Key Points
Question
Can the psoriatic microenvironment (PME) score, a novel bioinformatic analysis of the psoriatic microenvironment, be used to predict if psoriasis will respond to a new treatment before a clinical response is seen?
Findings
Analysis of whole-genome mRNA expression in skin biopsies identified 2 different immune profiles that correlate with psoriatic lesional and nonlesional skin; with systemic treatment, the immune phenotype of psoriatic lesional skin reverted to a nonlesional pattern. The PME score is a bioinformatic metric that encompasses these changes and correlates with future clinical responses 8 weeks prior to apparent clinical differences.
Meaning
The PME score may predict the therapeutic efficacy of systemic psoriasis therapies prior to apparent clinical changes and may serve as an example of personalized medicine.
This study examines a novel decision analytical model designed to measure mRNA gene expression changes to define the psoriatic microenvironment and assess medication response before a change in the Psoriasis Area and Severity Index score is evident.
Abstract
Importance
The ability to predict the efficacy of systemic psoriasis therapy based on immune profiles in skin biopsies could reduce the use of inappropriate treatment and its associated costs and adverse events. It could considerably decrease drug development trial costs as well.
Objective
To develop a bioinformatic gene signature score derived from skin mRNA to predict psoriasis treatment outcomes for a variety of therapies.
Design, Setting, and Participants
In this decision analytical model using 1145 skin samples from different cohorts of 12 retrospective psoriasis studies, samples were analyzed using the CIBERSORT algorithm to define the immune landscape of psoriasis lesions and controls. Random forest classification and principal component analysis algorithms were used to estimate psoriatic microenvironment (PME) signature genes and construct a PME score. Overall, 85 and 421 psoriasis lesions from 1 and 4 independent cohorts were used as discovery and validation studies, respectively. Among them, 157, 71, 89, and 90 psoriasis lesions were treated with etanercept, tofacitinib, adalimumab, and methotrexate, respectively.
Main Outcomes and Measures
Number of weeks after treatment initiation when responders and nonresponders could be predicted.
Results
Overall, 22 immune cell subtypes formed infiltration patterns that differentiated psoriasis lesions from healthy skin. In psoriasis lesions, the expression of 33 PME signature genes defined 2 immune phenotypes and in aggregate could be simplified to a numerical PME score. A high PME score, characterized by keratinocyte differentiation, correlated with a better treatment response (Psoriasis Area and Severity Index [PASI] reduction, 75.8%; 95% CI, 69.4% to 82.2%; P = .03), whereas a low PME score exhibited an immune activation signature and was associated with a worse response (PASI reduction, 53.5%; 95% CI, 45.3% to 61.7%; P = .03). The PME score at week 4 after treatment initiation correlated with future responder vs nonresponder to treatment status 8 to 12 weeks earlier than PASI reduction for etanercept, methotrexate plus adalimumab, and tofacitinib.
Conclusions and Relevance
The PME score is a biometric score that may predict clinical efficacy of systemic psoriasis therapy in advance of clinical responses. As an application of personalized medicine, it may reduce the exposure of patients with psoriasis to ineffective and expensive therapies.
Introduction
Psoriasis is a common1 autoimmune or autoinflammatory skin disease with dysfunction in both the innate and adaptive immune systems.2,3,4 Investigators have made important discoveries on the role of pathogenic cytokines like tumor necrosis factor, interleukin-12, -23, and -17,5,6,7,8,9 and immune cells like infiltrative type 17 and type 1 helper T cells.10,11,12,13,14,15,16,17,18,19 Effective biologic therapies for psoriasis block proinflammatory cytokines and lymphocyte activation, underscoring the significance of this psoriatic microenvironment (PME).20,21,22
An important clinical gap in psoriasis is the ability to predict which therapies will be effective for a given patient. After a patient commences a psoriasis therapy, there is often a delay of weeks to months between the earliest detectable tissue response in skin biopsy samples and the earliest point where a significant clinical response can be seen (defined as a 75% reduction in the Psoriasis Area and Severity Index [PASI] score [PASI75] was defined as clinical response).23,24 This gap may result in patients spending long periods of time using treatments that may not be effective for them, thereby increasing financial burden, risk of adverse events, and disease progression.25,26,27,28 This gap also elevates drug development costs in research trials.29 Because gene expression profiling can potentially detect a molecular response before a clinical response, defining the PME could be a good approach to characterizing the early molecular changes in response to treatment as well as predicting the long-term response.
To define the PME, we used CIBERSORT, a novel algorithm that identifies specific individual immune cell subsets using bulk mixed RNA samples containing diverse cell types.30 The validity of CIBERSORT is illustrated by its use in many previous studies of different organs, including chronic inflammatory skin.31,32 Using gene expression data from 1145 skin samples from psoriasis patients and controls from 12 studies,24,33,34,35 the diverse immune landscape of psoriatic skin was characterized. Two contrasting PME immune patterns and gene signatures were defined using bioinformatics. Based on these clusters, a numerical PME score was calculated, and its correlation with future response to systemic treatment was evaluated.
Methods
Psoriasis Data Sets and Preprocessing
Because all data used for the study were publicly available and completely deidentified, the study was deemed exempt from any institutional review board approval by Johns Hopkins School of Medicine. We benchmarked published psoriasis gene expression data sets that were available on Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) and selected data sets with more than 30 samples. Database searches were conducted in January 2018 and updated in April 2020. In total, we gathered 12 data sets with psoriasis for this study: GSE11903, GSE13355, GSE14905, GSE30999, GSE31652, GSE41664, GSE47751, GSE53552, GSE69967, GSE75343, GSE78097, and GSE85034. Using the Affy statistical software package (version 1.66.0; R Foundation, Inc), raw data from Affymetrix was processed using the RMA algorithm for background adjustment, quantile normalization, batch correction and final summarization.36 The lumi statistical software package was used to process raw data from Illumina. Data were processed and analyzed using the R Bioconductor package and R (version 3.5.0; R Foundation, Inc) between January 2018 and January 2020. Data processing techniques are detailed in eMethods in the Supplement.
