This phase 3, randomized, controlled, partially blinded interventional study evaluates the antitumor activity of pembrolizumab, pembrolizumab plus chemotherapy, or chemotherapy alone in patients with untreated, advanced gastric/gastroesophageal junction cancer with PD-L1 combined positive score ≥1.
Key Points
Question
What is the antitumor activity of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy in patients with advanced gastric/gastroesophageal junction (G/GEJ) cancer and programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 1 or greater in the first-line setting?
Findings
Among 763 patients with untreated, locally advanced/unresectable or metastatic G/GEJ cancer enrolled in the phase 3 KEYNOTE-062 randomized clinical trial, pembrolizumab was noninferior to chemotherapy for overall survival in patients with advanced G/GEJ cancer with PD-L1 CPS of 1 or greater, with patients receiving pembrolizumab experiencing fewer treatment-related adverse events.
Meaning
Pembrolizumab had a favorable benefit-to-risk profile in patients with advanced G/GEJ cancer with PD-L1 CPS of 1 or greater, including in the first-line setting.
Abstract
Importance
Safe and effective therapies for untreated, advanced gastric/gastroesophageal junction (G/GEJ) cancer remain an unmet need.
Objective
To evaluate the antitumor activity of pembrolizumab, pembrolizumab plus chemotherapy, or chemotherapy alone in patients with untreated, advanced G/GEJ cancer with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 1 or greater.
Design, Setting, and Participants
The phase 3 KEYNOTE-062 randomized, controlled, partially blinded interventional trial enrolled 763 patients with untreated, locally advanced/unresectable or metastatic G/GEJ cancer with PD-L1 CPS of 1 or greater from 200 centers in 29 countries between September 18, 2015, and May 26, 2017.
Interventions
Patients were randomized 1:1:1 to pembrolizumab 200 mg, pembrolizumab plus chemotherapy (cisplatin 80 mg/m2/d on day 1 plus fluorouracil 800 mg/m2/d on days 1 to 5 or capecitabine 1000 mg/m2 twice daily), or chemotherapy plus placebo, every 3 weeks.
Main Outcomes and Measures
Primary end points were overall survival (OS) and progression-free survival (PFS) in patients with PD-L1 CPS of 1 or greater or 10 or greater.
Results
A total of 763 patients were randomized to pembrolizumab (n = 256), pembrolizumab plus chemotherapy (n = 257), or chemotherapy (n = 250). The median (range) age of all patients in the study cohort was 62 (20-87) years; 554 of 763 (72.6%) were men. At final analysis, after a median (range) follow-up of 29.4 (22.0-41.3) months, pembrolizumab was noninferior to chemotherapy for OS in patients with CPS of 1 or greater (median, 10.6 vs 11.1 months; hazard ratio [HR], 0.91; 99.2% CI, 0.69-1.18). Pembrolizumab monotherapy was not superior to chemotherapy in patients with CPS of 1 or greater. Pembrolizumab prolonged OS vs chemotherapy in patients with CPS of 10 or greater (median, 17.4 vs 10.8 months; HR, 0.69; 95% CI, 0.49-0.97), but this difference was not statistically tested. Pembrolizumab plus chemotherapy was not superior to chemotherapy for OS in patients with CPS of 1 or greater (12.5 vs 11.1 months; HR, 0.85; 95% CI, 0.70-1.03; P = .05) or CPS of 10 or greater (12.3 vs 10.8 months; HR, 0.85; 95% CI, 0.62-1.17; P = .16) or for PFS in patients with CPS of 1 or greater (6.9 vs 6.4 months; HR, 0.84; 95% CI, 0.70-1.02; P = .04). Grade 3 to 5 treatment-related adverse event rates for pembrolizumab, pembrolizumab plus chemotherapy, and chemotherapy were 17%, 73%, and 69%, respectively.
Conclusions and Relevance
This phase 3 randomized clinical trial found that among patients with untreated, advanced G/GEJ cancer, pembrolizumab was noninferior to chemotherapy, with fewer adverse events observed. Pembrolizumab or pembrolizumab plus chemotherapy was not superior to chemotherapy for the OS and PFS end points tested.
Trial Registration
ClinicalTrials.gov Identifier: NCT02494583
Introduction
Gastric and gastroesophageal junction (G/GEJ) cancer is the third leading cause of cancer death worldwide, with an estimated 1 million new cases and 783 000 deaths in 2018.1 Doublet or triplet fluoropyrimidine-based and platinum-based chemotherapy combination regimens are recommended as first-line therapy in ERBB2 (formerly HER2)-negative, unresectable, locally advanced/metastatic G/GEJ cancer.2,3,4,5,6,7,8,9 These regimens are associated with median overall survival (OS) of 1 year or less and significant toxic effects.5,6,7,8
Pembrolizumab has been shown to provide antitumor activity with a manageable safety profile in patients with G/GEJ cancer.10,11 In cohort 1 of the phase 2 KEYNOTE-059 study, pembrolizumab provided an objective response rate (ORR) of 15.5% in patients with advanced G/GEJ cancer with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 1 or greater that progressed after 2 or more prior lines of chemotherapy.10 In cohorts 2 and 3 of KEYNOTE-059, ORR was 25.8% and 60.0% with pembrolizumab alone or with chemotherapy in patients with untreated, advanced G/GEJ cancer. In the phase 3 KEYNOTE-061 study of pembrolizumab vs paclitaxel in advanced G/GEJ cancer that progressed following first-line treatment, median OS with pembrolizumab vs paclitaxel was 9.1 vs 8.3 months in patients with tumors with PD-L1 CPS of 1 or greater (hazard ratio [HR], 0.82; 95% CI, 0.66-1.03).11 The treatment effect was enhanced in patients with tumors with PD-L1 CPS of 10 or greater, with median OS of 10.4 vs 8.0 months (HR, 0.64; 95% CI, 0.41-1.02). Of note, pembrolizumab was associated with fewer grade 3 or greater toxic effects than paclitaxel (14% vs 35%).
In the phase 3 KEYNOTE-062 study, we evaluated whether first-line pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy improved antitumor activity in patients with advanced G/GEJ cancer with PD-L1 CPS of 1 or greater and CPS of 10 or greater. We also assessed the noninferiority of pembrolizumab vs chemotherapy based on the more favorable toxicity profile.
