Abstract
This cross-sectional study used nasal epithelium collected in 2015-2018 to compare expression of TMPRSS2, a facilitator of SARS-CoV-2 viral entry and spread, among Asian, Black, Latino, and White patients as well as patients of mixed race/ethnicity within a New York City health system.
Coronavirus disease 2019 (COVID-19) has disproportionately affected communities of color.1,2 In many areas of the US, infection and death rates for COVID-19 are 2 to 3 times higher in Black individuals than their proportion of the population.1,2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spread by airway contact and uses transmembrane serine protease 2 (TMPRSS2) to facilitate viral entry and spread.3 Host-expressed TMPRSS2 on nasal and bronchial epithelium activates the SARS-CoV-2 spike protein and cleaves the angiotensin-converting enzyme 2 receptor to which the virus binds, enabling SARS-CoV-2 to enter the body.3
Racial/ethnic differences in TMPRSS2 gene–related activity in prostate tissue have been associated with disproportionately higher incidence of prostate cancer in Black men vs White men.4 Recognizing that many factors contribute to COVID-19 health disparities, we investigated TMPRSS2 nasal gene expression in a racially/ethnically diverse cohort.
Methods
This cross-sectional study used nasal epithelium collected during 2015-2018 from individuals within the Mount Sinai Health System (New York, New York), a cohort we have previously studied.5 Healthy individuals and individuals with asthma aged 4 to 60 years underwent nasal brushing for research on asthma biomarkers.
Self-identified race/ethnicity was queried given prior associations between race/ethnicity and asthma. RNA isolation of brushings followed by RNA sequencing, sequence alignment, and normalization were performed. The Mount Sinai institutional review board approved the study. Written informed consent was obtained from participants.
Linear regression modeling adjusted for age, sex, and asthma with TMPRSS2 expression in log2 counts per million as the dependent variable and self-identified race/ethnicity as the independent variable was performed using R version 3.6.0 (R Foundation for Statistical Computing). Two-sided tests and a significance threshold of P ≤ .05 were used.
Results
The cohort (n = 305) was 8.2% Asian individuals, 15.4% Black individuals, 26.6% Latino individuals, 9.5% individuals of mixed race/ethnicity, and 40.3% White individuals. Of the participants, 48.9% were male and 49.8% had asthma.
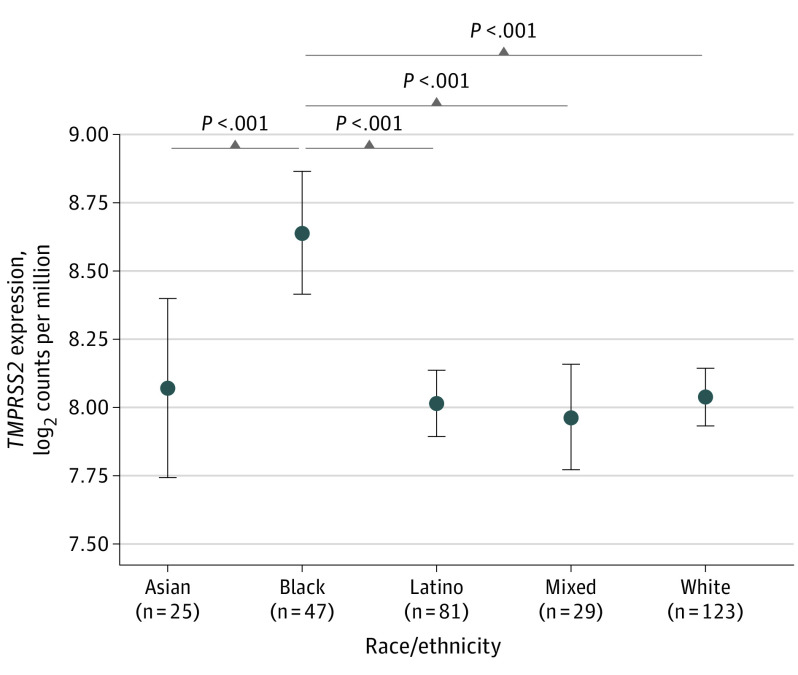
Among the racial/ethnic groups, nasal gene expression of TMPRSS2 was highest in Black individuals (n = 47; mean, 8.64 [95% CI, 8.41-8.86] log2 counts per million) compared with Asian individuals (n = 25; mean, 8.07 [95% CI, 7.74-8.40] log2 counts per million), Latino individuals (n = 81; mean, 8.02 [95% CI, 7.90-8.14] log2 counts per million), individuals of mixed race/ethnicity (n = 29; mean, 7.97 [95% CI, 7.77-8.16] log2 counts per million), and White individuals (n = 123; mean, 8.04 [95% CI, 7.94-8.15] log2 counts per million) (Figure).