Determination of Immune Cell Infiltration Patterns Among Multiple Cohorts
To construct a model of the PME, we used a bioinformatics workflow outlined in eFigure 1 in the Supplement. Overall, 1145 skin samples from patients with psoriasis and healthy controls in 12 studies were characterized as clinically annotated: psoriasis lesional (Pso-L); psoriasis nonlesional (Pso-NL); psoriasis treatment (Pso-T); and healthy control (Ctrl).24,33,34,35 To define the abundance of infiltrating immune cells in Pso-L, Pso-NL, Pso-T and Ctrl, the LM22 gene signature and the CIBERSORT algorithm were used.30 The CIBERSORT algorithm employs deconvolution based on 574 reference genes considered a minimal representation for 22 cell types, including natural killer cells, macrophages, neutrophils, dendritic cells (DCs), B cells, T cells, and subdivided resting and activated immune cell subtypes. After standardizing annotation files, gene expression profiles were uploaded to the CIBERSORT web service portal (http://cibersort.stanford.edu/). Data were run with 1000 permutations under the LM22 signature. This allowed the definition of 2 distinct cellular infiltration patterns: lesional and nonlesional phenotypes with contrasting immune signatures corresponding with psoriatic vs healthy skin.
Definition of PME Score and Classification of High and Low PME Score
The complete definition of PME gene signature and the calculation algorithm for the PME score are described in detail in the Supplement. Both lesional and nonlesional PME scores for a particular individual are determined through weighted calculations of that patient’s upregulated or downregulated genes and their association with each particular phenotype. A PME score is thus a representative capture of a patient’s disease profile at a given moment in time, comprising a patient’s nonlesional and lesional PME scores (ie, it is the difference between these 2 scores).
To distinguish between high and low PME scores, aggregate week 4 Δ PME scores were compared and divided into 2 groups around the median as either high or low PME scores. To describe changes in the immune landscape due to treatment, a Δ PME score, defined as PME at a particular time point (week N) minus the baseline (week 0), was calculated. The PME was assessed at weeks 1, 2, 4, 12, and 16 based on the data set. The Δ PME score was compared with response to treatment, defined as achievement of PASI75, at each of these time points as well.
Statistical Analysis
Variable normality was tested using Shapiro-Wilk normality test.37 Nonnormally distributed variables were measured by the Mann-Whitney U tests (also called the Wilcoxon rank-sum test) for 2 groups’ comparison. Statistical significance for normally distributed variables were analyzed using the unpaired student t-test. For comparisons of more than 2 groups, 1-way analysis of variance, and Kruskal-Wallis tests were used as parametric and nonparametric methods.38 To identify significant genes in DEGs, Benjamini-Hochberg method was applied to convert P values to false discover rates.39 All statistical analyses were performed using R (https://www.r-project.org/) statistical software (version 3.5.0; R foundation, Inc), and P values were 2-sided. P < .05 was considered statistically significant. Further details of the methods are described in the eMethods in the Supplement.