Methods
Patients
Eligible patients were 18 years or older with histologically confirmed G/GEJ adenocarcinoma. Selection criteria included locally advanced/unresectable or metastatic disease, no prior neoadjuvant or adjuvant therapy 6 or more months before randomization, measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) criteria by investigator assessment, and Eastern Cooperative Oncology Group performance status (ECOG PS)12 of 0 or 1. Patients provided either a newly obtained or archival tumor sample for PD-L1 analysis. Patients had to have tumors that were ERBB2 negative with a PD-L1 CPS of 1 or greater for randomization. Full eligibility criteria are in the protocol (Supplement 1). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design and Treatment
KEYNOTE-062 is a randomized, controlled, partially blinded phase 3 study. Patients were randomly allocated (1:1:1) via a central interactive voice response and integrated web response system (Almac Clinical Technologies) to pembrolizumab (200 mg every 3 weeks), pembrolizumab plus chemotherapy (cisplatin 80 mg/m2/d on day 1 plus fluorouracil 800 mg/m2/d on days 1-5 or capecitabine 1000 mg/m2 twice daily on days 1-14 every 3 weeks), or placebo plus chemotherapy. Randomization was stratified by Europe/North America/Australia vs Asia (South Korea/Hong Kong/Taiwan/Japan) vs rest of world (Mexico, Central and South America), disease status (locally advanced vs metastatic), and fluorouracil vs capecitabine. Treatment was allocated in blocks of 6 per stratum. The sponsor generated the allocation schedule using a computerized random list generator. Patients and site and sponsor personnel were blinded to pembrolizumab or placebo in the combination and chemotherapy groups. Investigators and patients were unblinded to pembrolizumab monotherapy. Treatment continued until documented progression, unacceptable toxic effects, physician/patient withdrawal, or 35 administrations (2 years) of pembrolizumab.
Assessments
Tumor response was assessed per RECIST 1.1 by blinded independent central review (BICR) every 6 weeks. Disease progression was verified by central review. Survival follow-up was assessed every 12 weeks. Adverse events were assessed throughout the study and at 30 days (90 days for serious adverse events and events of interest to pembrolizumab) after treatment discontinuation and graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Trial Oversight
The study was designed by academic investigators and employees of the sponsor (Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc). An external data monitoring committee reviewed interim study results to ensure patient safety and to recommend whether the study should continue per protocol. The protocol and all amendments were approved by the appropriate ethics body at each participating institution. All patients provided voluntary written informed consent before enrollment. All authors attest that the study was conducted in accordance with the protocol and all its amendments and Good Clinical Practice standards. All authors had full access to the study data and vouch for the accuracy and completeness of the data, were involved in writing or critical review and editing of the manuscript, and approved submission for publication with assistance of a medical writer employed by the sponsor.
End Points
Primary end points were OS in patients with PD-L1 CPS of 1 or greater (intention-to-treat population) and PD-L1 CPS of 10 or greater, and progression-free survival (PFS) per RECIST 1.1 by BICR in PD-L1 CPS of 1 or greater. Secondary end points included ORR, duration of response (DOR) per RECIST 1.1 by BICR in PD-L1 CPS of 1 or greater, safety and tolerability, and health-related quality of life.
Statistical Analyses
Efficacy was assessed in all randomized patients. Safety was assessed in randomized patients with 1 or more doses of study treatment. The OS, PFS, and DOR were estimated using the Kaplan-Meier method and rules for censoring. Between-group differences in OS and PFS were assessed using a stratified log-rank test. A stratified Miettinen and Nurminen method was used for ORR between-group comparison. A stratified Cox proportional hazards model with the Efron method of tie handling was used to estimate HRs and associated 95% CIs. Randomization stratification factors were applied to all stratified analyses.
The final protocol mandated 6 primary and 1 secondary hypotheses. The first 4 were tested in parallel, with remaining hypotheses tested only if preceding hypotheses with allocated α were positive (eFigure 1 in Supplement 2). The protocol prespecified 2 interim analyses (IAs) and a final analysis. The study was considered to have met its primary objective if 1 or more hypotheses were significant at interim or final analysis. The IA1 was planned for 10 or more months after all patients were enrolled and 317 or more OS events in the pembrolizumab plus chemotherapy and chemotherapy groups (PD-L1 CPS of 1 or greater) and IA2 for 6 months after IA1 and 369 or more OS events (data cutoff date of September 26, 2018). The data monitoring committee recommended continuing to final analysis after reviewing IA2. The protocol-specified final analysis was planned for 22 months after randomization of the last patient and 415 OS events in the pembrolizumab plus chemotherapy and chemotherapy groups (PD-L1 CPS of 1 or greater). The study was controlled at a 1-sided α of 2.5% using the Mauer and Bretz graphical approach (eFigure 1 in Supplement 2). The Hwang-Shih-DeCani α spending function with γ parameter was used to construct group sequential boundaries to control the type-1 error rate. The planned enrollment was 750 patients. Statistical analyses were done using SAS, version 9.4 (SAS Institute). The full statistical analysis plan is available in the protocol (Supplement 1).
Results
Patients
Between September 18, 2015, and May 26, 2017, a total of 763 patients from 200 sites in 29 countries were randomized to pembrolizumab (n = 256), pembrolizumab plus chemotherapy (n = 257), or chemotherapy (n = 250) (Figure 1). Per the protocol, investigator decision regarding the type of comparator (fluorouracil or capecitabine) was determined before randomization; patients continued on the fluoropyrimidine chosen before randomization throughout the study. Capecitabine was chosen by 159 patients (61.9%) who received pembrolizumab plus chemotherapy and 155 (62.0%) who received chemotherapy. Baseline characteristics were well balanced between groups (Table 1). The median (range) age of all patients in the study cohort was 62 (20-87) years. Most patients were male (554 of 763; 72.6%), 527 (69.1%) had adenocarcinoma of the stomach, 281 (36.8%) had tumors with PD-L1 CPS of 10 or greater, and 50 (6.6%) had microsatellite instability–high (MSI-H) tumors. At the data cutoff date of March 26, 2019, the median (range) study follow-up was 29.4 (22.0-41.3) months. Sixteen of 253 patients (6.3%) and 16 of 251 (6.4%) who received pembrolizumab and pembrolizumab plus chemotherapy completed 35 administrations compared with 4 of 244 (1.6%) who received chemotherapy; 4 of 253 (1.6%), 9 of 251 (3.6%), and 3 of 244 (1.2%) patients who received pembrolizumab, pembrolizumab plus chemotherapy, and chemotherapy, respectively, remained on therapy.
Figure 1. Study Profile.
