Abstract
This study investigates the use of cardiac magnetic resonance imaging in competitive athletes who recovered from coronavirus disease 2019 (COVID-10) to detect myocardial inflammation that would identify high-risk athletes for return to competitive play.
Myocarditis is a significant cause of sudden cardiac death in competitive athletes and can occur with normal ventricular function.1 Recent studies have raised concerns of myocardial inflammation after recovery from coronavirus disease 2019 (COVID-19), even in asymptomatic or mildly symptomatic patients.2 Our objective was to investigate the use of cardiac magnetic resonance (CMR) imaging in competitive athletes recovered from COVID-19 to detect myocardial inflammation that would identify high-risk athletes for return to competitive play.
Methods
We performed a comprehensive CMR examination including cine, T1 and T2 mapping, extracellular volume fraction, and late gadolinium enhancement (LGE), on a 1.5-T scanner (Magnetom Sola; Siemens Healthineers) using standardized protocols,3 in all competitive athletes referred to the sports medicine clinic after testing positive for COVID-19 (reverse transcriptase–polymerase chain reaction) between June and August 2020. The Ohio State University institutional review board approved the study, and informed consent in writing was obtained from participating athletes. Cardiac magnetic resonance imaging was performed after recommended quarantine (11-53 days). Electrocardiogram, serum troponin I, and transthoracic echocardiogram were performed on day of CMR imaging.
Results
We performed CMR imaging in 26 competitive college athletes (mean [SD] age, 19.5 [1.5] years; 15 male [57.7%]) from the following sports: football, soccer, lacrosse, basketball, and track. No athletes required hospitalization or received COVID-19–specific antiviral therapy. Twelve athletes (26.9%; including 7 female individuals) reported mild symptoms during the short-term infection (sore throat, shortness of breath, myalgias, fever), while others were asymptomatic. There were no diagnostic ST/T wave changes on electrocardiogram, and ventricular volumes and function were within the normal range in all athletes by transthoracic echocardiogram and CMR imaging. No athlete had elevated serum levels of troponin I. Four athletes (15%; all male individuals) had CMR findings consistent with myocarditis based on the presence of 2 main features of the updated Lake Louise Criteria: myocardial edema by elevated T2 signal and myocardial injury by presence of nonischemic LGE (Figure).4 Pericardial effusion was present in 2 athletes with CMR evidence of myocarditis. Two of these 4 athletes with evidence of myocardial inflammation had mild symptoms (shortness of breath), while the other 2 were asymptomatic. Twelve athletes (46%) had LGE (mean of 2 American Heart Association segments), of whom 8 (30.8%) had LGE without concomitant T2 elevation (Table). Mean (SD) T2 in those with suspected myocarditis was 59 (3) milliseconds compared with 51 (2) milliseconds in those without CMR evidence of myocarditis.
Figure. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From Coronavirus Disease 2019 Infection.
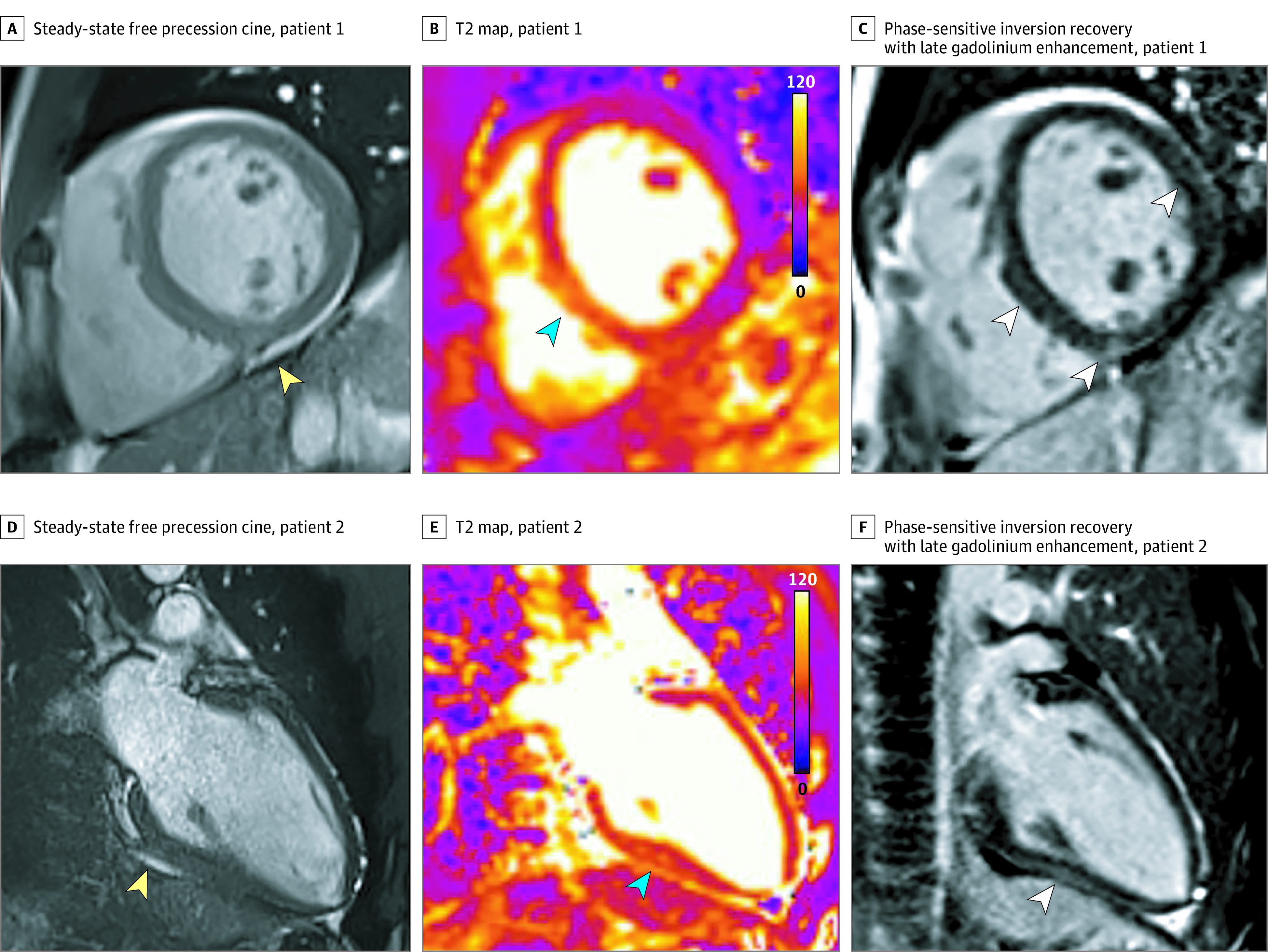
