Key Points
Question
Is the risk of severe cardiovascular events increased after the initiation of treatment with the anti–interleukin 12/23p40 (IL-12/23p40) monoclonal antibody ustekinumab?
Findings
This case-time-control analysis based on data from 9290 patients with records in the French national health insurance database from 2010 to 2016 suggests that the initiation of ustekinumab treatment is associated with increased risk of acute coronary syndrome or stroke in patients with a high baseline cardiovascular risk.
Meaning
The results of this case-time-control analysis suggest that ustekinumab should be prescribed with caution to patients at high cardiovascular risk.
Abstract
Importance
Ustekinumab, a monoclonal antibody targeting interleukin 12/23p40 (IL-12/23p40), is effective in the treatment of moderate to severe psoriasis, psoriatic arthritis, and Crohn disease. In 2011, a meta-analysis of randomized clinical trials reported a potential risk of severe cardiovascular events (SCEs) within the first few months after the initiation of anti–IL-12/23p40 antibodies.
Objective
To assess whether the initiation of ustekinumab treatment is associated with increased risk of SCEs.
Design, Setting, and Participants
This case-time-control study used data from the French national health insurance database, covering 66 million individuals, on all patients exposed to ustekinumab between April 1, 2010, and December 31, 2016, classified according to their cardiovascular risk level (high- and low-risk strata). The risk period was the 6 months before the SCE, defined as acute coronary syndrome or stroke, and the reference period was the 6 months before the risk period. Statistical analysis was performed from September 20, 2017, to July 6, 2018.
Exposure
The initiation of ustekinumab treatment was screened during the risk and reference periods.
Main Outcomes and Measures
Odds ratios for the risk of SCE after the initiation of ustekinumab treatment were calculated.
Results
Of the 9290 patients exposed to ustekinumab (4847 men [52%]; mean [SD] age, 43 [14] years), 179 experienced SCEs (65 cases of acute coronary syndrome, 68 cases of unstable angina, and 46 cases of stroke). Among patients with a high cardiovascular risk, a statisically significant association between initiaton of ustekinumab treatment and SCE occurrence was identified (odds ratio, 4.17; 95% CI, 1.19-14.59). Conversely, no statistically significant association was found among patients with a low cardiovascular risk (odds ratio, 0.30; 95% CI, 0.03-3.13).
Conclusions and Relevance
This study suggests that the initiation of ustekinumab treatment may trigger SCEs among patients at high cardiovascular risk. In line with the current mechanistic models for atherosclerotic disease, the period after the initiation of anti–IL-12/23p40 may be associated with atherosclerotic plaque destabilization via the inhibition of helper T cell subtype 17. Although the study interpretation is limited by its observational design, these results suggest that caution may be needed in the prescription of ustekinumab to patients at high cardiovascular risk.
This case-time-control analysis uses data from the French national health insurance database to assess whether the initiation of ustekinumab treatment is associated with triggering severe cardiovascular events.
Introduction
During the past 20 years, anticytokine therapies have transformed the management of many chronic immunoinflammatory diseases, including psoriasis, inflammatory rheumatic disorders, and inflammatory bowel disease. The recognition of atherosclerosis as an inflammatory process raises a question about the association of these anticytokine therapies with atherosclerosis progression and cardiovascular thrombotic complications.1,2,3,4,5,6,7,8 In patients with psoriasis, therapeutic successes have been obtained with antibodies blocking tumor necrosis factor, interleukin 12 (IL-12), IL-23, and IL-17A/F.9,10 However, their respective associations with atherosclerosis progression seem to differ.2 A cardiovascular protective association with long-term use of anti–tumor necrosis factor drugs has been reported in several studies,11,12,13 corroborating experiments in atherosclerosis-prone mouse models reporting a pathogenic role of tumor necrosis factor.14 Regarding the anti-IL12/23p40 treatments with ustekinumab and briakinumab, a 2011 meta-analysis of randomized clinical trials of patients with moderate to severe psoriasis reported on a possible excess of major adverse cardiovascular events compared with placebo-treated groups.15 This warning is in line with experimental studies suggesting a protective role of the IL-23 and IL-17 pathway on atherosclerotic plaque growth and vulnerability.16,17,18,19 After concerns about ischemic cardiovascular safety, briakinumab was withdrawn from further development, while ustekinumab was marketed. With the use of large-scale real-world data, the aim of this study was to investigate whether ustekinumab treatment is associated with triggering severe cardiovascular events (SCEs) during the 6-month period after treatment initiation.