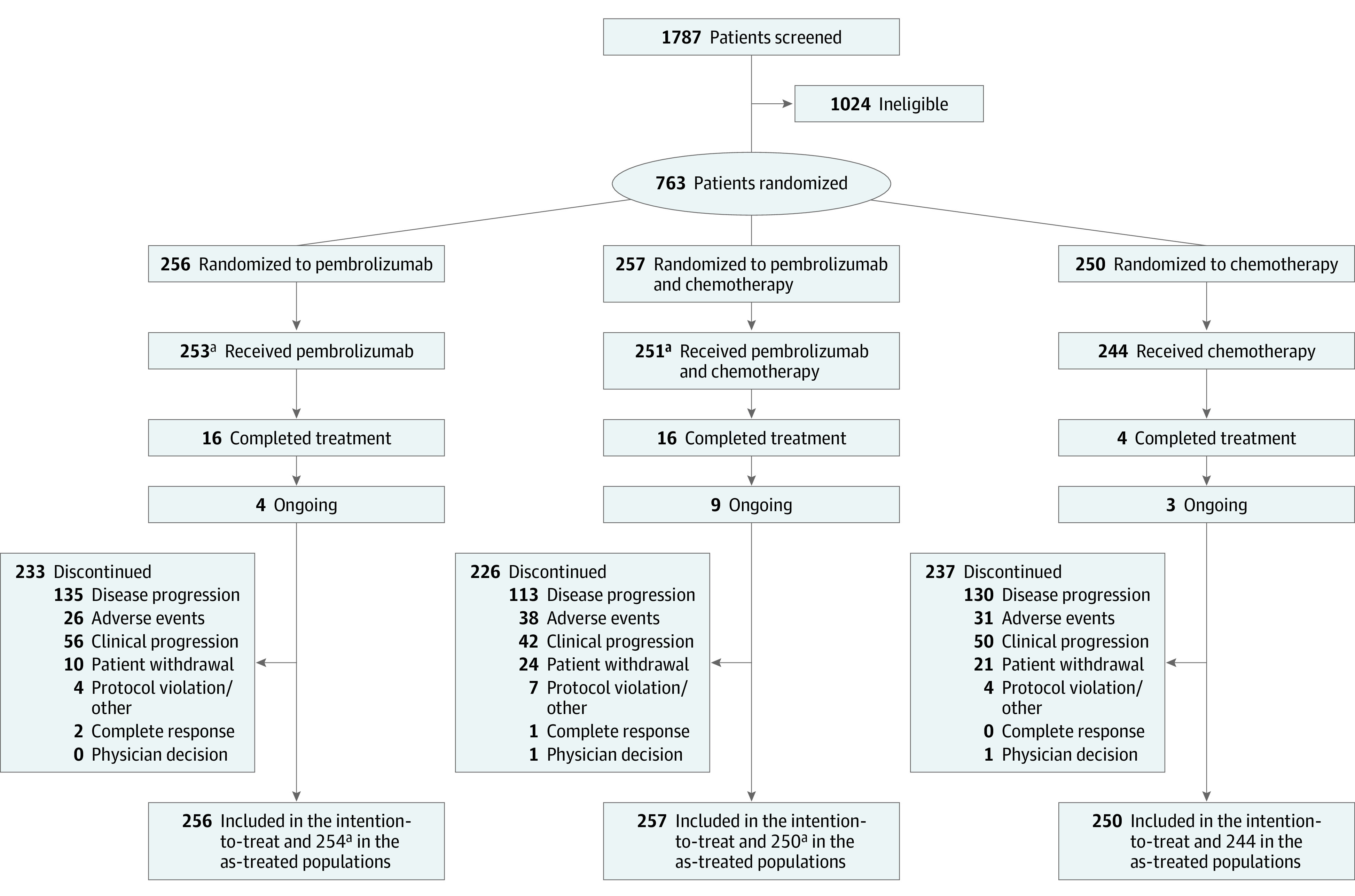
aOne patient who received pembrolizumab plus chemotherapy was counted in the pembrolizumab arm.
Table 1. Baseline Characteristics in the PD-L1 CPS of 1 or Greater Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Pembrolizumab (n = 256) | Pembrolizumab + chemotherapya (n = 257) | Chemotherapya (n = 250) | |
| Age, median (range), y | 61.0 (20-83) | 62.0 (22-83) | 62.5 (23-87) |
| Male | 180 (70.3) | 195 (75.9) | 179 (71.6) |
| ECOG PS 1b | 125 (48.8) | 138 (53.7) | 135 (54.0) |
| Metastatic disease | 245 (95.7) | 243 (94.6) | 235 (94.0) |
| CPS ≥10c | 92 (35.9) | 99 (38.5) | 90 (36.0) |
| MSI-H | 14 (5.5) | 17 (6.6) | 19 (7.6) |
| Europe/North America/Australia | 148 (57.8) | 148 (57.6) | 147 (58.8) |
| Asia | 62 (24.2) | 64 (24.9) | 61 (24.4) |
| Rest of world | 46 (18.0) | 45 (17.5) | 42 (16.8) |
| Adenocarcinoma of the stomach | 176 (68.8) | 170 (66.1) | 181 (72.4) |
| Adenocarcinoma of the GEJ | 79 (30.9) | 85 (33.1) | 67 (26.8) |
| Fluorouracil | 0 | 98 (38.1) | 95 (38.0) |
| Capecitabine | 0 | 159 (61.9) | 155 (62.0) |
Abbreviations: CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; GEJ, gastroesophageal junction; MSI-H, microsatellite instability high; PD-L1, programmed cell death ligand 1.
Patients in the chemotherapy group received cisplatin plus fluorouracil or capecitabine. Percentages may not total 100 because of rounding. There were no significant differences between treatment groups at baseline.
ECOG PS scores range from 0-5, with 0 indicating no symptoms and higher scores indicating greater disability.
The PD-L1 CPS was defined as the number of PD-L1–positive cells (tumor cells, macrophages, and lymphocytes) divided by the total number of tumor cells.
In total 254, 250, and 244 patients were treated with pembrolizumab, pembrolizumab plus chemotherapy, and chemotherapy for a median (range) duration of 2.1 (0.0-26.7) months, 5.9 (0.0-28.1) months, and 4.7 (0.0-28.5) months. Approximately 134 of 254 patients (52.8%), 118 of 250 patients (47.2%), and 132 of 244 patients (54.1%), respectively, received subsequent anticancer therapy. This included 12 of 254 patients (4.7%), 11 of 250 patients (4.4%), and 33 of 244 patients (13.5%) who received subsequent immunotherapy (eTable 2 in Supplement 2).