A, Cine mid short-axis images showing pericardial effusion indicated by yellow arrowhead. B, T2 map with color overlay mid short-axis showing myocardial edema (elevated T2, 61 milliseconds) indicated by blue arrowhead. C, Short-axis view showing late gadolinium enhancement in the mid inferoseptum, right ventricular insertion point, and mid anterolateral wall indicated by white arrowheads. D, Cine 2-chamber long-axis view showing pericardial effusion indicated by yellow arrowhead. E, T2 map with color overlay myocardial edema (elevated T2, 58 milliseconds) indicated by blue arrowhead. F, Right 2-chamber long-axis view showing epicardial late gadolinium enhancement in the inferior wall indicated by white arrowhead.
Table. Demographic Features and Echocardiographic and Cardiovascular Magnetic Resonance Parameters in Competitive Athletes Recovering From Coronavirus Disease 2019a.
| Athlete No. | Sex | Symptoms | Time CMR performed after positive test result, d | Echocardiography, mL/m2 | CMR, % | Native T1, ms | ECV, % | Maximal T2, ms, (AHA segments) | LGE (pattern/AHA segments) | CMR (updated Lake Louise Criteria) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEDV | RVEDV | LVEF | RVEF | |||||||||
| 1 | Male | No | 21 | Not done | Not done | 60 | 49 | 1034 | 21 | 51 (9) | Yes (RV insertion; 9) | Normal |
| 2 | Male | No | 22 | 51 | 46 | 56 | 59 | 964 | 24 | 48 (9) | Yes (patchy; 6, 8) | Normal |
| 3 | Male | No | 22 | 65 | 60 | 60 | 64 | 953 | 22 | 48 (10) | Yes (patchy, 5) | Normal |
| 4 | Male | No | 15 | 65 | 48 | 59 | 54 | 905 | 20 | 48 (9) | Yes (linear; 8, 12) | Normal |
| 5 | Male | No | 17 | 66 | 57 | 55 | 54 | 994 | 24 | 55 (9) | Yes (epicardial; 3, 9) | Myocarditis |
| 6 | Male | Yes | 23 | 73 | 52 | 61 | 62 | 947 | 26 | 63 (3, 9) | Yes (patchy; 3, 9) | Myocarditis |
| 7 | Male | Yes | 53 | 66 | 64 | 53 | 52 | 991 | 25 | 49 (7, 9) | Yes (linear, patchy; 8, 9, 12) | Normal |
| 8 | Male | No | 20 | 76 | 36 | 56 | 53 | 963 | 17 | 51 (10) | No | Normal |
| 9 | Male | Yes | 18 | 60 | 71 | 56 | 52 | 964 | 24 | 52 (7) | Yes (patchy; 3, 9) | Normal |
| 10 | Male | Yes | 11 | 67 | 70 | 61 | 58 | 929 | 25 | 58 (8, 9) | Yes (patchy; 2, 3, 8, 9) | Myocarditis |
| 11 | Male | No | 23 | 57 | 49 | 63 | 60 | 987 | 22 | 53 (7) | No | Normal |
| 12 | Male | Yes | 28 | 72 | 59 | 50 | 53 | 966 | 28 | 53 (7, 8) | No | Normal |
| 13 | Male | No | 28 | 81 | 52 | 33 | 53 | 925 | 25 | 53 (7, 8) | No | Normal |
| 14 | Male | No | 11 | 46 | 41 | 65 | 54 | 989 | 24 | 53 (8) | No | Normal |
| 15 | Male | No | 48 | 56 | 51 | 59 | 57 | 1003 | 25 | 53 (7) | Yes (RV insertion; 9) | Normal |
| 16 | Female | Yes | 23 | 68 | 50 | 64 | 58 | 1001 | 26 | 52 (8) | No | Normal |
| 17 | Female | Yes | 23 | 55 | 56 | 57 | 60 | 1030 | 28 | 48 (10) | No | Normal |
| 18 | Female | No | 21 | 53 | 35 | 65 | 66 | 1008 | 25 | 48 (9) | No | Normal |
| 19 | Female | Yes | 17 | 60 | 32 | 63 | 57 | 978 | 26 | 53 (8) | No | Normal |
| 20 | Female | No | 31 | 62 | 51 | 58 | 59 | 1002 | 25 | 52 (8) | No | Normal |
| 21 | Female | Yes | 31 | 52 | 40 | 60 | 60 | 946 | 28 | 53 (8) | No | Normal |
| 22 | Female | Yes | 30 | 67 | 49 | 59 | 64 | 1000 | 27 | 52 (8) | Yes (linear; 12) | Normal |
| 23 | Female | Yes | 30 | 58 | 57 | 57 | 55 | 964 | 26 | 53 (11) | No | Normal |
| 24 | Female | Yes | 26 | 52 | 49 | 55 | 57 | 1010 | 30 | 53 (10) | No | Normal |
| 25 | Female | No | 31 | 56 | 36 | 56 | 56 | 1027 | 28 | 50 (7) | No | Normal |
| 26 | Male | No | 12 | 80 | 44 | 60 | 53 | 969 | 21 | 61 (8) | Yes (linear; 8, 9) | Myocarditis |
Abbreviations: AHA, American Heart Association; CMR, cardiovascular magnetic resonance imaging; ECV, extracellular volume fraction; EDV, end-diastolic volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricular; RV, right ventricular.