Methods
Data Source and Population
This study was conducted using the French national health insurance database (SNDS [Système National des Données de Santé], formerly SNIIRAM [Système National d’Information Inter-Régimes de l’Assurance Maladie]).20 This database covers 98% of the population living in France (approximately 66 million inhabitants) and includes anonymous individual sociodemographic data and exhaustive data on all reimbursements for health-related expenditures, dispensed drugs with date of issue, any investigation (eg, imaging, surgery, and blood tests), and hospitalization discharge codes according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Data from public and private practices are recorded in the same way. Severe, costly, long-term diseases are recorded, with diagnoses encoded according to ICD-10, because individuals with such diseases are entitled to 100% health insurance coverage. The study protocol was authorized and approved by the French National Agency for Medicines and Health Products Safety (ANSM; Agence Nationale de Sécurité du Médicament), the National Institutional Review Board (INDS; Institut National des Données de Santé), and the French Data Protection Authority (CNIL; Commission Nationale de l’Informatique et des Libertés). Patient consent was waived by the CNIL because data in the SNDS are deidentified.
From the entire SNDS database, we extracted data on all the individuals who had at least 1 reimbursement for ustekinumab between April 1, 2010 (date of market release), and December 31, 2016. Thus, all patients included were incident users of ustekinumab. From this population, we selected patients who had a reported SCE.
Severe Cardiovascular Events
Severe cardiovascular events were defined as either acute coronary syndrome (ICD-10 codes I21 and I24) with hospitalization in the intensive care unit (ICU) or ischemic stroke (ICD-10 codes I63 and I64) with hospitalization in any department, identified using the hospitalization discharge codes. Cardiovascular deaths that occurred after hospitalization for acute coronary syndrome or ischemic stroke were included. The date of admission was used as the date of the event. Only SCEs occurring after April 1, 2011, were selected, to obtain at least 1 year for detecting initiation of ustekinumab treatment before the SCE. In addition, the validity criteria for the case-time-control design required the probability of observing ustekinumab initiations to be modified only by the association with an SCE.21 We did not include the SCE if the patient had already experienced an SCE in the previous 2 years because it could reduce the probability of initiation of ustekinumab treatment by the physician.
Drug Exposure
Because we focused on the triggering association of ustekinumab, exposure was defined as the initiation of treatment (ie, the first dispensation of ustekinumab). According to the product information,22 the first 2 subcutaneous injections of ustekinumab are administered at a 4-week interval; all subsequent subcutaneous injections are administered every 12 weeks. Ustekinumab is used as a long-term treatment, pursued until ineffectiveness or treatment-related toxic effects occur, or it is stopped after a long remission. Most patients receive only 1 course of ustekinumab, but some can receive several courses.
Covariates
Cardiovascular risk was considered high if the patient had at least 2 risk factors or a personal history of atherothrombotic disease. Otherwise, it was considered low. The indication for ustekinumab (psoriasis or Crohn disease) was assessed. The algorithms used are detailed in the eAppendix, eTable 1, and eTable 2 in the Supplement.