Efficacy
At final analysis (PD-L1 CPS of 1 or greater), median OS with pembrolizumab (10.6 months; 95% CI, 7.7-13.8) vs chemotherapy (11.1 months; 95% CI, 9.2-12.8) met the criteria for noninferiority (HR, 0.91; 99.2% CI, 0.69-1.18; noninferiority margin, 1.2) (Figure 2A). The 12-month and 24-month OS rates were 46.9% (95% CI, 40.7%-52.8%) and 26.5% (95% CI, 21.2%-32.1%) with pembrolizumab vs 45.6% (95% CI, 39.3%-51.6%) and 19.2% (95% CI, 14.6%-24.3%) with chemotherapy. Pembrolizumab was not superior to chemotherapy in patients with PD-L1 CPS of 1 or greater (HR, 0.91; 95% CI, 0.74-1.10). In the PD-L1 CPS of 10 or greater population, median OS with pembrolizumab was 17.4 (95% CI, 9.1-23.1) vs 10.8 months (95% CI, 8.5-13.8) with chemotherapy (HR, 0.69; 95% CI, 0.49-0.97) (Figure 2B). This difference was not tested per the statistical analysis plan because superiority was not demonstrated (eFigure 1 in Supplement 2). The 12-month and 24-month OS rates were 56.5% (95% CI, 45.8%-65.9%) and 39.0% (95% CI, 29.1%-48.8%) with pembrolizumab vs 46.7% (95% CI, 36.1%-56.5%) and 22.2% (95% CI, 14.3%-31.2%) with chemotherapy. Overall survival was consistent across most prespecified subgroups (eFigure 2A and 2B in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Overall Survival According to Programmed Cell Death Ligand 1 (PD-L1) Combined Positive Score (CPS).
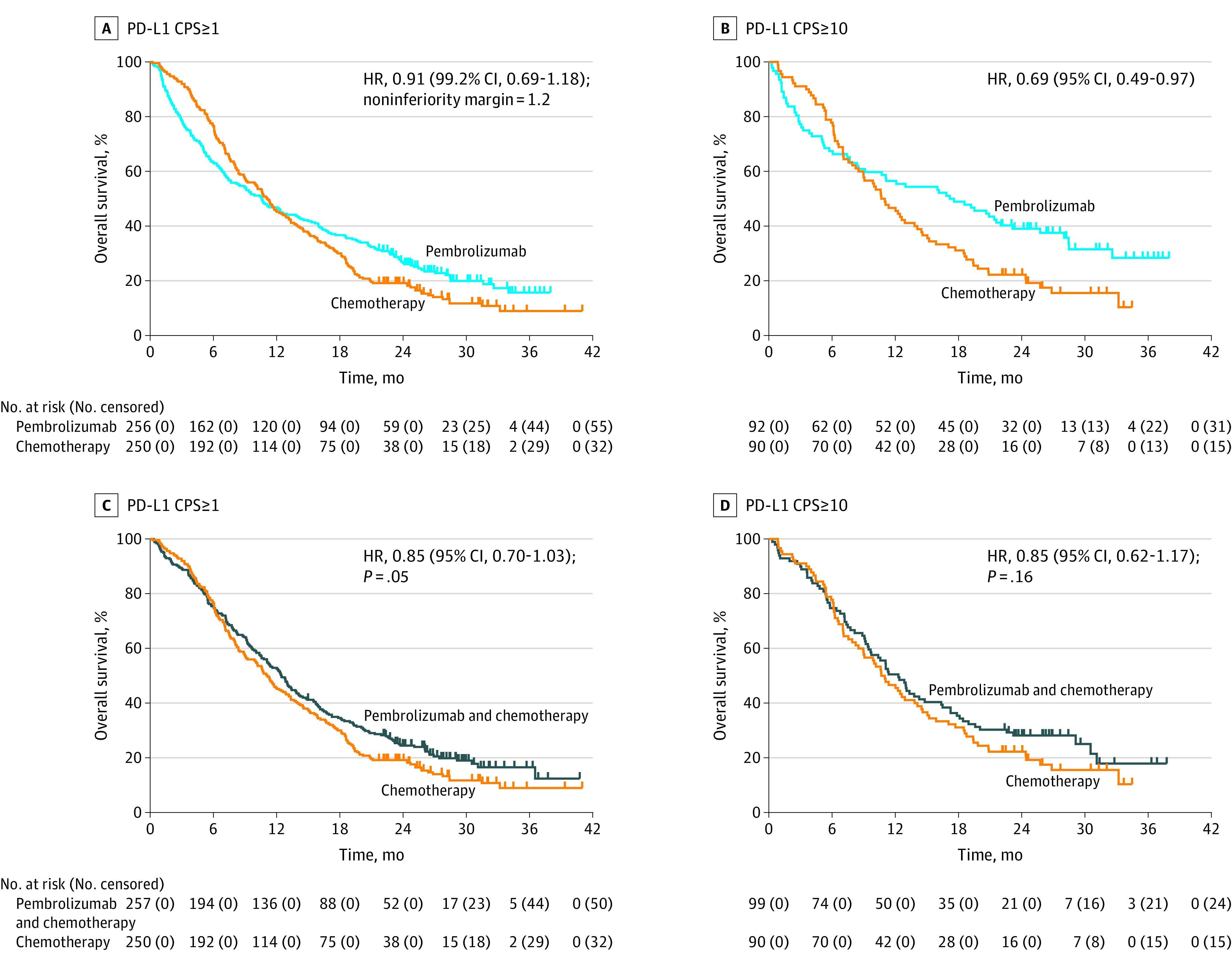
A, Overall survival in the PD-L1 CPS of 1 or greater population for pembrolizumab vs chemotherapy. B, Overall survival in the PD-L1 CPS of 10 or greater population for pembrolizumab vs chemotherapy. C, Overall survival in the PD-L1 CPS of 1 or greater population for pembrolizumab plus chemotherapy vs chemotherapy. D, Overall survival in the PD-L1 CPS of 10 or greater population for pembrolizumab plus chemotherapy vs chemotherapy. Tick marks represent data censored at the last time the patient was known to be alive.
