This cohort study assesses the association of miglustat therapy with swallowing dysfunction in individuals with Niemann-Pick disease, type C1.
Key Points
Question
Does miglustat use preserve swallowing function in individuals with Niemann-Pick disease, type C1?
Findings
In this cohort study of 50 individuals with Niemann-Pick disease, type C1 with neurologic disease onset before age 15 years, miglustat use was associated with stabilized swallowing function and reduced aspiration risk. Untreated patients were more likely to progress in swallowing dysfunction and have worse aspiration risk than those receiving miglustat therapy.
Meaning
This finding supports the efficacy of miglustat therapy in Niemann-Pick disease, type C1 in reducing aspiration risk and improving quality of life due to progressive oropharyngeal swallowing dysfunction aspiration pneumonia.
Abstract
Importance
Niemann-Pick disease, type C1 (NPC1) is a progressive neurovisceral disease with no US Food and Drug Administration–approved therapy. Miglustat, a drug used off-label in the United States for the treatment of NPC1, appears to stabilize neurologic disease progression. Several prospective trials suggest that miglustat stabilizes oropharyngeal swallowing function; however, its effect on dysphagia and aspiration risk has not been demonstrated instrumentally.
Objective
To determine if miglustat therapy is associated with stabilized swallowing dysfunction in individuals with NPC1.
Design, Setting, and Participants
Patients with confirmed NPC1 diagnoses were evaluated in a single-center cohort study of NPC1 from April 1997 to November 2019. Longitudinal data from individuals with neurologic disease onset prior to age 15 years were analyzed. The study population was divided into those with neurologic disease onset in early childhood (age <6 years) and late childhood (age ≥6 years and <15 years). Analysis began September 2019.
Exposures
Oral miglustat at baseline and at follow-up.
Main Outcomes and Measures
Oropharyngeal swallowing function was assessed with videofluoroscopic swallowing studies. Overall swallowing ability and aspiration risk were evaluated using the American Speech-Language-Hearing Association National Outcome Measurement System swallowing domain and an adapted Rosenbek aspiration-penetration scale, respectively.
Results
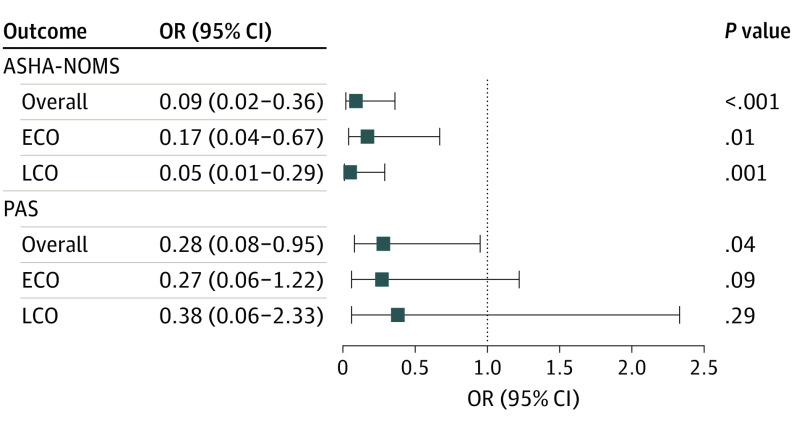
Overall, 50 participants were evaluated at baseline (median [interquartile range] age, 9.4 [3.4-16.4] years; 26 [52%] female). The median (interquartile range) duration of follow-up was 3.0 (1.1-4.4) years. Miglustat use was associated with decreased odds of worse American Speech-Language-Hearing Association National Outcome Measurement System swallowing domain outcomes in all 3 subsets (overall: odds ratio [OR], 0.09 [95% CI, 0.02-0.36); P < .001; early childhood: OR, 0.17 [95% CI, 0.04-0.67]; P = .01; late childhood: OR, 0.05 [95% CI, 0.01-0.29]; P = .001). Miglustat use was associated with decreased odds of worse Rosenbek aspiration-penetration scale outcomes in the overall cohort (OR, 0.28 [95% CI, 0.08-0.95]; P = .04) but not in each subgroup (early childhood: OR, 0.27 [95% CI, 0.06-1.22]; P = .09; late childhood: OR, 0.38 [95% CI, 0.06-2.33]; P = .29).