Results
A Distinct Immune Microenvironment Landscape in Psoriatic Lesional vs Healthy Skin
Bioinformatic analysis demonstrated 2 distinct immune cell infiltration phenotypes across multiple psoriasis cohorts. The nonlesional phenotype was associated with Pso-NL and Ctrl, whereas the lesional phenotype was chiefly seen with Pso-L (Figure 1A). Overall, 93% of Pso-NL and Ctrl belonged to the nonlesional phenotype whereas 80% of Pso-L mapped to the lesional phenotype category, confirming a high degree of segregation (Figure 1B). This indicated that—consistent with previous studies31,32—unsupervised clustering allows for good separation of psoriatic lesional and healthy skin based on the immune landscape inferred from gene expression.
Figure 1. Distinct Immune Microenvironment Landscapes Used to Define Psoriasis Plaques and Their Resolution.
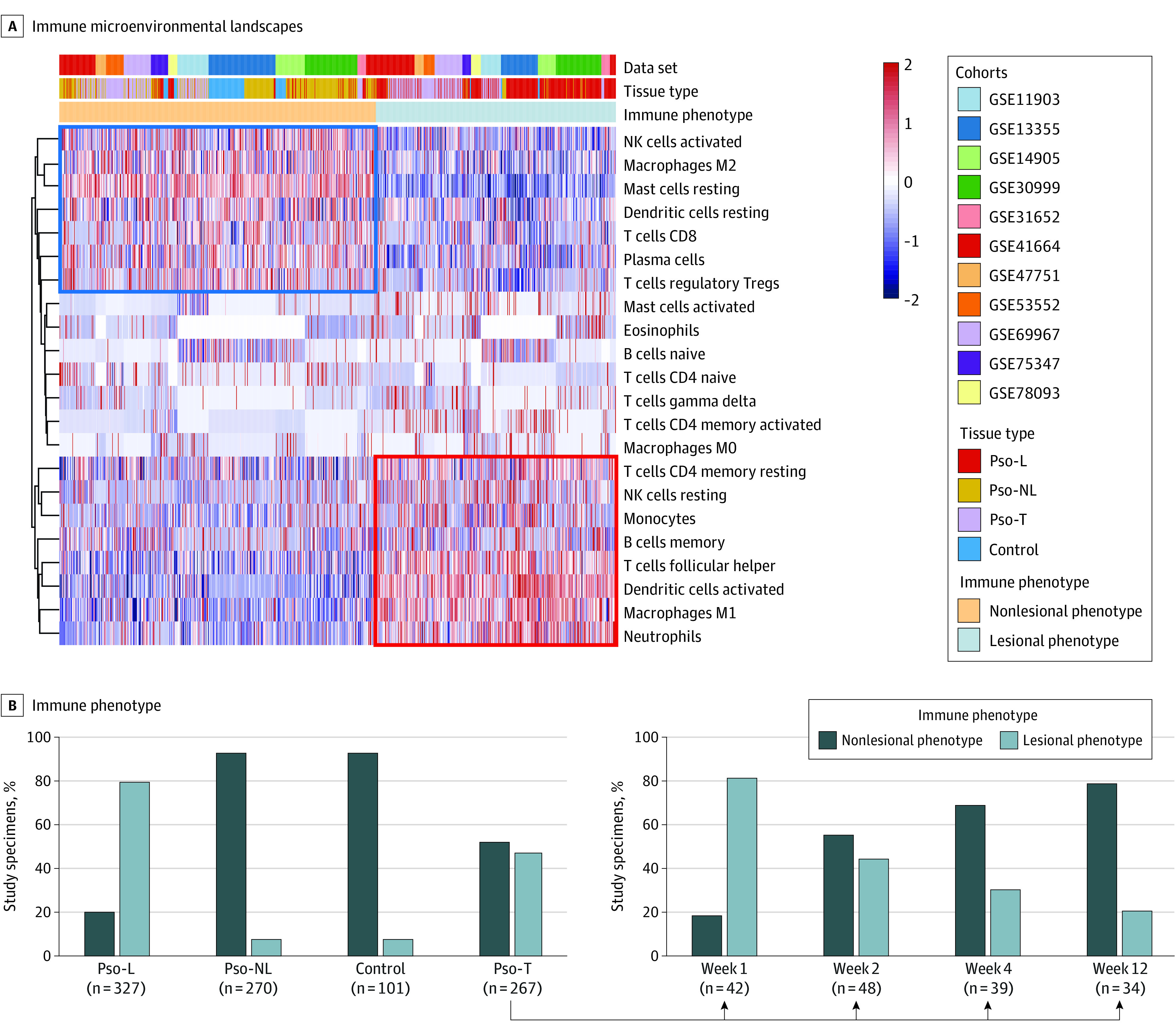
Pso-L indicates psoriasis lesional; Pso-NL, psoriasis nonlesional; Pso-T, psoriasis treatment specimens. A, Vertical lines represent signal strength for each cell type depicted in rows. Shown is unsupervised clustering of immune cell signal strength from 1145 skin samples in multiple cohorts (GSE11903, GSE13355, GSE14905, GSE30999, GSE31652, GSE41664, GSE47751,GSE53552, GSE69967, GSE75343, and GSE78097) each measuring biopsies from Pso-L, Pso-NL, Pso-T, and control specimens. Nonlesional phenotype is more associated with healthy tissue, whereas lesional phenotype is more associated with lesional tissue. B, Although, in aggregate, all psoriasis lesions under treatment show a roughly equal mix between nonlesional phenotype and lesional phenotype, nonlesional phenotype proportion rises with elapsed time after treatment initiation, whereas lesional phenotype decreases.
Among all psoriasis lesions under treatment, 52% expressed the nonlesional phenotype and 48% expressed the lesional phenotype showing roughly equal shares (Figure 1B). Stratifying these lesions based on duration of treatment demonstrated that the proportion of the non-lesional phenotype increased while the proportion of the lesional phenotype decreased over time (Figure 1B). This suggests that modification of the immune cell infiltration pattern reflects psoriasis treatment effect. In particular, increases in the infiltration of activated NK cells, M2 macrophages, resting mast cells, resting DCs, CD8+ T cells, Tregs, and plasma cells occurred with treatment (Figure 1, A and B). In contrast, decreases in infiltrating CD4-positive memory T cells, resting NK cells, monocytes, memory B cells, follicular helper T cells, activated DCs, M1 macrophages, and neutrophils occurred with treatment (Figure 1, A and B). These results demonstrate the capacity of mRNA expression to illustrate the shifting immune cell infiltration during psoriasis treatment.
Identification of a Simpler 33-Gene Set to Define the Psoriasis Immune Landscape
