Key Points
Question
Can treating patients with coronavirus disease 2019 (COVID-19) with recombinant human granulocyte colony-stimulating factor (rhG-CSF) increase their peripheral blood leukocyte and lymphocyte cell counts and lead to clinical improvement?
Findings
In this open-label, randomized clinical trial of 200 Chinese patients with COVID-19, lymphopenia, and no comorbidities, rhG-CSF treatment did not accelerate clinical improvement, but the number of patients progressing to critical illness or death may have been reduced, without an increased risk of serious adverse events.
Meaning
Preliminary findings from a randomized clinical trial suggest that rhG-CSF treatment should be studied in larger trials and in a broader range of patients with COVID-19.
Abstract
Importance
Lymphopenia is common and correlates with poor clinical outcomes in patients with coronavirus disease 2019 (COVID-19).
Objective
To determine whether a therapy that increases peripheral blood leukocyte and lymphocyte cell counts leads to clinical improvement in patients with COVID-19.
Design, Setting and Participants
Between February 18 and April 10, 2020, we conducted an open-label, multicenter, randomized clinical trial at 3 participating centers in China. The main eligibility criteria were pneumonia, a blood lymphocyte cell count of 800 per μL (to convert to ×109/L, multiply by 0.001) or lower, and no comorbidities. Severe acute respiratory syndrome coronavirus 2 infection was confirmed with reverse-transcription polymerase chain reaction testing.
Exposures
Usual care alone, or usual care plus 3 doses of recombinant human granulocyte colony-stimulating factor (rhG-CSF, 5 μg/kg, subcutaneously at days 0-2).
Main Outcomes and Measures
The primary end point was the time from randomization to improvement of at least 1 point on a 7-category disease severity score.
Results
Of 200 participants, 112 (56%) were men and the median (interquartile range [IQR]) age was 45 (40-55) years. There was random assignment of 100 patients (50%) to the rhG-CSF group and 100 (50%) to the usual care group. Time to clinical improvement was similar between groups (rhG-CSF group median of 12 days (IQR, 10-16 days) vs usual care group median of 13 days (IQR, 11-17 days); hazard ratio, 1.28; 95% CI, 0.95-1.71; P = .06). For secondary end points, the proportion of patients progressing to acute respiratory distress syndrome, sepsis, or septic shock was lower in the rhG-CSF group (rhG-CSF group, 2% vs usual care group, 15%; difference, −13%; 95%CI, −21.4% to −5.4%). At 21 days, 2 patients (2%) had died in the rhG-CSF group compared with 10 patients (10%) in the usual care group (hazard ratio, 0.19; 95%CI, 0.04-0.88). At day 5, the lymphocyte cell count was higher in the rhG-CSF group (rhG-CSF group median of 1050/μL vs usual care group median of 620/μL; Hodges-Lehmann estimate of the difference in medians, 440; 95% CI, 380-490). Serious adverse events, such as sepsis or septic shock, respiratory failure, and acute respiratory distress syndrome, occurred in 29 patients (14.5%) in the rhG-CSF group and 42 patients (21%) in the usual care group.
Conclusion and Relevance
In preliminary findings from a randomized clinical trial, rhG-CSF treatment for patients with COVID-19 with lymphopenia but no comorbidities did not accelerate clinical improvement, but the number of patients developing critical illness or dying may have been reduced. Larger studies that include a broader range of patients with COVID-19 should be conducted.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR2000030007
This randomized clinical trial examines the effect of recombinant human granulocyte colony-stimulating factor on peripheral blood leukocyte and lymphocyte cell counts and clinical improvement in Chinese patients with COVID-19.
Introduction
As of August 11, 2020, coronavirus disease 2019 (COVID-19) has led to a global pandemic, with more than 19 000 000 laboratory-confirmed cases and 720 000 deaths.1 To our knowledge, there are few effective therapies for COVID-19.
Lymphopenia is prevalent among patients with COVID-19.2,3,4,5,6,7 The magnitude and duration of peripheral blood lymphocyte (PBL) cell count decline is predictive of disease severity and death.2,3,4,5,6,7,8 In mouse models and people, recombinant human granulocyte colony-stimulating factor (rhG-CSF) increases peripheral blood leukocyte and lymphocyte (including lymphocyte subsets) cell counts.9,10 Although rhG-CSF did not alter the severity of acute lung injury in a sheep model,11 rhG-CSF normalized PBL cell counts in patients with severe acute respiratory syndrome.12 In an observational study, rhG-CSF was associated with improved clinical outcomes in patients with AIDS.13
The potential therapeutic effects of rhG-CSF for patients with COVID-19 are uncertain. In healthy persons, rhG-CSF reportedly increased PBL cell counts via mobilizing CD4+ and CD8+ T cells14,15; findings after autologous bone marrow transplant were similar.16 Nonetheless, rhG-CSF did not mobilize natural killer (NK) cells in vivo.17
In a clinical trial, we sought to determine whether patients with COVID-19 with lymphopenia would benefit from treatment with rhG-CSF. To minimize the potential adverse effects of other conditions on immune responses, we excluded patients who had comorbidities.