Conclusions and Relevance
These data suggest that miglustat use is associated with stabilized swallowing function and reduced aspiration risk in NPC1, thus supporting its use in this population. In addition, these data demonstrate that a quantification of swallowing dysfunction can be used as a clinically relevant, functional outcome measure in future therapeutic trials in NPC1.
Introduction
Niemann-Pick disease, type C1 (NPC1) is a progressive, autosomal recessive neurovisceral disease with an estimated incidence of approximately 1 in 100 000 live births.1 Defects in NPC1 lead to endolysosomal accumulation of unesterified cholesterol and glycosphingolipids, which are responsible for neurologic and visceral morbidities.2 Miglustat is thought to stabilize neurologic disease in NPC1 and has been approved in many countries.3 The US Food and Drug Administration has approved miglustat for the treatment of Gaucher disease; therefore, it is frequently prescribed off-label for the treatment of NPC1 in the US. Because miglustat is not a Food and Drug Administration–approved treatment for NPC1, patients in the US often have difficulty obtaining insurance coverage.
In NPC1, progressive neurologic deterioration leads to cerebellar ataxia, gelastic cataplexy, dementia, supranuclear vertical gaze palsy, dysarthria, and dysphagia.1 Dysphagia is a known morbidity in NPC1, affecting between 55% and 80% of this population.4 It is a risk factor for mortality because it is a common cause of aspiration pneumonia, which accounts for approximately two-thirds of deaths in this population.4,5 Because dysphagia is prevalent in NPC1 and is associated with mortality, it is a clinically relevant outcome measure to follow.6
Prospective therapeutic trials have assessed the effect of miglustat on dysphagia in NPC1 and suggest that this therapy may stabilize or improve swallowing function.7,8,9 However, their lack of instrumental assessments, comparative data, and natural history data of dysphagia in NPC1 limit their findings. Further evidence of the efficacy of miglustat on swallowing outcomes is needed to justify its use in this population and support insurance coverage in the US. Using videofluoroscopic swallow study (VFSS) data from an observational study of NPC1, we identify that miglustat may preserve swallowing function and decrease aspiration risk.
Methods
A total of 120 individuals met inclusion criteria and participated in research procedures under a Eunice Kennedy Shriver National Institute of Child Health and Human Development institutional review board–approved natural history study of NPC1 (NCT00001367) from April 1997 to November 2019. Participants and guardians provided consent and assent when appropriate. Individuals who had not yet developed neurologic symptoms (n = 6) or had neurologic disease onset in adulthood (age ≥15 years) (n = 11) and patients with only a single evaluation (n = 53) were excluded from the analysis.
To assess swallowing ability and safety, VFSS was performed when clinically indicated per protocol guidelines. Following each evaluation, a single speech-language pathologist (B.I.S.) summarized swallowing impairments and applied the American Speech-Language-Hearing Association National Outcome Measurement System (ASHA-NOMS) swallowing domain and an adapted Rosenbek penetration/aspiration scale (PAS) to assess for overall ability to eat and swallow safely and aspiration severity, respectively.10,11 Outcome measure scores were reversed to reflect increasing disease severity from low to high.
Data were described using frequency (percentage), descriptive statistics, and data variability. Patient and disease characteristics at baseline are reported. Longitudinal analyses of follow-up data were performed using generalized linear models for repeated measures (proc GENMOD with generalized estimating equation using cumulative logit link) with independent working correlations for ordinal multinomial models. Probabilities for severity of disease (disease progression) were modeled for the ASHA-NOMS and PAS outcomes. Models accounted for the association of miglustat use with outcomes, seizure history, duration of neurologic symptoms, and duration of follow-up in the overall cohort, early childhood onset (ECO; age <6 years) subgroup, and late childhood onset (LCO; age ≥6 years and <15 years) subgroup for a total of 6 models. One patient discontinued miglustat use during the study; data collected after discontinuation (1 visit) were excluded. Analyses were done using SAS version 9.4 (SAS Institute Inc). Two-sided P values were used with a significance threshold of .05. Analysis began September 2019.