Statistical Analysis
Statistical analysis was performed from September 20, 2017, to July 6, 2018. We performed a case-time-control study. This design is an extension of the case-crossover design, which was used to investigate the triggering role of different factors in acute adverse events. The rationale of these designs is to search for an excess of initiations of ustekinumab treatment immediately preceding SCE compared with a previous time period, which could reflect a triggering association of initiation of ustekinumab treatment with SCE. The case-crossover design is based on the principle that individuals serve as their own controls in a different time period. This enables time-invariant confounders to be controlled for. The case-time-control design has the additional benefit of adjusting for time trends in the general prescribing pattern of ustekinumab by incorporating matched time controls (Figure 1).23,24
Figure 1. Case-Crossover Study Design.
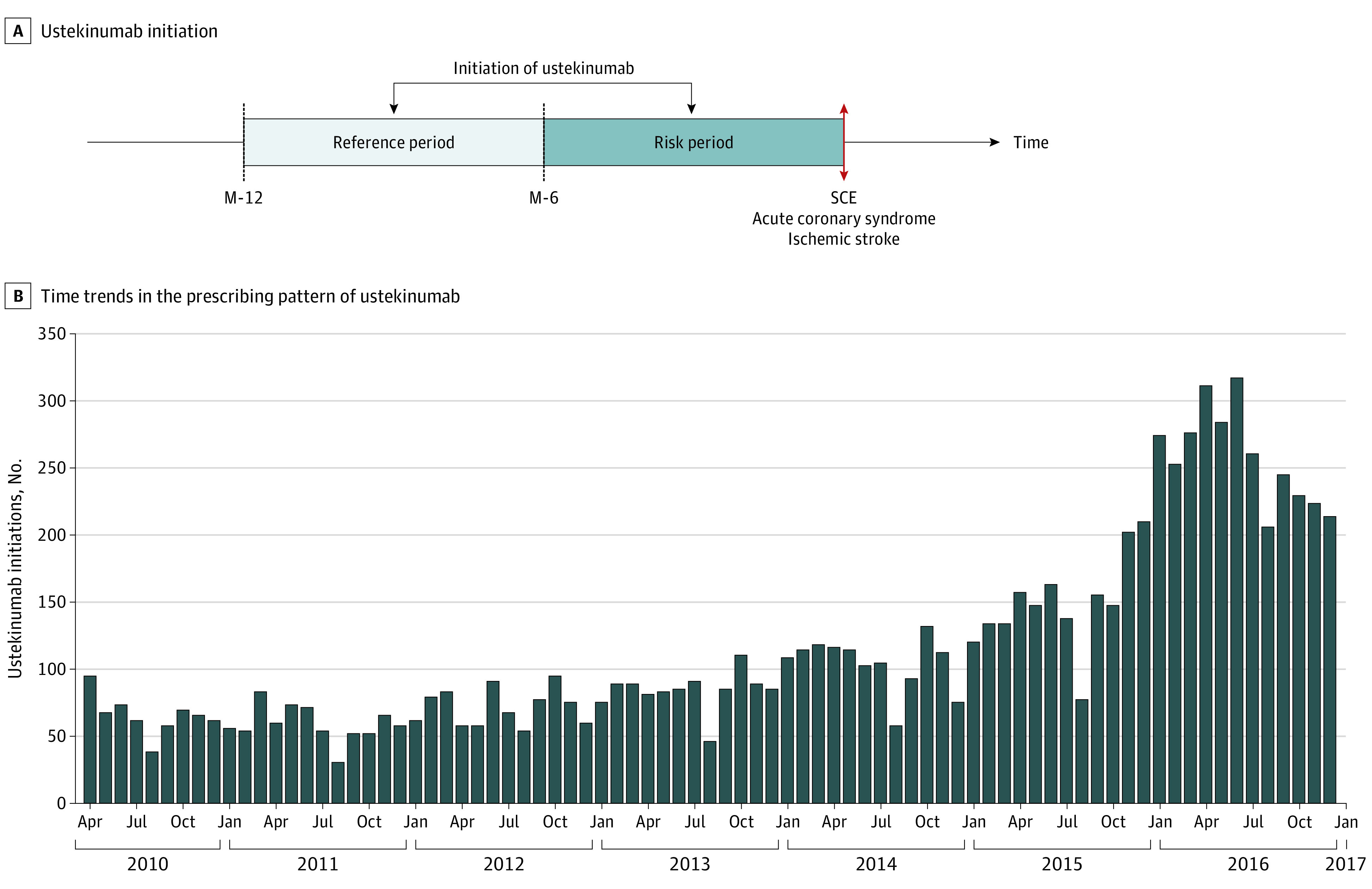
A, Among patients exposed to ustekinumab and having had a severe cardiovascular event (SCE), ustekinumab initiation was screened during 2 periods prior to SCE. The number of ustekinumab initiations during the risk period was compared with the number of ustekinumab initiations during the reference period, leading to assessment of the odds ratio (OR) of the case-crossover analysis. B, Time trends in the general prescribing pattern for ustekinumab between 2010 and 2016. Because the number of ustekinumab initiations was not constant over time, time controls were introduced in the model. The OR among time controls estimates how much the case-crossover analysis is associated with the time trends in the prescription of ustekinumab over time. The final OR of the case-time-control estimates the association between the initiation of ustekinumab and SCE (ie, the OR of the case-crossover analysis), adjusted for time trends (estimated among time controls). M-6 indicates the 6th month before the index date; and M-12, the 12th month before the index date.
The initiation of ustekinumab treatment was measured during 2 periods prior to the event.23 We defined the risk period as occurring 6 months or fewer before the SCE and the reference period as occurring 6 to 12 months before the SCE (Figure 1A).