Methods
Study Design
This study was an open-label, multicenter, parallel-group randomized clinical trial conducted from February 18, 2020, to April 10, 2020. We recruited patients from 3 trial sites (Guangzhou No. 8 People’s Hospital [Guangzhou, China], Wuhan Union Hospital, and Wuhan Hankou Hospital [Wuhan, China]).
Ethics approval was obtained from institutional review boards at each participating site. Patients (n = 156) or their legal representative (n = 44) provided written informed consent; legal representatives provided consent for patients requiring noninvasive ventilation who had illness too severe to consent themselves. The clinical trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization–Good Clinical Practice, and Consolidated Standards of Reporting Trials guidelines. Supplement 1 includes the study protocol and further details of the methods are described in Supplement 2.
Patients
Patients 18 years or older who had positive test results with reverse-transcription polymerase chain reaction (RT-PCR) assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in oropharyngeal samples were eligible if they had pneumonia as confirmed by chest imaging and a PBL cell count of 800 per μL (to convert to ×109/L, multiply by 0.001) or lower. Patients were excluded if they had a peripheral blood leukocyte cell count greater than 15 000 per μL, any comorbidity (eg, chronic obstructive pulmonary disease, hypertension, diabetes, coronary heart disease, and/or malignancy), required invasive ventilation, developed shock or other organ failure that required admission to an intensive care unit, had an allergy to rhG-CSF, or were breastfeeding or pregnant.
Randomization and Masking
Eligible patients were randomized in a 1:1 ratio to receive either usual care alone or usual care plus rhG-CSF (GRAN; Kyowa Hakko Kirin China Pharmaceutical Co Ltd; 5 μg/kg, subcutaneously once daily, from days 0 to 2). Usual care comprised supplemental oxygen, noninvasive ventilation, or intravenous antibiotics when indicated. Randomization was stratified by the center and oxygen therapy (nasal cannula or mask, or high-flow oxygen, noninvasive ventilation). A statistician who was masked to trial allocation generated the permuted block (4 patients per block) randomization sequence by using SAS software (version 9.4; SAS Institute). Randomization was conducted by using the Interactive Voice Response service provided by Guangzhou University. The allocation team was masked to the block size and random allocation table. A placebo-controlled trial was not performed because of the outbreak emergency.
Procedures
Patients were dynamically monitored daily by trained physicians. Diary cards were dispensed to investigators to collect a 7-category ordinal scale and safety score from day 0 to day 21, hospital discharge, or death. The remaining clinical data were gathered from the customized case report form on which information from the clinical charts was recorded.
Serial oropharyngeal swab specimens were obtained on days 1 (before rhG-CSF injection), 3, 5, 7, 10, 14, and 21 until discharge or death and were subject to real-time RT-PCR assays. Viral RNA was extracted with Nucleic Acid Isolation Kit (category DA0630) on an automatic workstation Smart 32 (Da’an Gene Corporation). The RT–PCR reagent (category DA0930; Da’an Gene Corporation) was used for viral detection. Briefly, 2 PCR primer and probe sets that targeted the ORF1ab (FAM reporter) and N genes (VIC reporter) separately were added. Positive and negative controls were included for each batch of detection. A cycle threshold (Ct) of less than 37 was defined as positive, and a Ct of 40 or greater was defined as negative and recorded as 40. Oropharyngeal swab samples were obtained for 200 patients at every point. Higher Cts indicated lower viral loads. Sampling was continued regardless of the findings.
Peripheral blood samples were obtained on days 1, 3, 5, 7, 10, and 14 and processed to isolate peripheral blood mononuclear cells by density gradient centrifugation. Isolated peripheral blood mononuclear cells were stained with BD multitest IMK kit (category 340503; BD Biosciences). The total T, CD4+ T, CD8+ T, B, and NK cells were enumerated by flow cytometry on an LSR Fortessa Cell Analyzer (BD Biosciences) and analyzed using the FlowJo software (TreeStar). Safety was also evaluated based on the Good Clinical Practice principles. Supplement 2 provides further details.
Outcome Measures
The primary end point was the time to clinical improvement,18 ie, the duration from randomization to the improvement of at least 1 point on a 7-category ordinal scale (adopted from a scale for hospitalized patients with severe influenza)19 or discharge from hospital, whichever occurred first. The 7-category ordinal scale was graded based on the following scheme: 1 for nonhospitalized with normal activities; 2 for nonhospitalized but unable to resume normal activities; 3 for hospitalized but not requiring supplemental oxygen; 4 for hospitalized and requiring supplemental oxygen; 5 for hospitalized and requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; 6 for hospitalized and requiring extracorporeal membrane oxygenation, invasive ventilation, or both; and 7 for death.
Secondary outcomes included lymphocyte cell counts on day 5 posttreatment, mortality at day 21, the proportions of patients progressing to critical conditions (eg, acute respiratory distress syndrome, sepsis, or septic shock), and viral loads (reflected by the Ct values as measured with real-time RT-PCR assays) at day 21. Safety outcomes included adverse events, serious adverse events, and premature discontinuation of treatment. Classifications were based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
A detailed description of the statistical analysis plan is provided in Supplement 3. Assuming a 2-sided significance level of P < .05, the median duration of illness of 14 days in usual care group, and 140 patients (70%) achieving clinical improvement, 192 patients (96%) would be needed to provide a power of 90% to detect a 6-day difference in the median time to clinical improvement.