In an exploratory analysis of patients with MSI-H tumors with PD-L1 CPS of 1 or greater, median OS with pembrolizumab was not reached (95% CI, 10.7-not reached) vs 8.5 months (95% CI, 5.3-20.8) with chemotherapy (HR, 0.29; 95% CI, 0.11-0.81) (Figure 3A). Median OS was prolonged with pembrolizumab (median, not reached; 95% CI, 10.7-not reached) vs chemotherapy (median, 13.6 months; 95% CI, 3.8-25.8) in patients with MSI-H tumors in the PD-L1 CPS of 10 or greater population (HR, 0.21; 95% CI, 0.06-0.83) (eFigure 3A in Supplement 2). This effect was maintained for pembrolizumab (median, 16.0 months; 95% CI, 7.7-20.6) vs chemotherapy (median, 10.8 months; 95% CI, 8.2-13.8) following a post-hoc exploratory analysis in the PD-L1 CPS of 10 or greater population after exclusion of MSI-H tumors (HR, 0.76; 95% CI, 0.54-1.09) (eFigure 3B in Supplement 2).
Figure 3. Overall Survival in Patients With MSI-H Tumors and PD-L1 CPS of 1 or Greater.
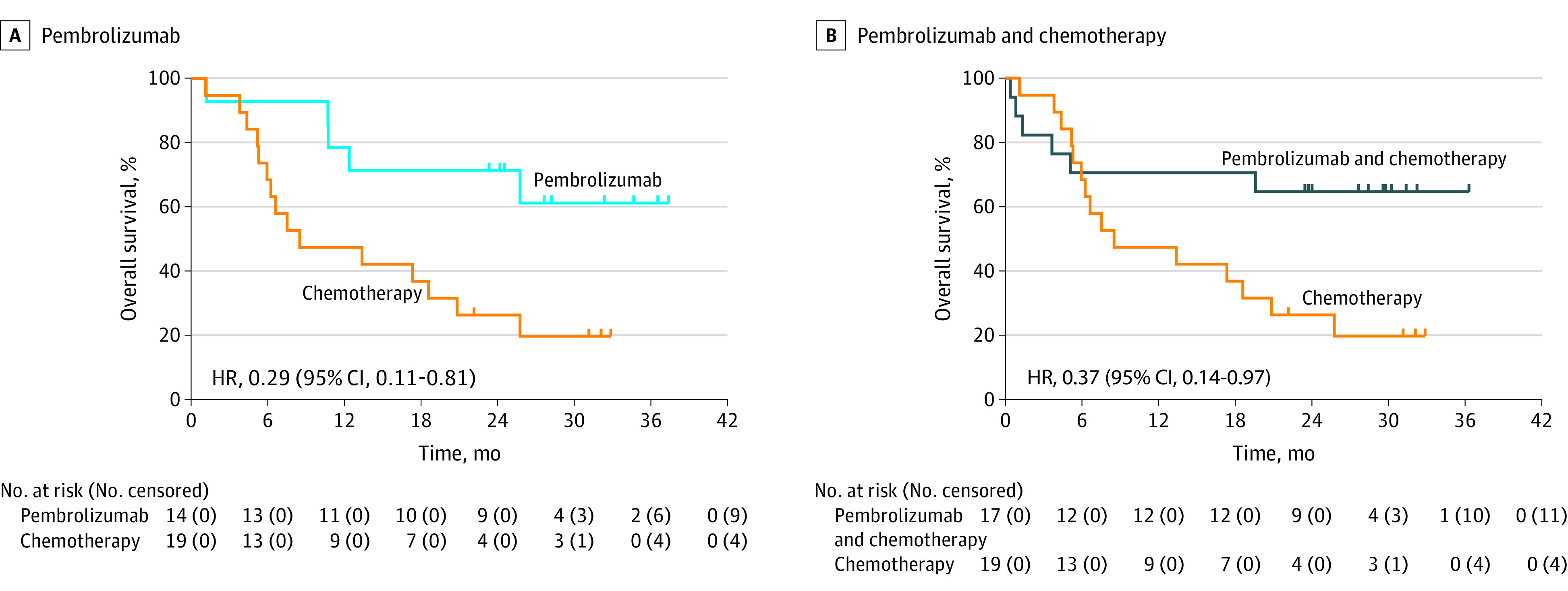
Kaplan-Meier estimates of overall survival in patients with microsatellite instability–high (MSI-H) tumors in the programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 1 or greater population for pembrolizumab (A) and pembrolizumab plus chemotherapy (B) vs chemotherapy. Analysis of overall survival in patients with MSI-H tumors was not adjusted for multiplicity.
In the population with PD-L1 CPS of 1 or greater, median PFS with pembrolizumab was 2.0 months (95% CI, 1.5-2.8) vs 6.4 months (95% CI, 5.7-7.0) with chemotherapy (HR, 1.66; 95% CI, 1.37-2.01) (eFigure 4A in Supplement 2). In the population with PD-L1 CPS of 10 or greater, median PFS was 2.9 months (95% CI, 1.6-5.4) vs 6.1 months (95% CI, 5.3-6.9) (HR, 1.10; 95% CI, 0.79-1.51) (eFigure 4B in Supplement 2).
A total of 38 of 256 patients (14.8%) who received pembrolizumab vs 93 of 250 (37.2%) who received chemotherapy had an objective response, with complete responses in 9 of 256 patients (3.5%) vs 14 of 250 (5.6%), respectively. Median DOR was 13.7 months (95% CI, 8.3-21.2) vs 6.8 months (95% CI, 5.4-8.2). In the population with PD-L1 CPS of 10 or greater, 23 of 92 patients (25.0%) who received pembrolizumab vs 34 of 90 (37.8%) who received chemotherapy had an objective response, with complete responses in 7 of 92 patients (7.6%) vs 4 of 90 (4.4%), respectively. Median DOR was 19.3 months (95% CI, 8.3-not reached) vs 6.8 months (95% CI, 4.8-8.7) (eTable 3 in Supplement 2). All outcomes were enhanced in the MSI-H population (eTable 4 in Supplement 2).
At final analysis (PD-L1 CPS of 1 or greater), median OS with pembrolizumab plus chemotherapy (12.5 months; 95% CI, 10.8-13.9) vs chemotherapy (11.1 months; 95% CI, 9.2-12.8) did not meet the criteria for superiority (HR, 0.85; 95% CI, 0.70-1.03; P = .05) (Figure 2C). The 12-month OS rate was 52.9% (95% CI, 46.6%-58.8%) with pembrolizumab plus chemotherapy vs 45.6% (95% CI, 39.3%-51.6%) with chemotherapy. In the population with PD-L1 CPS of 10 or greater, median OS with pembrolizumab plus chemotherapy was 12.3 months (95% CI, 9.5-14.8) vs 10.8 months (95% CI, 8.5-13.8) with chemotherapy and did not meet the criteria for superiority (HR, 0.85; 95% CI, 0.62-1.17; P = .16) (Figure 2D). The 12-month OS rate was 50.5% (95% CI, 40.3%-59.8%) in the pembrolizumab plus chemotherapy arm vs 46.7% (95% CI, 36.1%-56.5%) in the chemotherapy arm. Overall survival with pembrolizumab plus chemotherapy vs chemotherapy was consistent across most prespecified subgroups (eFigure 2C in Supplement 2). Overall survival was enhanced in patients with MSI-H tumors in the PD-L1 CPS of 1 or greater population with median OS not reached (95% CI, 3.6-not reached) with pembrolizumab plus chemotherapy vs 8.5 months (95% CI, 5.3-20.8) with chemotherapy (Figure 3B).