Symptoms refer to symptoms during short-term infection. Echo volumes were calculated by 3-dimensional method. Cardiovascular magnetic resonance imaging–derived left and right ventricular volumes and function were measured from contiguous short-axis cine images using semiautomated software for endocardial segmentation using endocardial and epicardial contours at end systole and end diastole per standard protocol. Cardiovascular magnetic resonance imaging–derived myocardial T1 and T2 mapping and ECV were done per standard guidelines. Mean (SD) native T1 less than 999 (31) milliseconds, native T2 of less than 53 milliseconds, and ECV of less than 29% were considered normal per institutional protocol based on phantom and human volunteer experiments. T2 and LGE were only considered significant if seen in 2 orthogonal planes.
Discussion
Of 26 competitive athletes, 4 (15%) had CMR findings suggestive of myocarditis and 8 additional athletes (30.8%) exhibited LGE without T2 elevation suggestive of prior myocardial injury. COVID-19–related myocardial injury in competitive athletes and sports participation remains unclear. Cardiac magnetic resonance imaging has the potential to identify a high-risk cohort for adverse outcomes and may, importantly, risk stratify athletes for safe participation because CMR mapping techniques have a high negative predictive value to rule out myocarditis.4 A recent study by Puntmann et al2 demonstrated cardiac involvement in a significant number of patients who had recovered from COVID-19. A recent expert consensus article recommended 2-week convalescence followed by no diagnostic cardiac testing if asymptomatic and an electrocardiogram and transthoracic echocardiogram in mildly symptomatic athletes with COVID-19 to return to play for competitive sports.5 However, emerging knowledge and CMR observations question this recommendation. Cardiac magnetic resonance imaging evidence of myocardial inflammation has been associated with poor outcomes, including myocardial dysfunction and mortality.6 Study limitations include lack of baseline CMR imaging and variable timing of CMR imaging from a positive COVID-19 test result. Athletic cardiac adaptation could be responsible for these abnormalities; however, in this cohort, mean (SD) T2 in those with suspected myocarditis was 59 (3) milliseconds vs 51 (2) milliseconds in those without, favoring pathology. Additionally, the rate of LGE (42%) is higher than in previously described normative populations. To conclude, while long-term follow-up and large studies including control populations are required to understand CMR changes in competitive athletes, CMR may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.
References
- 1.Maron BJ, Udelson JE, Bonow RO, et al. ; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology . Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273-e280. [DOI] [PubMed] [Google Scholar]
- 2.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online July 27, 2020. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). [published correction appears in J Cardiovasc Magn Reson. 2018;20(1):9]. J Cardiovasc Magn Reson. 2017;19(1):75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 5.Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.2136 [DOI] [PubMed] [Google Scholar]
- 6.Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. [published correction appears in J Am Coll Cardiol. 2017;70(21):2736]. J Am Coll Cardiol. 2017;70(16):1964-1976. doi: 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]