Efficacy assessment was based on the intention-to-treat population, ie, all patients who were randomized. A sensitivity analysis was performed based on the actual treatment exposure (2 patients [1%] randomized to the rhG-CSF group who did not receive rhG-CSF were included in the usual care group). The safety population consisted of participants who received at least 1 dose of the study drug.
The time to clinical improvement was assessed by reviewing all patients who had reached day 21. Right censoring at day 21 was conducted in case of a failure to reach clinical improvement. Death that occurred before day 21 was treated as not improved and analyzed as the competing risk event. The cumulative incidence function plot was used to demonstrate the time to clinical improvement, with the Gray test being applied for the between-group comparisons. The Fine and Gray proportional subdistribution hazards model adjusting for the center and the oxygen therapy was used to calculate the hazard ratios (HRs) and the 95% confidence intervals.20 The time to death was compared by the log-rank test, and the HR with 95% CIs were estimated by the Cox proportional hazards model. The difference in the day-21 mortality and proportion of patients progressing to critical conditions was expressed as the difference of the rate and the Newcombe hybrid score 95% CIs.21 The difference in the median hospital stay and oxygen support days were analyzed by using the Hodges-Lehmann estimate, along with 95% CIs.22 The change from baseline in the viral load, posttreatment PBL, leukocyte, CD8+T, and NK cell counts was compared by using a linear mixed-effect model, with the baseline level being the covariate. Because lymphocyte cell counts reportedly correlated with the outcomes of viral pneumonia, a predefined subgroup analysis was performed based on the cutoff of 400 per μL (patients requiring high-flow nasal cannula oxygen therapy or noninvasive mechanical ventilation consistently had PBL cell counts ≤400/μL). A predefined subgroup analysis based on age, sex, and oxygen therapy was also performed. Safety analyses were based on actual treatment exposure. As we did not adjust for the multiplicity of secondary analyses, the uncorrected 95% CIs cannot be used to definitively infer therapeutic effects. All analyses were conducted with SAS software (version 9.4; SAS Institute).
Results
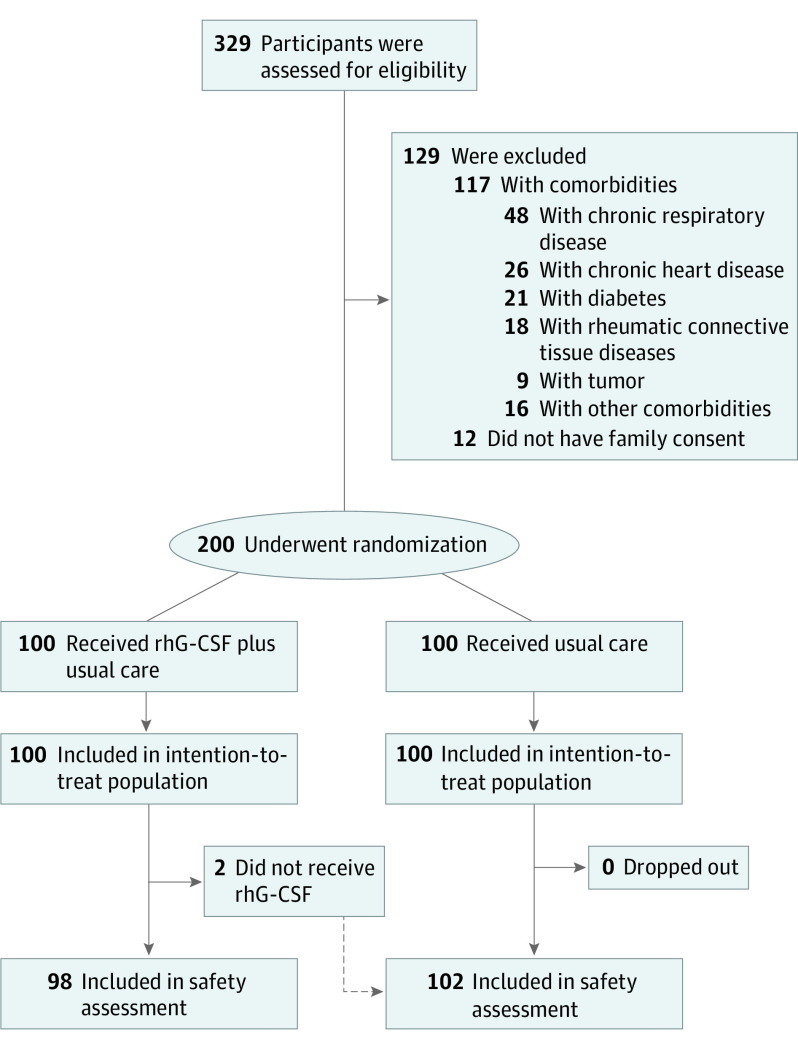
Of 329 patients with COVID-19 who underwent screening, 117 (35.6%) did not meet eligibility criteria, and consent was not obtained from family guardians for 12 (3.6%). Therefore, 100 patients were assigned to receive rhG-CSF plus usual care and 100 usual care alone. Ninety-eight patients in the rhG-CSF group received treatment as assigned and were included in the safety population (Figure 1). Two patients did not receive rhG-CSF, as the treatment was declined after randomization.