Our next goal was to distill the PME changes above into a short list of specific genes amenable to easier analysis and clinical use. Within a training cohort: GSE30999 of untreated psoriasis lesions, the nonlesional phenotype showed a high infiltration of plasma cells, CD8 T cells, Tregs, activated NK cells, resting DCs, resting mast cells, and other cell types (Figure 2A). In contrast, the lesional phenotype was associated with memory B cells, follicular helper T cells, resting NK cells, M1 macrophages, activated mast cells, and eosinophils (Figure 2A). These cells largely overlap with the analysis in Figure 1A for the nonlesional phenotype and the lesional phenotype and confirmed the validity of this approach.
Figure 2. Immune Phenotype in Psoriasis Distilled to the Expression of 33 Genes.
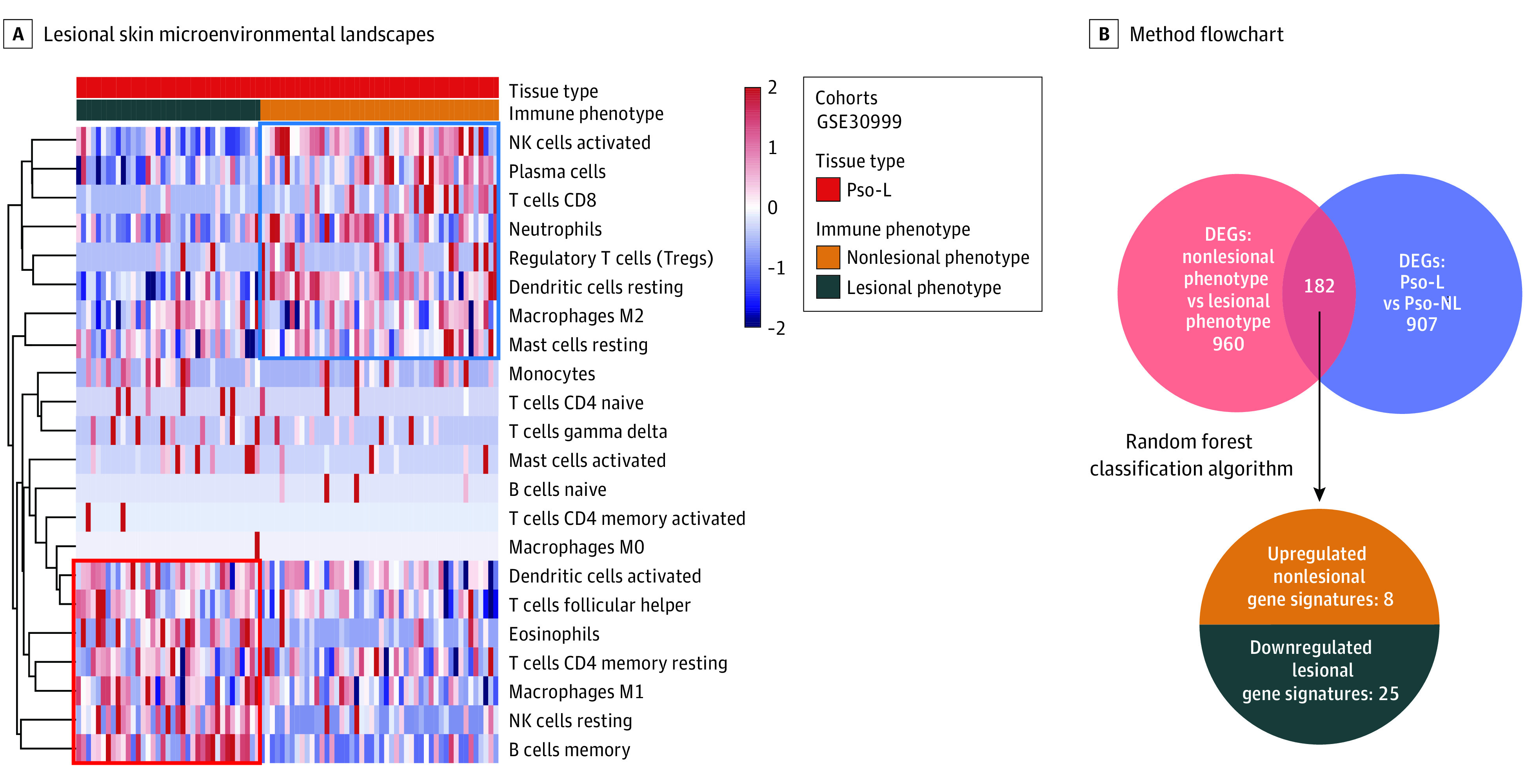
A, An analysis of nonlesional phenotype and lesional phenotype among just psoriasis lesions shows similar immune cells to the analysis including both psoriatic lesional and nonlesional samples in Figure 1A. Shown is unsupervised clustering of immune cells for 85 psoriasis lesions (Pso-L) samples in the GSE30999 as a training cohort. B, DEGs indicates differentially expressed genes. Flowchart of the method to determine the final 33-gene set that defines the psoriatic microenvironment.
To identify the gene signature associated with each PME cell infiltration pattern, we found 182 common genes were differentially expressed in the nonlesional phenotype vs the lesional phenotype and between Pso-L vs Pso-NL (Figure 2B). We distilled this group to 33 PME genes (eMethods in the Supplement). Eight were upregulated in the nonlesional phenotype, whereas 25 were upregulated in the lesional phenotype. This minimum set of genes represents a candidate list for further investigation as well as potential future therapeutic targets for psoriasis.
Using the PME Gene Signature Score to Evaluate the Biology of Healthy vs Psoriatic Skin
Based on these 33 PME gene expression values, patients were separated into 2 clusters through unsupervised sorting. Patients with high expression of the nonlesional gene signature had a higher PME score and were classified as the nonlesional phenotype. In contrast, patients with high expression of the lesional gene signature had a lower PME score and were classified as the lesional phenotype (Figure 3A).
Figure 3. Biologic Categories of the 33 Genes Match Current Models of Psoriasis and Can Be Further Simplified to a Single Numerical Psoriatic Microenvironment (PME) Score.
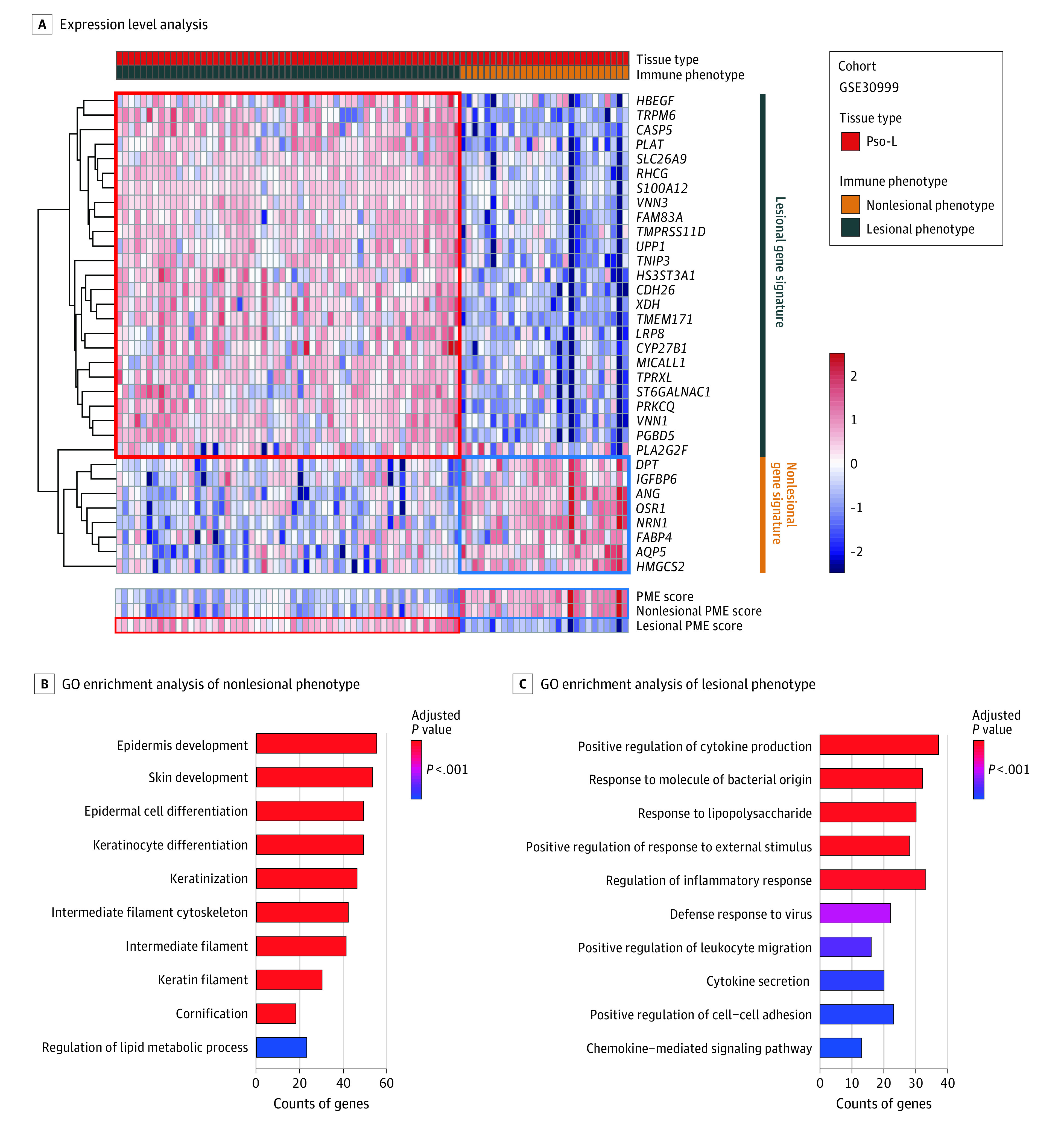
A, Pso-L indicates psoriasis lesional specimen. Expression levels of the 33-gene set as summarized by the PME score accurately separates nonlesional phenotype vs lesional phenotype. Shown is unsupervised analysis and hierarchical clustering of lesional gene signature and nonlesional gene signature derived from the GSE30999 to classify Pso-L samples into 2 groups with gene expression levels depicted. B and C, GO indicates gene ontology. Overrepresented gene categories associated with heathy tissue are present in nonlesional phenotype, whereas those associated with psoriasis are associated with lesional phenotype. Shown are GO enrichment analysis of the differently expressed genes between nonlesional phenotype and lesional phenotype.