In the population with PD-L1 CPS of 1 or greater, median PFS with pembrolizumab plus chemotherapy (6.9 months; 95% CI, 5.7-7.3) vs chemotherapy (6.4 months, 95% CI, 5.7-7.0) did not meet the threshold for superiority (HR, 0.84; 95% CI, 0.70-1.02; P = .04) (eFigure 4C in Supplement 2). A total of 125 of 257 (48.6%) patients receiving pembrolizumab plus chemotherapy and 93 of 250 (37.2%) receiving chemotherapy had an objective response, with complete responses in 24 of 257 (9.3%) vs 14 of 250 patients (5.6%), respectively. Median DOR was 6.8 months (95% CI, 5.5-8.3) vs 6.8 months (95% CI, 5.4-8.2), respectively (eTable 3 in Supplement 2). Efficacy outcomes were enhanced in the MSI-H population (eTable 4 in Supplement 2).
Safety
Adverse events attributed to the drug by investigator occurred in 138 of 254 (54.3%), 235 of 250 (94.0%), and 224 of 244 patients (91.8%) receiving pembrolizumab, pembrolizumab plus chemotherapy, and chemotherapy, respectively (Table 2). Grade 3 or greater drug-related events occurred in 43 of 254 (16.9%), 183 of 250 (73.2%), and 169 of 244 patients (69.3%), respectively. Drug-related events resulted in discontinuation in 10 of 254 (3.9%), 69 of 250 (27.6%), and 44 of 244 patients (18.0%). Three of 254 (1.2%), 5 of 250 (2.0%), and 3 of 244 patients (1.2%) died of drug-related events. Immune-mediated adverse events and infusion reactions occurred in 54 of 254 (21.3%), 60 of 250 (24.0%), and 19 of 244 patients (7.8%) with pembrolizumab, pembrolizumab plus chemotherapy, and chemotherapy, respectively (eTable 5 in Supplement 2). Grade 3 or greater immune-mediated events occurred in 15 of 254 (5.9%), 14 of 250 (5.6%), and 4 of 244 patients (1.6%), respectively. Immune-mediated events resulted in discontinuation in 4 of 254 (1.6%), 11 of 250 (4.4%), and 2 of 244 patients (0.8%). One patient in each arm died of an immune-mediated event.
Table 2. Adverse Events in All Treated Patients in the PD-L1 CPS of 1 or Greater Population.
| Event, No. (%) | All grade | Grade 3-5 | All grade | Grade 3-5 | All grade | Grade 3-5 |
|---|---|---|---|---|---|---|
| Pembrolizumab (n = 254) | Pembrolizumab + chemotherapy (n = 250) | Chemotherapy (n = 244) | ||||
| Any | 242 (95.3) | NA | 244 (97.6) | NA | 240 (98.4) | NA |
| Drug-related | 138 (54.3) | NA | 235 (94.0) | NA | 224 (91.8) | NA |
| Grade 3-5 | NA | 43 (16.9) | NA | 183 (73.2) | NA | 169 (69.3) |
| Led to discontinuation | 10 (3.9) | NA | 69 (27.6) | NA | 44 (18.0) | NA |
| Led to deatha | 3 (1.1) | NA | 5 (2.0) | NA | 3 (1.2) | NA |
| Drug-related events in ≥5% of patients in either group | ||||||
| Nausea | 9 (3.5) | 1 (0.4) | 145 (58.0) | 19 (7.6) | 120 (49.2) | 18 (7.4) |
| Fatigue | 25 (9.8) | 1 (0.4) | 90 (36.0) | 19 (7.6) | 63 (25.8) | 14 (5.7) |
| Anemia | 13 (5.1) | 4 (1.6) | 90 (36.0) | 30 (12.0) | 80 (32.8) | 35 (14.3) |
| Neutropenia | 0 | 0 | 88 (35.2) | 63 (25.2) | 95 (38.9) | 68 (27.9) |
| Decreased appetite | 19 (7.5) | 3 (1.2) | 75 (30.0) | 11 (4.4) | 74 (30.3) | 17 (7.0) |
| Vomiting | 9 (3.5) | 0 | 66 (26.4) | 12 (4.8) | 71 (29.1) | 14 (5.7) |
| Diarrhea | 16 (6.3) | 3 (1.2) | 63 (25.2) | 12 (4.8) | 62 (25.4) | 14 (5.7) |
| PPE syndrome | 0 | 0 | 59 (23.6) | 12 (4.8) | 44 (18.0) | 8 (3.3) |
| Neutrophil count decreased | 2 (0.8) | 2 (0.8) | 56 (22.4) | 34 (13.6) | 37 (15.2) | 22 (9.0) |
| Mucosal inflammation | 2 (0.8) | 0 | 41 (16.4) | 11 (4.4) | 34 (13.9) | 15 (6.1) |
| Peripheral sensory neuropathy | 1 (0.4) | 0 | 32 (12.8) | 3 (1.2) | 15 (6.1) | 0 |
| Stomatitis | 4 (1.6) | 0 | 31 (12.4) | 11 (4.4) | 34 (13.9) | 9 (3.7) |
| White blood cell count decreased | 2 (0.8) | 0 | 30 (12.0) | 7 (2.8) | 24 (9.8) | 10 (4.1) |
| Rash | 16 (6.3) | 0 | 27 (10.8) | 1 (0.4) | 10 (4.1) | 0 |
| Thrombocytopenia | 0 | 0 | 27 (10.8) | 7 (2.8) | 24 (9.8) | 6 (2.5) |
| Hypothyroidism | 19 (7.5) | 1 (0.4) | 26 (10.4) | 1 (0.4) | 7 (2.9) | 0 |
| Asthenia | 7 (2.8) | 1 (0.4) | 26 (10.4) | 9 (3.6) | 39 (16.0) | 10 (4.1) |
| Neuropathy peripheral | 0 | 0 | 26 (10.4) | 4 (1.6) | 15 (6.1) | 2 (0.8) |
| Blood creatinine increased | 2 (0.8) | 1 (0.4) | 25 (10.0) | 0 | 31 (12.7) | 1 (0.4) |
| Hypomagnesemia | 1 (0.4) | 0 | 24 (9.6) | 8 (3.2) | 22 (9.0) | 8 (3.3) |
| Constipation | 4 (1.6) | 0 | 23 (9.2) | 0 | 26 (10.7) | 0 |
| Platelet count decreased | 2 (0.8) | 0 | 23 (9.2) | 8 (3.2) | 16 (6.6) | 2 (0.8) |
| Weight decreased | 2 (0.8) | 0 | 23 (9.2) | 2 (0.8) | 15 (6.1) | 1 (0.4) |
| Dysgeusia | 2 (0.8) | 0 | 22 (8.8) | 0 | 26 (10.7) | 0 |
| Leukopenia | 0 | 0 | 21 (8.4) | 6 (2.4) | 24 (9.8) | 1 (0.4) |
| Pruritus | 20 (7.9) | 0 | 18 (7.2) | 0 | 8 (3.3) | 0 |
| Alopecia | 0 | 0 | 17 (6.8) | 0 | 11 (4.5) | 0 |
| Tinnitus | 0 | 0 | 16 (6.4) | 0 | 18 (7.4) | 1 (0.4) |
| Hypokalemia | 0 | 0 | 16 (6.4) | 4 (1.6) | 20 (8.2) | 10 (4.1) |