Figure 1. Trial Profile.
Some of the patients had more than 1 comorbidity, and therefore the sum of patients with each category of comorbidities exceeded 117. rhG-CSF indicates recombinant human granulocyte colony-stimulating factor.
Table 1 presents the baseline demographic and clinical characteristics of the participants. The median age was 45 years (interquartile range [IQR], 40-55 years), and 112 (56%) were men. The median interval was 6 days (IQR, 5-8 days) from symptom onset to randomization and 2 days (IQR, 2-3 days) from hospital admission to randomization. Both groups were well matched for baseline variables. Systemic corticosteroids were administered for 25 patients (12.5%) in the rhG-CSF group and 32 patients (16%) in the usual care group. Lopinavir-ritonavir, arbidol, and α-interferon inhalation were administered in 14%, 16%, and 8% of patients in the rhG-CSF group before enrollment, respectively. These figures were 17%, 20%, and 10% in the usual care group, respectively (Table 1). Other treatments at enrollment are shown in eTable 1 in Supplement 2.
Table 1. Baseline Demographic Characteristics and Laboratory Findings and Treatments Received Before Enrollmenta.
| Characteristic | No. (%) | ||
|---|---|---|---|
| rhG-CSF (n = 100) | Usual care (n = 100) | Total (N = 200) | |
| Age, median (IQR), y | 45 (40-55) | 46 (38-54) | 45 (40-55) |
| Male sex | 58 (58) | 54 (54) | 112 (56) |
| Body temperature, median (IQR), °F | 99.5 (98.4-100.0) | 99.0 (98.2-100.0) | 99.3 (98.2-100.0) |
| Fever on admission | 73 (73) | 67 (67) | 140 (70) |
| Respiratory rate (IQR), /min | 21 (19-24) | 23 (19-25) | 22 (19-25) |
| Pao2:FiO2 ratio (IQR), mm Hg | 272.5 (235.0-344.0) | 266.0 (236.5-328.0) | 271.0 (235.0-337.0) |
| White cell count, median (IQR), /μL | 4250 (3550-5650) | 5050 (3900-6850) | 4550 (3800-6250) |
| >10 000 | 3 (3) | 7 (7) | 10 (5) |
| ≤4000 | 40 (40) | 27 (27) | 67 (33.5) |
| Lymphocyte count (IQR), /μL | 430 (338-623) | 420 (340-593) | 430 (340-620) |
| <4000 | 46 (46) | 49 (49) | 95 (47.5) |
| Platelet count (IQR), ×103/μL | 197.5 (156.0-242.0) | 210.0 (138.5-263.0) | 201.0 (150.0-253.5) |
| <100 | 4 (4) | 7 (7) | 11 (5.5) |
| C-reactive protein, ≥1 mg/dL | 67/96 (69.8) | 76/97 (78.4) | 146/193 (75.6) |
| Procalcitonin ≥0.5 ng/mL | 20/93 (21.5) | 25/93 (26.9) | 46/186 (24.7) |
| Aspartate aminotransferase >40 U/L | 27/94 (28.7) | 24/92 (26.1) | 51/186 (27.4) |
| Alanine aminotransferase >40 U/L | 30/94 (31.9) | 32/92 (34.8) | 62/186 (33.3) |
| Lactate dehydrogenase ≥250 U/L | 36/95 (37.9) | 39/92 (42.4) | 75/187 (40.1) |
| Creatine kinase ≥200 U/L | 21/95 (22.1) | 24/92 (26.1) | 47/187 (25.1) |
| Seven-category scale at day 1b | |||
| 3: Hospitalization, not requiring supplemental oxygen | 11 (11) | 15 (15) | 26 (13) |
| 4: Hospitalization, requiring supplemental oxygen | 63 (63) | 57 (57) | 120 (60) |
| 5: Hospitalization, requiring HFNC or noninvasive mechanical ventilation | 26 (26) | 28 (28) | 54 (27) |
| Days from illness onset to randomization, median (IQR) | 6 (5-8) | 6 (5-7) | 6 (5-8) |
| Viral load, cycle threshold values of Orf1ab gene by RT-PCR at day 1c | 29.3 (5.2) | 31.0 (4.2) | 30.1 (4.8) |
| Use of interferon α before enrollment | 8 (8) | 10 (10) | 18 (9) |
| Use of lopinavir–ritonavir before enrollment | 14 (14) | 17 (17) | 31 (15.5) |
| Use of arbidol before enrollment | 16 (16) | 20 (20) | 36 (18) |
Abbreviations: FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; IQR, interquartile range; Pao2, partial pressure of oxygen; rhG-CSF, recombinant human granulocyte colony-stimulating factor; RT-PCR, reverse-transcription polymerase chain reaction.
SI conversion factors: To convert alanine aminotransferase, aspartate aminotransferase, creatine kinase, and lactate dehydrogenase to μkat/L, multiply by 0.0167; C-reactive protein to mg/L, multiply by 10; platelet count to ×109/L, multiply by 1; white blood cell and lymphocyte count to ×109/L, multiply by 0.001.
None of the study participants had documented comorbidities according to our exclusion criteria.