Figure. Nasal Gene Expression of TMPRSS2 in Self-identified Racial/Ethnic Groups.
The data points indicate means and the error bars indicate 95% CIs for transmembrane serine protease 2 (TMPRSS2) gene expression in self-identified racial/ethnic groups. The P values were calculated using linear regression modeling in which TMPRSS2 gene expression was the dependent variable and race/ethnicity was the independent variable.
TMPRSS2 expression was significantly higher in Black individuals compared with Asian, Latino, mixed race/ethnicity, and White individuals (all P < .001) based on linear regression (Figure and Table). There were no significant associations between TMPRSS2 expression and sex, age, or asthma.
Table. β Coefficients for Race/Ethnicity From Linear Regression Modelinga.
| Race/ethnicity | Unadjusted β coefficient (95% CI)b | P value | Adjusted β coefficient (95% CI)b,c | P value |
|---|---|---|---|---|
| Black | [Reference] | [Reference] | ||
| Asian | −0.57 (−0.87 to −0.27) | <.001 | −0.63 (−0.94 to −0.32) | <.001 |
| Latino | −0.62 (−0.85 to −0.40) | <.001 | −0.64 (−0.86 to −0.42) | <.001 |
| Mixed race | −0.67 (−0.96 to −0.39) | <.001 | −0.66 (−0.95 to −0.37) | <.001 |
| White | −0.60 (−0.81 to −0.39) | <.001 | −0.60 (−0.81 to −0.39) | <.001 |
TMPRSS2 expression was the dependent variable and self-identified race/ethnicity was the independent variable.
β coefficients indicate the difference in TMPRSS2 expression in log2 counts per million between a given race/ethnicity and Black individuals.
Adjusted for age, sex, and asthma.
Discussion
This study of nasal epithelial gene expression in a racially/ethnically diverse cohort showed significantly higher expression of TMPRSS2 in Black individuals compared with other self-identified races/ethnicities. Given the essential role of TMPRSS2 in SARS-CoV-2 entry,3 higher nasal expression of TMPRSS2 may contribute to the higher burden of COVID-19 among Black individuals. TMPRSS2 inhibitors such as camostat mesylate3 are undergoing clinical trials to test their utility for COVID-19 treatment. The finding of racial/ethnic variation in TMPRSS2 expression emphasizes that inclusion of diverse participants and analyses stratified by race/ethnicity should be incorporated into such trials.
The limitations of this study include its modest cohort size, constraint to 1 metropolitan region, and participant age range of 4 to 60 years. Although this study suggests one factor that may partially contribute to COVID-19 risk among New York–area Black individuals, many additional factors are likely, especially because gene expression and race/ethnicity reflect multiple social, environmental, and geographic factors.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. doi: 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 2.Oppel RA, Gebeloff R, Lai KKR, Wright W, Smith M The fullest look yet at the racial inequality of coronavirus. Accessed August 31, 2020. https://www.nytimes.com/interactive/2020/07/05/us/coronavirus-latinos-african-americans-cdc-data.html
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, et al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan J, Kensler KH, Hu Z, et al. . Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 2020;16(2):e1008641. doi: 10.1371/journal.pgen.1008641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427-2429. doi: 10.1001/jama.2020.8707 [DOI] [PMC free article] [PubMed] [Google Scholar]