| Dry skin | 3 (1.2) | 0 | 12 (4.8) | 0 | 10 (4.1) | 0 |
| Dizziness | 2 (0.8) | 0 | 12 (4.8) | 1 (0.4) | 9 (3.7) | 1 (0.4) |
| Dehydration | 1 (0.4) | 0 | 12 (4.8) | 7 (2.8) | 16 (6.6) | 6 (2.5) |
| Skin hyperpigmentation | 0 | 0 | 12 (4.8) | 0 | 6 (2.5) | 0 |
Abbreviations: CPS, combined positive score; NA, not applicable; PD-L1, programmed cell death ligand 1; PPE, palmar-plantar erythrodysesthesia.
Grade 5 drug-related events were pneumonitis, malignant neoplasm progression, and pericardial effusion in 1 patient each in the pembrolizumab group; febrile neutropenia, myocardial ischemia, colitis, sepsis, and malignant progression in 1 patient each in the pembrolizumab plus chemotherapy group; and multiple organ failure, pneumonitis, and pulmonary embolism in 1 patient each in the chemotherapy group.
Discussion
To our knowledge, this is the first global, randomized, phase 3 study of a checkpoint inhibitor as a single agent or in combination with chemotherapy in patients with advanced ERBB2-negative G/GEJ adenocarcinoma in the first-line setting. In this final analysis, pembrolizumab was noninferior to chemotherapy for OS in patients with PD-L1 CPS of 1 or greater (HR, 0.91; 99.2% CI, 0.69-1.18). Pembrolizumab monotherapy was not superior to chemotherapy in patients with PD-L1 CPS of 1 or greater. In patients with PD-L1 CPS of 10 or greater, pembrolizumab provided a clinically meaningful benefit in OS vs chemotherapy, although this hypothesis could not be tested per the statistical analysis plan. This benefit was maintained in patients without MSI-H tumors. Pembrolizumab plus chemotherapy was not superior to chemotherapy for OS in populations with PD-L1 CPS of 1 or greater or PD-L1 CPS of 10 or greater.
Median OS with chemotherapy in patients with PD-L1 CPS of 1 or greater is consistent with that reported in prior first-line global studies in patients with G/GEJ cancer.6,8,13,14 Examination of Kaplan-Meier OS curves for pembrolizumab vs chemotherapy showed an early favorable trend toward chemotherapy. Meanwhile, the separation in favor of pembrolizumab was sustained after 12 months, indicating a long-term survival benefit with pembrolizumab (2-year OS rates of 27% vs 19%). This violation of the proportional hazards assumption suggests that alternative statistical approaches to addressing changes in risk over time will be necessary in future clinical studies evaluating the delayed onset of benefit typically observed with immunotherapies. The survival benefit with pembrolizumab was consistent across most prespecified subgroups including in patients with diffuse type tumors, although Asian patients appeared to have an enhanced survival benefit with pembrolizumab vs chemotherapy. Further analysis into factors influencing outcomes in Asian vs non-Asian patients are ongoing. In patients with PD-L1 CPS of 10 or greater, durability of the survival benefit is illustrated by 2-year OS rates of 39% and 22%, with response duration 3 times longer with pembrolizumab vs chemotherapy. These data are consistent with those in KEYNOTE-061 and reinforce the utility of high PD-L1 expression for selecting patients for treatment with pembrolizumab in an earlier line of therapy.11 Additional biomarker or multifactorial analyses would help to define an optimal population of patients with advanced gastric cancer who would benefit from pembrolizumab monotherapy in the first-line setting.
As observed previously with anti–programmed cell death 1 (PD-1) therapy, the OS benefit with pembrolizumab vs chemotherapy did not directly correlate with PFS and ORR in patients with PD-L1 CPS of 1 or greater, with shorter PFS and lower ORRs observed with pembrolizumab vs chemotherapy.15,16,17 However, the similar proportions of patients with subsequent therapy between arms suggested that the relative survival benefit was driven by pembrolizumab. This is consistent with the hypothesis that anti–PD-1 therapy may enhance the antitumor response to subsequent chemotherapy and prolong further disease progression.18,19,20 Conversely, higher ORR and better PFS with pembrolizumab plus chemotherapy did not translate into an OS benefit. Preclinical studies have suggested that cycles of fluorouracil could prevent acquisition of T-cell cytotoxic effector functions and impair the antitumor immune response21,22; however, additional exploration is needed. Further evaluation of optimal sequencing and choice of chemotherapy combinations in the first-line setting is needed to determine the effect of first-line combination regimens that include a PD-1 or PD-L1 inhibitor on response and survival. Chemotherapy combinations are being evaluated in the CheckMate 649 (NCT02872116), KEYNOTE-811 (NCT03615326), and KEYNOTE-859 (NCT03675737) studies.