At 1 day after randomization but before the treatment.
Data are presented as mean (SD). Viral load is measured with cycle thresholds.
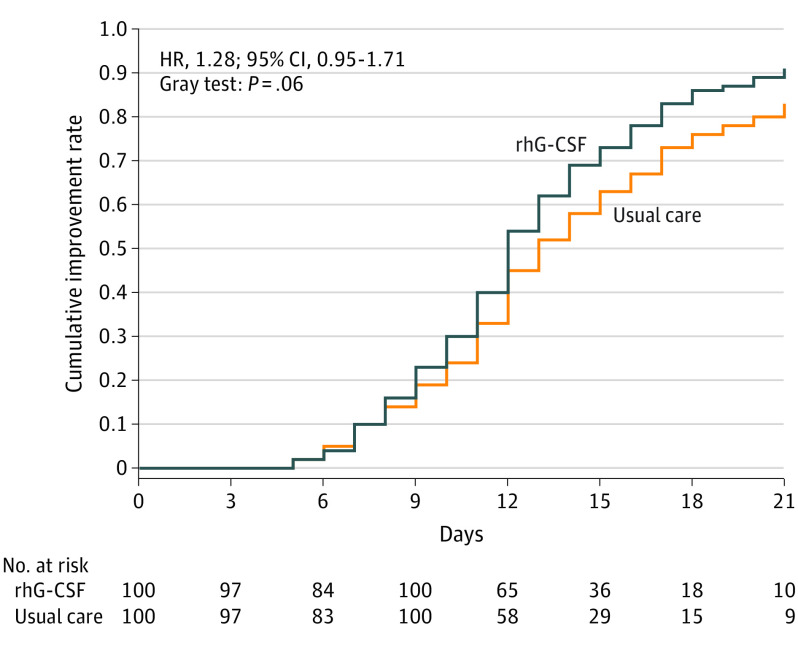
In the intention-to-treat analysis, the time to clinical improvement did not differ between the rhG-CSF group and the usual care groups (median [IQR]: 12 [10-16] vs 13 [11-17] days; HR, 1.28; 95% CI, 0.95-1.71; Figure 2; Table 2). However, a sensitivity analysis based on actual treatment exposure showed a modest difference in the time to clinical improvement in the rhG-CSF group (median [IQR], 12 [10-16] vs 13 [11-17] days; HR, 1.34; 95% CI, 1.00-1.79) (eTable 2 in Supplement 2). In the subgroup analysis, rhG-CSF had greater effects on time to clinical improvement in patients with a PBL cell count 400 per μL or less (rhG-CSF group median [IQR] of 12 [9-15] vs usual care group median of 14 [11-18] days; HR, 1.86; 95% CI, 1.23-2.83) compared with patients with a PBL cell count of more than 400 per μL (rhG-CSF group median [IQR] of 12 [11-17] vs usual care group median [IQR] of 12 [10-17] days; HR, 0.92; 95% CI, 0.64-1.33). The rhG-CSF also had greater effects on the time to clinical improvement in patients requiring high-flow nasal cannula oxygen therapy or noninvasive mechanical ventilation (rhG-CSF group median [IQR] of 9.5 [8-11] vs usual care group median of 11 days; HR, 2.06; 95% CI, 1.16-3.67). No significant inconsistent treatment effects on the time to clinical improvement across age and sex subgroups were observed (eFigure 1 in Supplement 2).
Figure 2. Time to Clinical Improvement at Day 21.
The blue curve indicates the recombinant human granulocyte colony-stimulating factor (rhG-CSF) group, whereas the orange curve denotes the control group (usual care). The hazard ratio (HR) of achieving clinical improvement, along with the 95% CI and the P value, is also reported. The hazards ratio with the 95% CI was estimated by using the Fine and Gray proportional subdistribution hazards model with treatment group, center, and oxygen therapy being included in the model.
Table 2. Therapeutic Outcomes in the Intention-to-Treat Population.
| Characteristic | rhG-CSF (n = 100) | Usual care (n = 100) | Difference (95% CI)a |
|---|---|---|---|
| Primary outcome | |||
| Time to clinical improvement, median (IQR), d | 12 (10 to 16) | 13 (11 to 17) | 1.28 (0.95 to 1.71)b |
| Secondary outcomes | |||
| Patients progressing to critical condition, No. (%)c | 2 (2.0) | 15 (15.0) | −13.0 (−21.4 to −5.4) |
| Day 21 fatality, No. (%) | 2 (2.0) | 10 (10.0) | −8.0 (−15.6 to −1.3) |
| Oxygen support duration, median (IQR), d | 10 (9 to 12) | 10 (8 to 13) | 0 (−1 to 1) |
| Hospital stay, median (IQR), d | 13 (11 to 16) | 14 (11 to 17) | −1 (−2 to 0) |
| Lymphocyte cell count on Day 5, median (IQR), /μL | 1050 (900 to 1200) | 620 (480 to 740) | 440 (380 to 490) |
| Viral load, cycle threshold values of Orf1b gene by RT-PCR at day 10, mean (SD) | 37.9 (2.5) | 36.9 (3.0) | 1.2 (0.1 to 2.3)d |
Abbreviations: IQR, interquartile range; rhG-CSF, recombinant human granulocyte colony-stimulating factor; RT-PCR, reverse-transcription polymerase chain reaction.