Results
We collected longitudinal data on 50 participants (median [interquartile range] age, 9.4 [3.4-16.4] years; 26 [52%] female) for a median (IQR) of 3.0 (2.0-4.0) visits over 3.0 (1.1-4.4) years (range, 0.6-10.3 years). At baseline, 21 participants (42%) were receiving standard-dose miglustat therapy orally or parenterally. Fifteen participants (30%) initiated miglustat therapy during the study. The median (IQR) duration of miglustat therapy was 3.1 (1.2-5.4) years. Miglustat use coincided with 109 of 182 swallowing evaluations (60%) (Table).
Table. Patient Enrollment and Demographicsa.
| Characteristic | Overall cohort | ECO cohort | LCO cohort |
|---|---|---|---|
| Individuals with NPC1, No. | 50 | 30 | 20 |
| Sex, No. (%) | |||
| Male | 24 (48) | 14 (47) | 10 (50) |
| Female | 26 (52) | 16 (53) | 10 (50) |
| Age at neurologic disease onset, median (IQR), y | 3.5 (2.0-7.7) | 2.0 (1.5-3.0) | 8.0 (6.5-11.0) |
| Age at baseline evaluation, median (IQR), y | 9.4 (3.4-16.4) | 4.4 (2.4-10.0) | 16.6 (11.6-21.6) |
| Individuals taking miglustat, No. (%) | |||
| Baseline | 21 (42) | 11 (37) | 10 (50) |
| Follow-up | 36 (72) | 21 (70) | 15 (75) |
| Duration of miglustat treatment, median (IQR), y | 3.1 (1.2-5.4) | 3.2 (1.2-5.5) | 2.9 (1.8-5.2) |
| Evaluations of individuals with seizures, No. (%) | 69 (38) | 51 (43) | 18 (28) |
| Evaluations of individuals taking miglustat, No. (%) | 109 (60) | 66 (56) | 43 (67) |
| Total No. of evaluations | 182 | 118 | 64 |
| Duration of follow-up, median (IQR), y | 3.0 (1.1-4.4) | 3.3 (1.1-4.4) | 2.8 (1.1-4.5) |
Abbreviations: ECO, early childhood onset; IQR, interquartile range; LCO, late childhood onset; NPC1, Niemann-Pick disease, type C1.
Neurologic disease presented in individuals younger than 15 years in the overall cohort, younger than 6 years in the ECO cohort, and 6 or older and younger than 15 years in the LCO cohort.
Figure 1 shows predicted cumulative probabilities of disease progression and detailed results from multivariate longitudinal models. Comparing the curves for each severity level across time corresponds with lower probability of disease progression for miglustat use vs no use for both outcomes.
Figure 1. Predicted Cumulative Probabilities of Disease Progression for Miglustat Use vs No Use From Longitudinal Models .