To define the biologic characteristics of these 33 genes, we next determined their common features (gene ontology). We found overexpression of genes involved in keratinocyte differentiation in the nonlesional phenotype, as expected, to be associated with healthy tissue and better treatment response (Figure 3B). In contrast, upregulated immune activation genes, which are enriched in the lesional phenotype, were associated with psoriatic lesional skin and poor treatment response (Figure 3B).
PME Score Associated With Treatment Response Prior to Detectable Clinical Changes
Our final goal was to examine the clinical utility of the PME score. We observed a time-dependent effect such that the nonlesional PME score increased and the lesional PME score decreased during treatment among all 4 treatment data sets. In an analysis of these 4 data sets combined, the PME score increased with treatment, whereas the PASI clinical score decreases over time (Figure 4, A-C).
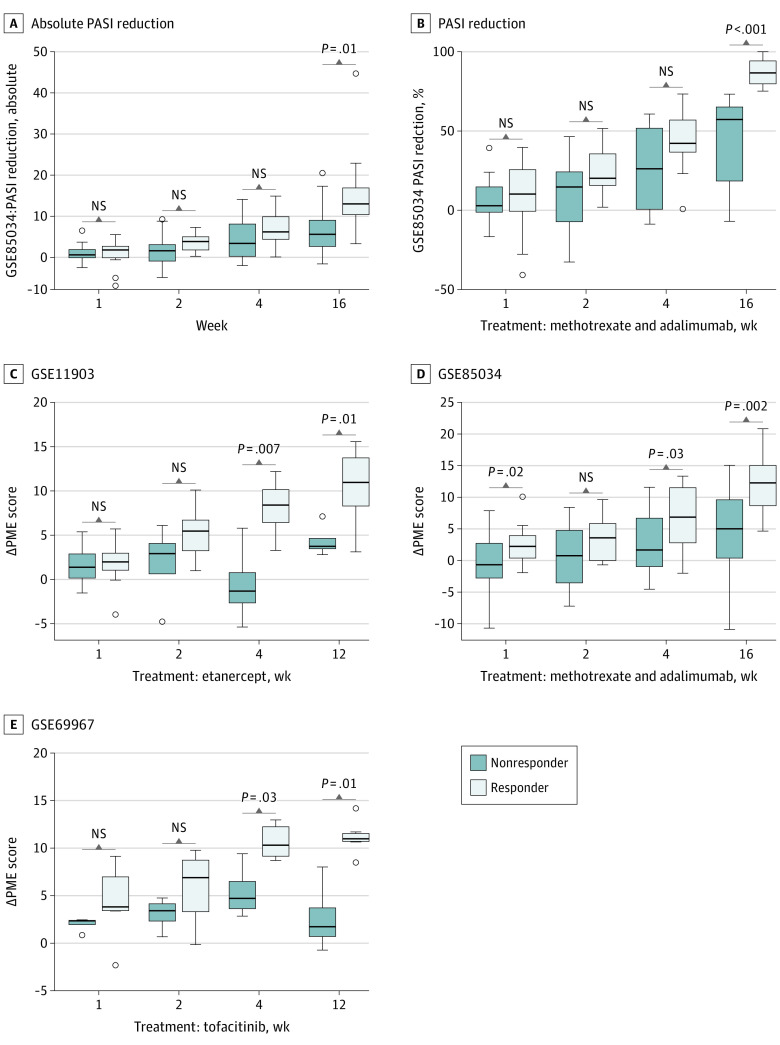
Figure 4. PME Score as a Predictive Biometric Score of Treatment Success, Prior to Clinical Changes.