SI conversion factor: To convert lymphocyte cell count to ×109/L, multiply by 0.001.
The difference in the primary or secondary end points was expressed as the difference of the rate or median levels and the 95% CIs.
The hazard ratio for clinical improvement was estimated by the Fine and Gray proportional subdistribution hazards model, with the treatment group, participating center, and oxygen therapy being included in the model.
Critical conditions included acute respiratory distress syndrome, sepsis, or septic shock.
The change from baseline in the viral load was compared, and the mean difference of least-squares means was estimated with the mixed-effect model, with the baseline level being the covariate.
The proportion of patients developing critical conditions was lower in the rhG-CSF group compared with the usual care group (2% vs 15%; difference, −13%; 95% CI, −21.4% to −5.4%). The 21-day fatality rate was 2% in the rhG-CSF group compared with 10% in the usual care group (HR, 0.19; 95% CI, 0.04-0.88; Table 2). eTable 3 in Supplement 2 summarizes the information about the patients who died; eTable 4 in Supplement 2 presents more information about death rates. There were no significant differences between the groups for the duration of hospitalization and oxygen support (Table 2).
The dynamic changes in leukocyte and lymphocyte cell counts are shown in eFigures 2, 3, and 4 in Supplement 2. Leukocyte cell counts increased rapidly and then generally plateaued after day 5 in the rhG-CSF group (eFigure 2 in Supplement 2). At day 5, the median lymphocyte cell count was significantly higher in the rhG-CSF group (rhG-CSF group median [IQR] of 1050/μL [900-1200/μL] vs a usual care group median [IQR] of 620/μL [480-740/μL]; Hodges-Lehmann estimate of the difference in medians, 440; 95% CI, 380-490). Similar findings applied at days 7 and 10. The rhG-CSF rapidly increased CD8+ cell counts (which plateaued at day 5), although the difference was less pronounced at day 14. There was a significant, albeit modest, increase in NK cell counts at days 5, 7, and 10 in the rhG-CSF group compared with the usual care group (eFigure 3 in Supplement 2). The trend of increases in peripheral blood CD4+ T cell and B cell counts was comparable between the 2 groups despite more prominent increases in rhG-CSF group at certain points (eFigure 4 in Supplement 2).
Sixty-one patients in the rhG-CSF group (30.5%) and 58 in the usual group (29%) had available virologic data, whereas the others had undetectable viral RNA on oropharyngeal swabs. Mean (SD) baseline Cts of SARS-CoV-2 in rhG-CSF group were lower than that in the usual care group (29.3 [5.2] vs 31.0 [4.2]) (Table 1). Overall, viral RNA loads over time did not differ between the 2 groups. However, the viral RNA loads declined more rapidly in rhG-CSF group at day 10 (Mean [SD] Ct of Orf1b gene, 37.9 [2.5] vs 36.9 [3.0]; mean difference, 1.2; 95% CI, 0.1-2.3) (eFigure 5 in Supplement 2).
Adverse events occurred in 91 of 98 patients (92.9%) in the rhG-CSF group and 47 of 102 patients (46.1%) in the usual care group. Muscular soreness, osteodynia, rash, fatigue, nausea, and vomiting were most common. The incidence of osteodynia, muscular soreness, fatigue, increased lactate dehydrogenase, and alkaline phosphatase levels was higher in the rhG-CSF group. Serious adverse events occurred in 29 of 98 (29.6%) in the rhG-CSF group and 42 of 102 patients (41.2%) in the usual care group. Respiratory failure (defined by the partial pressure of arterial blood oxygen being <60 mm Hg while breathing room air), acute respiratory distress syndrome, sepsis, and septic shock were more common in the usual care group. Six patients in the rhG-CSF group developed severe leukocytosis based on a leukostasis grading score.23 There were no anaphylactic or delayed hypersensitivity reactions (Table 3).
Table 3. Summary of Adverse Events in the Safety Population.