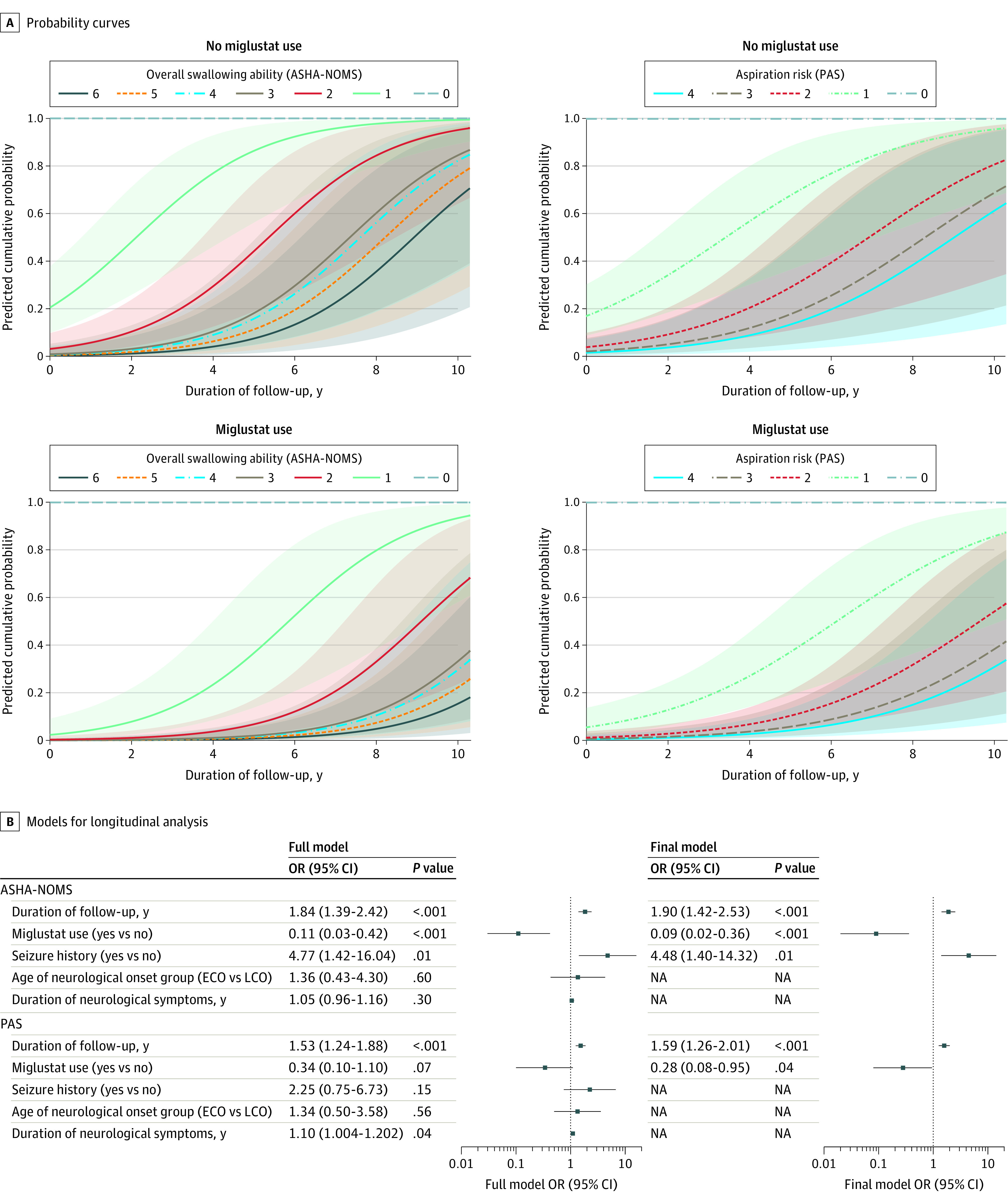
A, Shaded regions indicate 95% CI for American Speech-Language-Hearing Association National Outcome Measurement System swallowing domain (ASHA-NOMS; range, 0 to 6 where 0 indicates ability to eat independently and 6 indicates swallowing is not safe by mouth) and Rosenbek aspiration-penetration scale (PAS; range, 0 to 4 where 0 indicates no obvious aspiration risk or penetration and 4 indicates profound aspiration). B, Analyses were performed using generalized linear models for repeated measures with independent working correlations for ordinal multinomial models. Each model also adjusted for baseline levels of the outcome variable. The full model was based on a priori hypothesis, and the final model retained variables following iterative model fitting, which are reflected in panel A. ECO indicates early childhood onset (age <6 years); LCO, late childhood onset (age ≥6 and <15 years); NA, not applicable; OR, odds ratio.
