K, Bentley DJ, Beltrame JF, Aromataris EC. Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart Lung Circ. 2015;24(2):149-157. doi: 10.1016/j.hlc.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 5.Pattyn N, Coeckelberghs E, Buys R, Cornelissen VA, Vanhees L. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Med. 2014;44(5):687-700. doi: 10.1007/s40279-014-0158-x [DOI] [PubMed] [Google Scholar]
- 6.Mezzani A, Hamm LF, Jones AM, et al. ; European Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation . Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20(3):442-467. doi: 10.1177/2047487312460484 [DOI] [PubMed] [Google Scholar]
- 7.Taylor J, Keating SE, Leveritt MD, Holland DJ, Gomersall SR, Coombes JS. Study protocol for the FITR Heart Study: feasibility, safety, adherence, and efficacy of high intensity interval training in a hospital-initiated rehabilitation program for coronary heart disease. Contemp Clin Trials Commun. 2017;8:181-191. doi: 10.1016/j.conctc.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher GF, Ades PA, Kligfield P, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873-934. doi: 10.1161/CIR.0b013e31829b5b44 [DOI] [PubMed] [Google Scholar]
- 9.Borg G. Borg's Perceived Exertion and Pain Scales. Human Kinetics; 1998. [Google Scholar]
- 10.Mikkelsen N, Cadarso-Suárez C, Lado-Baleato O, et al. Improvement in VO2peak predicts readmissions for cardiovascular disease and mortality in patients undergoing cardiac rehabilitation. Eur J Prev Cardiol. 2020;27(8):811-819. doi: 10.1177/2047487319887835 [DOI] [PubMed] [Google Scholar]
- 11.Costa EC, Hay JL, Kehler DS, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018;48(9):2127-2142. doi: 10.1007/s40279-018-0944-y [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 13.Sosner P, Gayda M, Drigny J, et al. Effects of long-term lifestyle and high-intensity interval training intervention on blood pressure reduction in patients with abdominal obesity. Eur Heart J. 2013;34(suppl 1):3648. doi: 10.1093/eurheartj/eht309.P3648 [DOI] [Google Scholar]
- 14.Keteyian SJ, Hibner BA, Bronsteen K, et al. Greater improvement in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. J Cardiopulm Rehabil Prev. 2014;34(2):98-105. doi: 10.1097/HCR.0000000000000049 [DOI] [PubMed] [Google Scholar]
- 15.Moholdt T, Aamot IL, Granøien I, et al. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: a randomized controlled study. Clin Rehabil. 2012;26(1):33-44. doi: 10.1177/0269215511405229 [DOI] [PubMed] [Google Scholar]
- 16.Rognmo Ø, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436-1440. doi: 10.1161/CIRCULATIONAHA.112.123117 [DOI] [PubMed] [Google Scholar]
- 17.Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203-210. doi: 10.1016/j.ijcard.2014.10.155 [DOI] [PubMed] [Google Scholar]
- 18.Taylor JL, Holland DJ, Spathis JG, et al. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog Cardiovasc Dis. 2019;62(2):140-146. doi: 10.1016/j.pcad.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 19.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216-222. doi: 10.1097/01.hjr.0000131677.96762.0c [DOI] [PubMed] [Google Scholar]
- 20.Joo KC, Brubaker PH, MacDougall A, Saikin AM, Ross JH, Whaley MH. Exercise prescription using resting heart rate plus 20 or perceived exertion in cardiac rehabilitation. J Cardiopulm Rehabil. 2004;24(3):178-184. doi: 10.1097/00008483-200405000-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eMethods.
eTable 1. Medication adjustments throughout the study period.
eTable 2. Serious adverse events.
eTable 3. Exercise intensity during stages 1 and 2.
eTable 4. Adherence to exercise training protocols.
eTable 5. Per-protocol analyses.
eTable 6. Participant feasibility.
eTable 7. Sensitivity analyses.
eTable 8. Habitual physical activity and dietary intake.
Data sharing statement.