| Adverse event | No. (%) | |||
|---|---|---|---|---|
| rhG-CSF (n = 98) | Usual care (n = 102) | |||
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Any adverse event | 91 (92.9) | 20 (20.4) | 47 (46.1) | 21 (20.6) |
| Neutrophilia | 87 (88.8) | 16 (16.3) | 5 (4.9) | 0 |
| Osteodynia | 17 (17.3) | 1 (1.0) | 6 (5.9) | 0 |
| Muscular soreness | 29 (29.6) | 2 (2.0) | 15 (14.7) | 2 (2.0) |
| Rash | 5 (5.2) | 0 | 3 (2.9) | 0 |
| Fatigue | 12 (12.4) | 1 (1.0) | 10 (9.8) | 1 (1.0) |
| Nausea | 5 (5.1) | 0 | 8 (7.9) | 0 |
| Vomiting | 3 (3.1) | 0 | 1 (1.0) | 0 |
| Facial flushing | 5 (5.1) | 0 | 1 (1.0) | 0 |
| Tachycardia | 15 (15.3) | 2 (2.0) | 22 (21.6) | 5 (4.9) |
| Increased lactate dehydrogenase levels | 36 (36.7) | 3 (3.1) | 35 (34.3) | 7 (6.9) |
| Increased alkaline phosphatase levels | 18 (18.4) | 1 (1.0) | 15 (14.7) | 2 (2.0) |
| Increased aspartate aminotransferase levels | 29 (29.6) | 2 (2.0) | 37 (36.3) | 5 (4.9) |
| Increased alanine transaminase levels | 29 (29.6) | 2 (2.0) | 37 (36.3) | 3 (2.9) |
| Increased creatinine levels | 3 (3.1) | 0 | 1 (1.0) | 0 |
| Serious adverse event | 29 (29.6) | 29 (37.1) | 42 (41.2) | 42 (41.2) |
| Sepsis or septic shock | 2 (2.0) | 2 (2.0) | 10 (9.8) | 10 (9.8) |
| Respiratory failure | 29 (29.6) | 29 (29.6) | 40 (39.2) | 40 (39.2) |
| Acute respiratory distress syndrome | 2 (2.0) | 2 (2.0) | 15 (14.7) | 15 (14.7) |
| Acute kidney injury | 0 | 0 | 2 (2.0) | 2 (2.0) |
| Pneumothorax | 0 | 0 | 2 (2.0) | 1 (1.0) |
| Disseminated intravascular coagulation | 0 | 0 | 1 (1.0) | 1 (1.0) |
| Acute heart failure | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) |
Abbreviation: rhG-CSF, recombinant human granulocyte colony-stimulating factor.
The therapeutic effects of rhG-CSF were inconsistent among the subgroups with different PBL cell counts and oxygen therapy. Because our main study assumption was that rhG-CSF modulated the trafficking of PBL (including lymphocyte subpopulations) and patients requiring high-flow nasal cannula oxygen therapy or noninvasive mechanical ventilation consistently had PBL cell counts of 400 per μL or less (eTable 5 in Supplement 2), we performed a post hoc subgroup analysis based on PBL cell counts (≤400/μL and >400/μL) (eTables 6 and 7 and eFigures 6, 7, 8, and 9 in Supplement 2). In these analyses, rhG-CSF was associated with accelerated clinical improvement and a decreased rate of progression to critical conditions or death in patients with a PBL cell count of 400 per μL or less (eTable 6 in Supplement 2).
Discussion
In preliminary findings from a randomized clinical trial, treatment of patients with COVID-19 with lymphopenia and no comorbidities did not change the time to clinical improvement. However, rhG-CSF treatment led to rapid restoration of the lymphocyte (including subsets) and NK cell counts and appeared to decrease the frequency of patients progressing to critical illness or death.
The aberrant immune responses, characterized by severe lymphopenia and leukopenia and exhaustion of NK cells,24 contribute to the poor outcomes of some patients with COVID-19. In this study, rhG-CSF led to a sustained increase in lymphocyte (including subsets) and NK cell counts. By contrast, in an observational study, no significant effect of rhG-CSF on NK cell count was identified,17 which might have stemmed from the difference in study design and population.
Adverse events were mostly classified as mild to moderate. Serious adverse events (ie, sepsis or septic shock, respiratory failure, and acute respiratory distress syndrome) were less common in the rhG-CSF group. Because patients with comorbidities might have more contraindications for rhG-CSF treatment, this study was enriched for young patients without comorbidities. The exclusion of patients without comorbidities might have accounted for the low fatality rate (approximately 7%). Furthermore, the lower incidence of sepsis in the rhG-CSF group might be associated with the increased leukocyte cell count. Our findings also suggest that caution should be exercised in patients with leukocytosis. Leukocytosis was observed in nearly 90% of patients receiving rhG-CSF (6 of whom had severe leukocytosis). However, capillary obstruction leading to organ infarction was not documented.
Limitations
This study has limitations. First, it was limited by its small size and the observational time frame of 21 days, within which discharge from hospital or death might not have been documented. Second, the open-label trial design might have affected the outcome measures. However, most of the measures on the 7-category ordinal scare were objective, thus the risk of biased findings was minimized. Third, we did not exclude patients who had received antiviral therapy or systemic corticosteroids before enrollment, which could have confounded the findings. Finally, we excluded patients who had comorbidities to minimize the potential adverse effects of other comorbidities on the immune responses of patients and also the potential for adverse events related to rhG-CSF treatment.
Conclusions
Whether our findings may apply to other groups of patients with COVID-19 merits further investigation. Larger studies that include a broader range of patients with COVID-19 should be conducted.