Longitudinal analyses also showed that miglustat was associated with decreased odds of worse ASHA-NOMS outcomes in all 3 subsets (overall: odds ratio [OR], 0.09 [95% CI, 0.02-0.36]; P < .001; ECO: OR, 0.17 [95% CI, 0.04-0.67]; P = .01; LCO: OR, 0.05 [95% CI, 0.01-0.29]; P = .001). Miglustat use was also associated with decreased odds of worse PAS outcomes in the overall cohort (OR, 0.28 [95% CI, 0.08-0.95]; P = .04); however, this association was not observed in either subgroup (ECO: OR, 0.27 [95% CI, 0.06-1.22]; P = .09; LCO: OR, 0.38 [95% CI, 0.06-2.33]; P = .29) (Figure 2). In the overall cohort, the ECO subgroup, and the LCO subgroup, miglustat therapy was associated with reduced risk for the deterioration of swallowing function (ASHA-NOMS) by 91%, 83%, and 95%, respectively (Figure 2). Similarly, miglustat use was associated with a decreased penetration/aspiration risk in the overall population (by 72%).
Figure 2. Association of Miglustat Use With Overall Ability to Eat and Swallow Safely and Aspiration Risk .
Data are odds ratio (OR) and 95% CIs for miglustat use vs not. Longitudinal analyses of follow-up data were performed using generalized linear models for repeated measures with independent working correlations for ordinal multinomial models. Probabilities for severity of disease (disease progression) were modeled for the American Speech-Language-Hearing Association National Outcome Measurement System swallowing domain (ASHA-NOMS) and Rosenbek aspiration-penetration scale (PAS) outcomes. Models accounted for the association of miglustat use outcomes, seizure history, duration of neurologic symptoms, and duration of follow-up in the overall cohort, the early childhood onset subgroup (ECO; age <6 years), and late childhood onset subgroup (LCO; age ≥6 years and <15 years).
Discussion
Data from 2 independent, interpretive rating scales suggest that miglustat may stabilize oropharyngeal swallowing function and decrease aspiration risk in individuals with NPC1. Longitudinal analyses using ASHA-NOMS and PAS outcomes identify a potentially clinically meaningful association of miglustat with outcomes in individuals with neurologic disease onset prior to adulthood (age <15 years) while accounting for other factors that also affect the outcomes (Figure 1). Miglustat therapy was associated with reduced risk for the deterioration of swallowing function in the overall cohort and both subgroups, and miglustat use was associated with a decreased penetration/aspiration risk in the overall population; however, no association of miglustat with outcomes and PAS was observed in the ECO and LCO subgroups (Figure 2). This was likely influenced by a combination of smaller subgroup sample sizes, NPC1 heterogeneity, compact PAS scale range, and scores that were mostly mild and few that were severe.
These findings verify the results of previous clinical trials of miglustat in NPC1 and address their limitations. An international multicenter randomized clinical trial with 29 patients noted stabilization of swallowing function after 24 months of miglustat therapy.7 However, swallowing function was assessed via clinical swallowing examination rather than by direct instrumental assessment of swallowing physiology and aspiration. Individuals with neurodegenerative diseases are at an increased risk for silent aspiration, which was observed in studies of NPC1.12 Although the clinical swallowing examination may provide useful information on overall swallowing severity,13 it cannot detect silent aspiration. Using clinical swallowing examinations, an Italian multicenter trial followed up 20 patients treated with miglustat for 48 to 96 months9; they evaluated 4 pediatric patients with NPC1 with VFSS.8 Similarly, a Taiwanese study assessed the long-term effects of miglustat use in 5 pediatric patients with NPC1 using VFSS.14 These studies noted improvement in dysphagia for a period of time; however, there were no comparative control groups.9
Limitations
Although our data are from a nonrandomized observational study, our instrumental approach, functional interpretive analysis, and comparative natural history data address the shortcomings of these trials and support their results. While follow-up was not at the same intervals or evenly distributed, variability in follow-up duration as well as within-patient correlations were accounted for in our models. There were no baseline differences between patients with single vs follow-up visits.