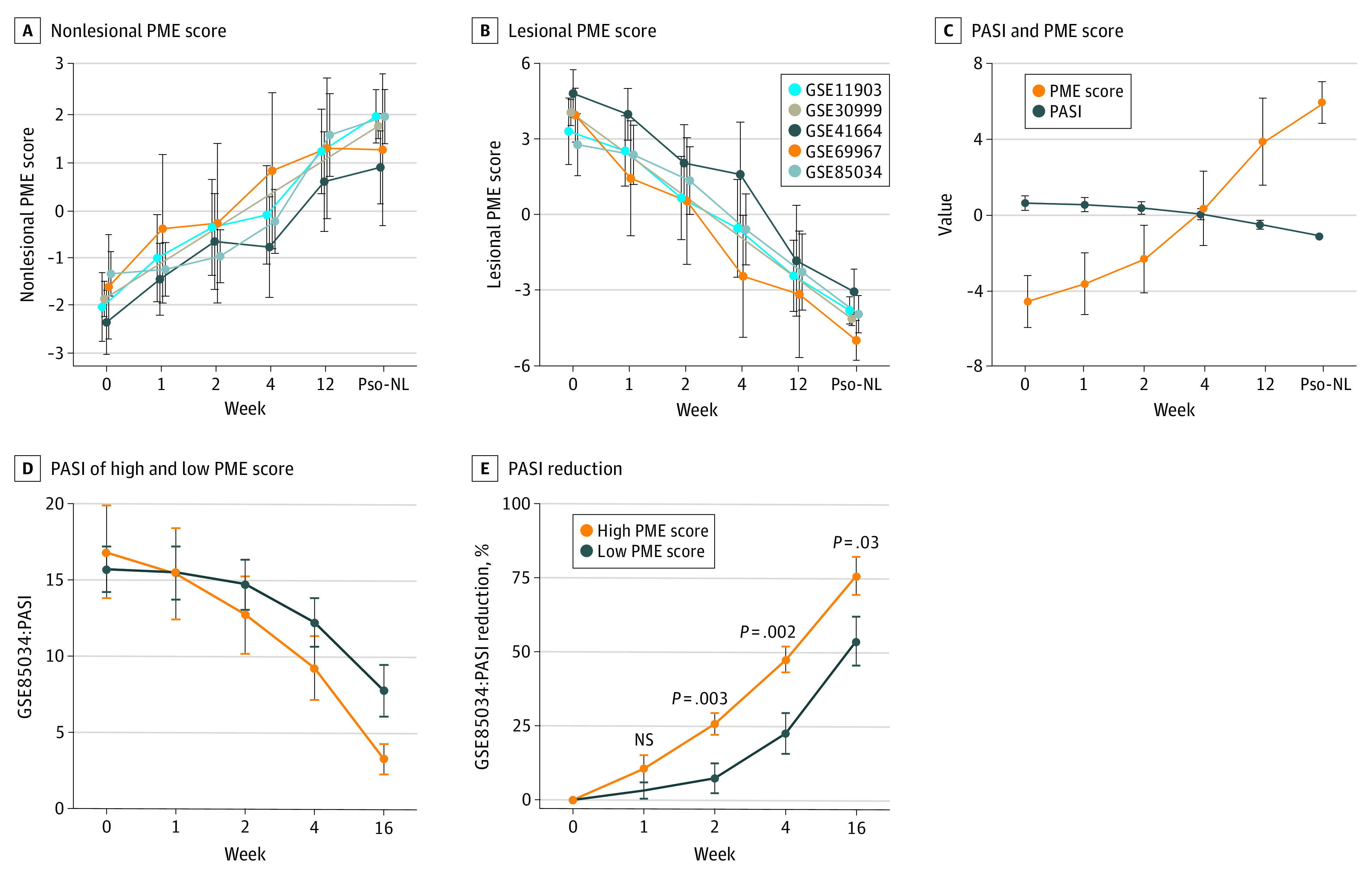
NS indicates not a significant difference; PASI, Psoriasis Area and Severity Index; PME, psoriasis microenvironment; Pso-NL, psoriasis nonlesional. A, The value of nonlesional PME score increased with treatment time. B, The value of lesional PME score decreased with treatment time. C, The PME score increased while PASI decreased. D, The value of PASI in high PME score versus low PME score at different treatment times in GSE85034 cohort. E, The value of PASI reduction in high PME score versus low PME score at different treatment times in the GSE85034 cohorts.
To better describe the immune landscape changes by treatment and remove confounders from distinct and unique patient baseline scores, we used a Δ PME score for each patient (eMethods in the Supplement). Predictive effect analysis showed that week 4 is the best time to predict therapeutic efficacy (eFigure 2B in the Supplement). At week 4, in a comparison among other known psoriasis signatures, Δ PME score has the highest area under the curve (0.78) revealing that the PME score model is a promising correlate to future treatment success (eFigure 2B in the Supplement). Using psoriasis patients’ week 4 Δ PME scores, patients can be divided into 2 groups: high PME score and low PME score. Although the high PME score group had a slightly higher baseline PASI than the low-PME score group at week 0, PASI reduction at week 16 in the high-PME score group was 75.8% (95% CI, 69.4%-82.2%; P = .03), whereas it was 53.5% (95% CI, 45.3%-61.7%; P = .03) in the low-PME score group (Figure 4, D and E). These results highlight the better clinical improvements in patients with a high PME score.
Finally, we sought to carefully test if PME score correlated with future treatment response before obvious clinical changes are apparent. For patients receiving methotrexate or adalimumab, the difference between responders and nonresponders in PASI reduction was not significant in the first 4 weeks of treatment and became significant at week 16 (Figure 5, A and B). However, the difference of the Δ PME score was noted in all 3 treatment cohorts (etanercept, methotrexate, adadlimumab, and tofacitinib) at 4 weeks and broadened by 12 or 16 weeks of therapy (Figure 5, C-E). Thus, the change in PME score may precede PASI changes by 8 weeks (Figure 5).
Figure 5. Δ PME Score Between Responder and Nonresponder.
NS indicates not a significant difference; PASI, Psoriasis Area and Severity Index; PME, psoriasis microenvironment. A, Absolute PASI reduction between responder and nonresponder in the GSE85034 cohorts was not significant until week 16. B, PASI reduction between responder and nonresponder in the GSE85034 cohorts was not significant until week 16 either. C, Δ PME score between responder and nonresponder in the GSE11903 cohorts showed significance by week 4. D, Δ PME score between responder and nonresponder in the GSE85034 cohorts showed significance by week 4. E, Δ PME score between responder and nonresponder in the GSE69967 cohorts showed significance by week 4.
Discussion
The ability to predict the effectiveness of systemic therapy for psoriasis in an individual may improve quality of life, reduce medication adverse effects, and decrease unnecessary costs; for the health care system it may also shorten drug development time. The strategy outlined here measures mRNA gene expression changes to define the PME and assess medication response before a change in PASI score is evident. The PME score represents a robust approach to apply personalized medicine to improve the treatment of patients with psoriasis.