Consistent with prior reports, approximately 7% of patients in KEYNOTE-062 trial had MSI-H tumors.23 The survival benefit was enhanced with pembrolizumab (HR, 0.29) and pembrolizumab plus chemotherapy (HR, 0.37) vs chemotherapy, consistent with data showing improved outcomes in patients with MSI-H tumors in the second-line setting.11 Although limited numbers preclude testing for superiority of pembrolizumab vs chemotherapy, to our knowledge, this study is the first to show survival benefit of an anti–PD-1 therapy vs chemotherapy for MSI-H tumors in the first-line setting. Moreover, the predictive value of PD-L1 CPS of 10 or greater was maintained after exclusion of MSI-H tumors, suggesting the independent value of these biomarkers.
Fewer drug-related (138 of 254 [54.3%] vs 224 of 244 [92.0%]) and grade 3 or greater drug-related adverse events (43 of 254 [16.9%] vs 169 of 244 [69.3%]) were reported with pembrolizumab vs chemotherapy, supporting consideration of pembrolizumab in patients with untreated G/GEJ who may be unsuitable for chemotherapy, although the patients enrolled in KEYNOTE-062 were fit for chemotherapy. The incidence of immune-mediated events was similar to that previously observed, with no new safety signals.10,11 The safety profile for pembrolizumab plus chemotherapy vs chemotherapy was also similar to that previously reported.10,24
Limitations
This study has limitations. A potential limitation of the current study is the unblinded administration of pembrolizumab monotherapy vs chemotherapy, which may have influenced adherence and biased patient management.
Conclusions
In conclusion, pembrolizumab was noninferior to chemotherapy, with fewer adverse events in patients with untreated, advanced G/GEJ adenocarcinoma with PD-L1 CPS of 1 or greater. The survival benefit was clinically meaningful in patients with PD-L1 CPS of 10 or greater or with MSI-H tumors. There was no clinically meaningful benefit of pembrolizumab plus chemotherapy vs chemotherapy.
Trial Protocol
eMethods.
eTable 1. KEYNOTE-062 Investigators
eTable 2. Post-discontinuation anticancer therapy in the PD-L1 CPS ≥1 population
eTable 3. Antitumor activity in the intention-to-treat population
eTable 4. Antitumor activity in patients with MSI-H tumors in the PD-L1 CPS ≥1 population
eTable 5. Immune-mediated events and infusion reactions in all treated patients in the PD-L1 CPS ≥1 population
eFigure 1. Type 1 error reallocation strategy
eFigure 2. Overall survival in pre-specified subgroups for pembrolizumab in (A) PD-L1 CPS ≥1 and (B) PD-L1 CPS ≥10 populations and pembrolizumab-chemotherapy versus chemotherapy in (C) PD-L1 CPS ≥1
eFigure 3. Kaplan-Meier estimates of overall survival in patients with (A) MSI-H tumors and (B) without MSI-H tumors in the PD-L1 CPS ≥10 population for pembrolizumab versus chemotherapy
eFigure 4. Kaplan-Meier estimates of progression-free survival for pembrolizumab versus chemotherapy in the (A) PD-L1 CPS ≥1 and (B) PD-L1 CPS ≥10 populations and for pembrolizumab-chemotherapy versus chemotherapy in the (C) PD-L1 CPS ≥1 and (D) CPS ≥10 population
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: esophageal and esophagogastric junction cancers. Version 1.2019. Accessed March 31, 2018. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [DOI] [PubMed]
- 3.Roth AD, Fazio N, Stupp R, et al. ; Swiss Group for Clinical Cancer Research . Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25(22):3217-3223. doi: 10.1200/JCO.2006.08.0135 [DOI] [PubMed] [Google Scholar]
- 4.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee . Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49. doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. ; V325 Study Group . Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991-4997. doi: 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. ; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom . Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36-46. doi: 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28(9):1547-1553. doi: 10.1200/JCO.2009.25.4706 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs CS, Shitara K, Di Bartolomeo M, et al. ; RAINFALL Study Group . Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420-435. doi: 10.1016/S1470-2045(18)30791-5 [DOI] [PubMed] [Google Scholar]
- 9.Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):19-33. doi: 10.1093/annonc/mdy502 [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shitara K, Özgüroğlu M, Bang YJ, et al. ; KEYNOTE-061 investigators . Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123-133. doi: 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19(1):234-244. doi: 10.1007/s10120-015-0457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968-3976. doi: 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 17.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1–positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717-726. doi: 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 18.Szabados B, van Dijk N, Tang YZ, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73(2):149-152. doi: 10.1016/j.eururo.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer. 2019;10(4):775-781. doi: 10.1111/1759-7714.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carreau NA, Diefenbach CS. Immune targeting of the microenvironment in classical Hodgkin’s lymphoma: insights for the hematologist. Ther Adv Hematol. 2019;10:2040620719846451. doi: 10.1177/2040620719846451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Deng Z, Wang H, Ma W, Zhou C, Zhang S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016;17(1):29. doi: 10.1186/s12865-016-0167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quéméneur L, Beloeil L, Michallet MC, et al. Restriction of de novo nucleotide biosynthesis interferes with clonal expansion and differentiation into effector and memory CD8 T cells. J Immunol. 2004;173(8):4945-4952. doi: 10.4049/jimmunol.173.8.4945 [DOI] [PubMed] [Google Scholar]
- 23.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828-837. doi: 10.1007/s10120-018-00909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eTable 1. KEYNOTE-062 Investigators
eTable 2. Post-discontinuation anticancer therapy in the PD-L1 CPS ≥1 population
eTable 3. Antitumor activity in the intention-to-treat population
eTable 4. Antitumor activity in patients with MSI-H tumors in the PD-L1 CPS ≥1 population
eTable 5. Immune-mediated events and infusion reactions in all treated patients in the PD-L1 CPS ≥1 population
eFigure 1. Type 1 error reallocation strategy
eFigure 2. Overall survival in pre-specified subgroups for pembrolizumab in (A) PD-L1 CPS ≥1 and (B) PD-L1 CPS ≥10 populations and pembrolizumab-chemotherapy versus chemotherapy in (C) PD-L1 CPS ≥1
eFigure 3. Kaplan-Meier estimates of overall survival in patients with (A) MSI-H tumors and (B) without MSI-H tumors in the PD-L1 CPS ≥10 population for pembrolizumab versus chemotherapy
eFigure 4. Kaplan-Meier estimates of progression-free survival for pembrolizumab versus chemotherapy in the (A) PD-L1 CPS ≥1 and (B) PD-L1 CPS ≥10 populations and for pembrolizumab-chemotherapy versus chemotherapy in the (C) PD-L1 CPS ≥1 and (D) CPS ≥10 population
Data Sharing Statement