Conclusions
These findings from our observational study demonstrate the robust stabilization of swallowing function with treatment of miglustat in NPC1 and underscore its potential to decrease aspiration risk, reduce respiratory morbidities, and improve quality of life. Because miglustat is used off-label for the treatment of NPC1 and is not approved by the Food and Drug Administration, many insurers are hesitant to cover its cost, thus reducing its availability to patients in the US. There is growing clinical evidence demonstrating the efficacy of miglustat in NPC1,15 and our results support its use in this population.
References
- 1.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ory DS. Niemann-Pick type C: a disorder of cellular cholesterol trafficking. Biochim Biophys Acta. 2000;1529(1-3):331-339. doi: 10.1016/S1388-1981(00)00158-X [DOI] [PubMed] [Google Scholar]
- 3.Patterson MC, Mengel E, Wijburg FA, et al. Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis. 2013;8:12. doi: 10.1186/1750-1172-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walterfang M, Chien YH, Imrie J, Rushton D, Schubiger D, Patterson MC. Dysphagia as a risk factor for mortality in Niemann-Pick disease type C: systematic literature review and evidence from studies with miglustat. Orphanet J Rare Dis. 2012;7:76. doi: 10.1186/1750-1172-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petroianni A, Ceccarelli D, Conti V, Terzano C. Aspiration pneumonia: pathophysiological aspects, prevention and management: a review. Panminerva Med. 2006;48(4):231-239. [PubMed] [Google Scholar]
- 6.Food and Drug Administration Center for Drug Evaluation and Research Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) to evaluate ZAVESCA (miglustat) for the treatment of progressive neurological manifestations in adult and pediatric patients with Niemann-Pick type C disease. Published January 12, 2010.
- 7.Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99(4):351-357. doi: 10.1016/j.ymgme.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Fecarotta S, Amitrano M, Romano A, et al. The videofluoroscopic swallowing study shows a sustained improvement of dysphagia in children with Niemann-Pick disease type C after therapy with miglustat. Am J Med Genet A. 2011;155A(3):540-547. doi: 10.1002/ajmg.a.33847 [DOI] [PubMed] [Google Scholar]
- 9.Fecarotta S, Romano A, Della Casa R, et al. Long term follow-up to evaluate the efficacy of miglustat treatment in Italian patients with Niemann-Pick disease type C. Orphanet J Rare Dis. 2015;10:22. doi: 10.1186/s13023-015-0240-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adults in Healthcare: Inpatient Rehab: National Data Report 2012-2016. American Speech-Language-Hearing Association; 2019. [Google Scholar]
- 11.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93-98. doi: 10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- 12.Garon BR, Sierzant T, Ormiston C. Silent aspiration: results of 2,000 video fluoroscopic evaluations. J Neurosci Nurs. 2009;41(4):178-185. doi: 10.1097/JNN.0b013e3181aaaade [DOI] [PubMed] [Google Scholar]
- 13.Rangarathnam B, McCullough GH. Utility of a clinical swallowing exam for understanding swallowing physiology. Dysphagia. 2016;31(4):491-497. doi: 10.1007/s00455-016-9702-1 [DOI] [PubMed] [Google Scholar]
- 14.Chien YH, Peng SF, Yang CC, et al. Long-term efficacy of miglustat in paediatric patients with Niemann-Pick disease type C. J Inherit Metab Dis. 2013;36(1):129-137. doi: 10.1007/s10545-012-9479-9 [DOI] [PubMed] [Google Scholar]
- 15.Patterson MC, Mengel E, Vanier MT, Moneuse P, Rosenberg D, Pineda M. Treatment outcomes following continuous miglustat therapy in patients with Niemann-Pick disease Type C: a final report of the NPC Registry. Orphanet J Rare Dis. 2020;15(1):104. doi: 10.1186/s13023-020-01363-2 [DOI] [PMC free article] [PubMed] [Google Scholar]