With the advancement of bioinformatics, efficient algorithms have accelerated the transition of clinical treatment targets from discovery to application. The CIBERSORT algorithm, as one of the most common, efficient, and robust algorithms for analyzing immune cell infiltration, has been used in thousands of studies. Its importance is proven in medication guidance of precision medicine,40,41 exploration of pathogenesis,42,43,44 and the development of new targets for clinical drugs.45,46,47 We used CIBERSORT to advance psoriasis therapy evaluation.
In this retrospective study, we describe different immune cell infiltration patterns in different skin microenvironments. Most of these immune cell infiltration patterns were consistent with previous studies,18,48,49,50,51,52,53 whereas the less studied cells illustrate the value of unsupervised bioinformatics analyses of these large data sets. We defined and tested the PME score through 12 pooled studies to look for a common transcriptome signature of psoriasis and its response to diverse treatments. The result of differences in immune cell infiltration, the PME score accurately captures the pathogenesis and resolution of psoriasis after treatment with 4 distinct therapies: etanercept, methotrexate, adalimumab, or tofacitinb. Based on the expression of only 33 genes, the PME score from the molecular data available at week 4 correlates with future clinical outcomes 12 or 16 weeks after starting treatment. Although it is the case that these varied modalities will have distinct biologic effects, our results illustrate that the PME score can detect improvements in these disparate contexts because it measures a core psoriasis molecular signature that must be normalized for clinical improvements to occur. The potential future rollout of this test is highly feasible given the small final set of 33 genes used. For example, the NanoString nCounter gene-expression platform can be used to test these 33 genes as a cost-effective tool for trials or standard clinical use.
The PME score can be viewed as part of a novel and emerging movement in medicine to tailor individual treatments to an individual’s genetic expression profile. This approach could be used to identify responders and nonresponders before clinical changes to more rapidly switch therapeutic strategies in nonresponders. Rather than using a uniform therapy for all patients or disease subtypes, our approach used molecular information belonging to an individual patient to develop a personalized treatment. This could improve psoriatic clinical practice through enhanced patient drug evaluation/treatment, clinical trial conduct, and drug development design.
Finally, our research provides a general approach to skin disease research. Other immune skin diseases like atopic dermatitis, vitiligo, and systemic lupus erythematosus likely have immune microenvironment changes that can likewise be segregated into patient subgroups of responder or nonresponder. Predictive signatures of treatment outcome can be defined by dimension reduction analysis of differential expression genes as shown in the present study. Therefore, the work we describe herein can be a template to better understand and treat other inflammatory skin diseases.
Limitations
There are several limitations to this study. First, it employed public data sets, and we cannot obtain or verify clinical findings on each participant. Second, we used PASI75 as our indicator to identify whether the patient was a responder or nonresponder. Although our decision relied on the increased granularity of PASI75 and its standing as the standard in many previous studies, many drugs can now reach PASI90 or even PASI100. The use of more stringent standards is necessary for future prospective research. Third, the interpretation of immune cell infiltration with particular cut-off values was determined empirically and should be standardized for this emerging technology. Finally, because the captured participants in our work were defined retrospectively, all the biases from the previous studies are carried over to this one.
Conclusions
We have defined a novel biometric score, the PME score, that characterizes the psoriatic microenvironment for psoriasis treatment response and provides new insights into psoriasis pathogenesis. The PME score is a possible platform to predict psoriasis treatment responses before they are detectable clinically. As an example of personalized medicine for psoriasis, this tool has the potential to shorten the treatment duration of ineffective medications and limit drug development time, health care costs, medication adverse effects, and general patient burden.
eMethods.
eFigure1. Overview of study design
eFigure2. PMEscore predicts the effect of psoriasis treatment
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385. doi: 10.1038/jid.2012.339 [DOI] [PubMed] [Google Scholar]
- 2.Nickoloff BJ. Skin innate immune system in psoriasis: friend or foe? J Clin Invest. 1999;104(9):1161-1164. doi: 10.1172/JCI8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schön MP, Erpenbeck L. The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front Immunol. 2018;9:1323. doi: 10.3389/fimmu.2018.01323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188(7):3150-3159. doi: 10.4049/jimmunol.1103414 [DOI] [PubMed] [Google Scholar]
- 5.Funk J, Langeland T, Schrumpf E, Hanssen LE. Psoriasis induced by interferon-alpha. Br J Dermatol. 1991;125(5):463-465. doi: 10.1111/j.1365-2133.1991.tb14774.x [DOI] [PubMed] [Google Scholar]
- 6.Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222-226. doi: 10.1038/nature14175 [DOI] [PubMed] [Google Scholar]
- 7.Griffiths CE, Reich K, Lebwohl M, et al. ; UNCOVER-2 and UNCOVER-3 investigators . Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541-551. doi: 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 8.Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A. 2005;102(52):19057-19062. doi: 10.1073/pnas.0509736102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273-290. doi: 10.1086/511051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamian F, Lowes MA, Lin SL, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 2005;102(6):2075-2080. doi: 10.1073/pnas.0409569102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eidsmo L, Martini E. Human langerhans cells with pro-inflammatory features relocate within psoriasis lesions. Front Immunol. 2018;9:300. doi: 10.3389/fimmu.2018.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207-1211. doi: 10.1038/sj.jid.5701213 [DOI] [PubMed] [Google Scholar]
- 13.Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621 [DOI] [PubMed] [Google Scholar]
- 14.Kolbinger F, Loesche C, Valentin MA, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139(3):923-932.e8, e8. doi: 10.1016/j.jaci.2016.06.038 [DOI] [PubMed] [Google Scholar]
- 15.Reich K, Papp KA, Matheson RT, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015;24(7):529-535. doi: 10.1111/exd.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94(1):202-209. doi: 10.1172/JCI117308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564-569. doi: 10.1038/nature06116 [DOI] [PubMed] [Google Scholar]