Trial protocol
eMethods
eResults
eTable 1. Treatments received after enrollment
eTable 2. Sensitivity analysis results for the primary outcome
eTable 3. A list of the details pertaining to the patients who progressed to death during the study
eTable 4. Fatality rate in the intention-to-treat population
eTable 5. Association between oxygen therapy and PBL count
eTable 6. Subgroup analysis results
eTable 7. Sensitivity post hoc subgroup analysis for the primary outcome
eFigure 1. Subgroup analysis of the primary outcome
eFigure 2. The dynamic changes in peripheral blood white blood cell count
eFigure 3: Dynamic changes in the mean T lymphocyte, CD8+ T cell and natural killer cell count
eFigure 4. The dynamic changes in peripheral blood CD4+ T cell and B cell count
eFigure 5. SARS-CoV-2 viral load by reverse transcription polymerase chain reaction on throat swabs
eFigure 6. Subgroup analysis of the time to clinical improvement at day 21
eFigure 7. Subgroup analysis of the dynamic changes in peripheral blood leukocyte count
eFigure 8. Subgroup analysis of the dynamic changes in the mean T lymphocyte subset count
eFigure 9. Subgroup analysis of the dynamic changes in peripheral blood lymphocyte subset count
Statistical analysis plan
Data sharing statement
Reference
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. Accessed August 11, 2020. https://covid19.who.int/
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Guo M, Sun X, et al. Effects of recombinant human granulocyte colony-stimulating factor on central and peripheral T lymphocyte reconstitution after sublethal irradiation in mice. J Radiat Res. 2013;54(1):83-91. doi: 10.1093/jrr/rrs082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang CC, Tay YK. Hematological abnormalities and the use of granulocyte-colony-stimulating factor in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Dermatol. 2011;50(12):1570-1578. doi: 10.1111/j.1365-4632.2011.05007.x [DOI] [PubMed] [Google Scholar]
- 11.Koizumi T, Kubo K, Koyama S, et al. Neutrophils pretreated with granulocyte colony-stimulating factor (G-CSF) are not related to the severity of endotoxin-induced lung injury. Exp Lung Res. 1997;23(5):393-404. doi: 10.3109/01902149709039234 [DOI] [PubMed] [Google Scholar]
- 12.Li JG, Zhang YJ, Wu W, et al. Effect of recombinant human granulocyte colony-stimulating factor on leukopenia in severe acute respiratory syndrome patients. Yi Shi Jin Xiu Za Zhi. 2004; 27:21-22. doi: 10.3760/cma.j.issn.1673-4904.2004.23.008 [DOI] [Google Scholar]
- 13.Kimura S, Matsuda J, Ikematsu S, et al. Efficacy of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with AIDS. AIDS. 1990;4(12):1251-1255. doi: 10.1097/00002030-199012000-00011 [DOI] [PubMed] [Google Scholar]
- 14.Zhao SS, Fang S, Zhu CY, Wang LL, Gao CJ. Effect of G-CSF in vitro stimulation on distribution of peripheral lymphocyte subsets in the healthy persons [article in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(1):258-262. [DOI] [PubMed] [Google Scholar]
- 15.Melve GK, Ersvaer E, Eide GE, Kristoffersen EK, Bruserud Ø. Peripheral blood stem cell mobilization in healthy donors by granulocyte colony-stimulating factor causes preferential mobilization of lymphocyte subsets. Front Immunol. 2018;9:845. doi: 10.3389/fimmu.2018.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.San Miguel JF, Hernández MD, Gonzalez M, et al. A randomized study comparing the effect of GM-CSF and G-CSF on immune reconstitution after autologous bone marrow transplantation. Br J Haematol. 1996;94(1):140-147. doi: 10.1046/j.1365-2141.1996.d01-1756.x [DOI] [PubMed] [Google Scholar]
- 17.Rondelli D, Raspadori D, Anasetti C, et al. Alloantigen presenting capacity, T cell alloreactivity and NK function of G-CSF-mobilized peripheral blood cells. Bone Marrow Transplant. 1998;22(7):631-637. doi: 10.1038/sj.bmt.1701413 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Coronavirus disease (COVID-2019). Accessed September 1, 2020. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/
- 19.International Severe Acute Respiratory and Emerging Infections Consortium. ISARIC home page. Accessed September 1, 2020. https://isaric.tghn.org/
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 21.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 22.Hodges JL Jr, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat. 1963;34:598-611. doi: 10.1214/aoms/1177704172 [DOI] [Google Scholar]
- 23.Piccirillo N, Laurenti L, Chiusolo P, et al. Reliability of leukostasis grading score to identify patients with high-risk hyperleukocytosis. Am J Hematol. 2009;84(6):381-382. doi: 10.1002/ajh.21418 [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eResults
eTable 1. Treatments received after enrollment
eTable 2. Sensitivity analysis results for the primary outcome
eTable 3. A list of the details pertaining to the patients who progressed to death during the study
eTable 4. Fatality rate in the intention-to-treat population
eTable 5. Association between oxygen therapy and PBL count
eTable 6. Subgroup analysis results
eTable 7. Sensitivity post hoc subgroup analysis for the primary outcome
eFigure 1. Subgroup analysis of the primary outcome
eFigure 2. The dynamic changes in peripheral blood white blood cell count
eFigure 3: Dynamic changes in the mean T lymphocyte, CD8+ T cell and natural killer cell count
eFigure 4. The dynamic changes in peripheral blood CD4+ T cell and B cell count
eFigure 5. SARS-CoV-2 viral load by reverse transcription polymerase chain reaction on throat swabs
eFigure 6. Subgroup analysis of the time to clinical improvement at day 21
eFigure 7. Subgroup analysis of the dynamic changes in peripheral blood leukocyte count
eFigure 8. Subgroup analysis of the dynamic changes in the mean T lymphocyte subset count
eFigure 9. Subgroup analysis of the dynamic changes in peripheral blood lymphocyte subset count
Statistical analysis plan
Data sharing statement