- 18.Di Meglio P, Villanova F, Navarini AA, et al. Targeting CD8(+) T cells prevents psoriasis development. J Allergy Clin Immunol. 2016;138(1):274-276.e6, e6. doi: 10.1016/j.jaci.2015.10.046 [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Peters T, Kess D, et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116(8):2105-2114. doi: 10.1172/JCI27180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124(5):1022-10.e1, 395. doi: 10.1016/j.jaci.2009.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn R, Gupta R, Lai K, Chopra N, Arron ST, Liao W. Network analysis of psoriasis reveals biological pathways and roles for coding and long non-coding RNAs. BMC Genomics. 2016;17(1):841. doi: 10.1186/s12864-016-3188-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828-3836. doi: 10.4049/jimmunol.1301737 [DOI] [PubMed] [Google Scholar]
- 23.Bachelez H, Viguier M, Tebbey PW, et al. The mechanistic basis for psoriasis immunopathogenesis: translating genotype to phenotype. Report of a workshop, Venice, 2012. Br J Dermatol. 2013;169(2):283-286. doi: 10.1111/bjd.12347 [DOI] [PubMed] [Google Scholar]
- 24.Correa da Rosa J, Kim J, Tian S, Tomalin LE, Krueger JG, Suárez-Fariñas M. Shrinking the Psoriasis Assessment Gap: Early Gene-Expression Profiling Accurately Predicts Response to Long-Term Treatment. J Invest Dermatol. 2017;137(2):305-312. doi: 10.1016/j.jid.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 25.Feldman SR, Zhao Y, Gilloteau I, et al. Higher psoriasis skin clearance is associated with lower annual indirect costs in the United States: a post hoc analysis from the CLEAR Study. J Manag Care Spec Pharm. 2018;24(7):617-622. doi: 10.18553/jmcp.2018.24.7.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick Howard L. National Psoriasis Foundation: a patient-centric approach to improve access to psoriatic disease treatment. Am J Manag Care. 2016;22(4)(suppl):s104-s107. [PubMed] [Google Scholar]
- 27.Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm. 2017;23(8):859-867. doi: 10.18553/jmcp.2017.16272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Sawah S, Foster SA, Goldblum OM, et al. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20(9):982-990. doi: 10.1080/13696998.2017.1345749 [DOI] [PubMed] [Google Scholar]
- 29.D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72(4):589-598. doi: 10.1016/j.jaad.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 30.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453-457. doi: 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Félix Garza ZC, Lenz M, Liebmann J, et al. Characterization of disease-specific cellular abundance profiles of chronic inflammatory skin conditions from deconvolution of biopsy samples. BMC Med Genomics. 2019;12(1):121. doi: 10.1186/s12920-019-0567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng D, Li M, Zhou R, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7(5):737-750. doi: 10.1158/2326-6066.CIR-18-0436 [DOI] [PubMed] [Google Scholar]
- 33.Nair RP, Duffin KC, Helms C, et al. ; Collaborative Association Study of Psoriasis . Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199-204. doi: 10.1038/ng.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Tsoi LC, Swindell WR, et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol. 2014;134(7):1828-1838. doi: 10.1038/jid.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552-2564. doi: 10.1038/jid.2012.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307-315. doi: 10.1093/bioinformatics/btg405 [DOI] [PubMed] [Google Scholar]
- 37.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486-489. doi: 10.5812/ijem.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazra A, Gogtay N. Biostatistics series module 3: comparing groups: numerical variables. Indian J Dermatol. 2016;61(3):251-260. doi: 10.4103/0019-5154.182416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Yosef H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal R Stat Soc. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 40.Fakih M, Ouyang C, Wang C, et al. Immune overdrive signature in colorectal tumor subset predicts poor clinical outcome. J Clin Invest. 2019;129(10):4464-4476. doi: 10.1172/JCI127046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammerl D, Massink MPG, Smid M, et al. Clonality, antigen recognition, and suppression of CD8+ T cells differentially affect prognosis of breast cancer subtypes. Clin Cancer Res. 2020;26(2):505-517. doi: 10.1158/1078-0432.CCR-19-0285 [DOI] [PubMed] [Google Scholar]
- 42.Kashyap AS, Fernandez-Rodriguez L, Zhao Y, et al. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019;28(13):3367-3380.e8, e8. doi: 10.1016/j.celrep.2019.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolea I, Gella A, Sanz E, et al. Defined neuronal populations drive fatal phenotype in a mouse model of Leigh syndrome. Elife. 2019;8:e47163. doi: 10.7554/eLife.47163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jessa S, Blanchet-Cohen A, Krug B, et al. Stalled developmental programs at the root of pediatric brain tumors. Nat Genet. 2019;51(12):1702-1713. doi: 10.1038/s41588-019-0531-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zemek RM, De Jong E, Chin WL, et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci Transl Med. 2019;11(501):eaav7816. doi: 10.1126/scitranslmed.aav7816 [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran P, Dobie R, Wilson-Kanamori JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512-518. doi: 10.1038/s41586-019-1631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misselbeck K, Parolo S, Lorenzini F, et al. A network-based approach to identify deregulated pathways and drug effects in metabolic syndrome. Nat Commun. 2019;10(1):5215. doi: 10.1038/s41467-019-13208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunphy SE, Sweeney CM, Kelly G, Tobin AM, Kirby B, Gardiner CM. Natural killer cells from psoriasis vulgaris patients have reduced levels of cytotoxicity associated degranulation and cytokine production. Clin Immunol. 2017;177:43-49. doi: 10.1016/j.clim.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Lin Y, Li C, et al. IL-35 decelerates the inflammatory process by regulating inflammatory cytokine secretion and M1/M2 macrophage ratio in psoriasis. J Immunol. 2016;197(6):2131-2144. doi: 10.4049/jimmunol.1600446 [DOI] [PubMed] [Google Scholar]
- 50.Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015;29(9):1791-1796. doi: 10.1111/jdv.13027 [DOI] [PubMed] [Google Scholar]
- 51.Singh TP, Zhang HH, Borek I, et al. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat Commun. 2016;7:13581. doi: 10.1038/ncomms13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Lei J, Yang L, et al. Dysregulation of Akt-FOXO1 pathway leads to dysfunction of regulatory T cells in psoriasis patients. J Invest Dermatol. 2019;139(10):2098-2107. doi: 10.1016/j.jid.2018.12.035 [DOI] [PubMed] [Google Scholar]
- 53.Shao S, Fang H, Dang E, et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol. 2019;10:746. doi: 10.3389/fimmu.2019.00746 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure1. Overview of study design
eFigure2. PMEscore predicts the effect of psoriasis treatment