Time controls were not compared with cases. They were used to check for time trends in the general prescribing pattern for ustekinumab between 2010 and 2016. The increasing number of prescriptions of ustekinumab over time after its market authorization could otherwise have led to a false association between ustekinumab initiation and the outcome (Figure 1B).25 For each case, 4 time-controls of the same sex, age (±2 years), and cardiovascular risk were randomly sampled from the individuals exposed to ustekinumab. The odds ratio (OR) for the case-time-control analysis was derived from the interaction coefficient between exposure and case or time-control status introduced into the conditional logistic regression models. It estimated the association between the initiation of ustekinumab treatment and SCE, controlling for time trends. Statistical tests were 2-tailed, and results were deemed statistically significant at P < .05.
A differential association of ustekinumab initiation according to cardiovascular risk was anticipated, on the hypothesis that patients at low cardiovascular risk were less prone to atherosclerotic plaques or that their plaques were less prone to rupture. We planned in the protocol of the study to stratify the analyses accordingly, into low vs high cardiovascular risk strata. By testing a possible interaction between cardiovascular risk and ustekinumab initiation, we also evaluated the homogeneity between the ORs obtained from the 2 cardiovascular risk strata. Because interaction tests are less powerful, we planned in the protocol to consider P < .15 to be statistically significant for an interaction.26,27 Statistical analyses were performed with SAS Enterprise Guide Software, version 7.1 (SAS Institute Inc). Incidence rates and the estimated risk of SCE are provided in eTable 4 in the Supplement.
Sensitivity Analyses
We tested the robustness of our study findings in sensitivity analyses using alternative assessment criteria for exposure and outcome. First, we added reinitiation and dose increase as exposure. Reinitiation was defined as a dispensation of ustekinumab without any dispensation in the previous 6 months among patients who had previously received ustekinumab. Second, we added unstable angina (ICD-10 codes I200 and I200 + 0) and acute coronary syndrome among patients hospitalized in a unit other than the ICU to the definition of SCE.
Results
Severe Cardiovascular Events
Of the 66 million French inhabitants, 9290 (4847 men [52%]; mean [SD] age, 43 [14] years) were exposed to ustekinumab between April 1, 2010, and December 31, 2016. Their main characteristics are presented in eTable 3 in the Supplement. A total of 7974 patients (86%) received ustekinumab for psoriasis, and 1110 patients (12%) received ustekinumab for Crohn disease.
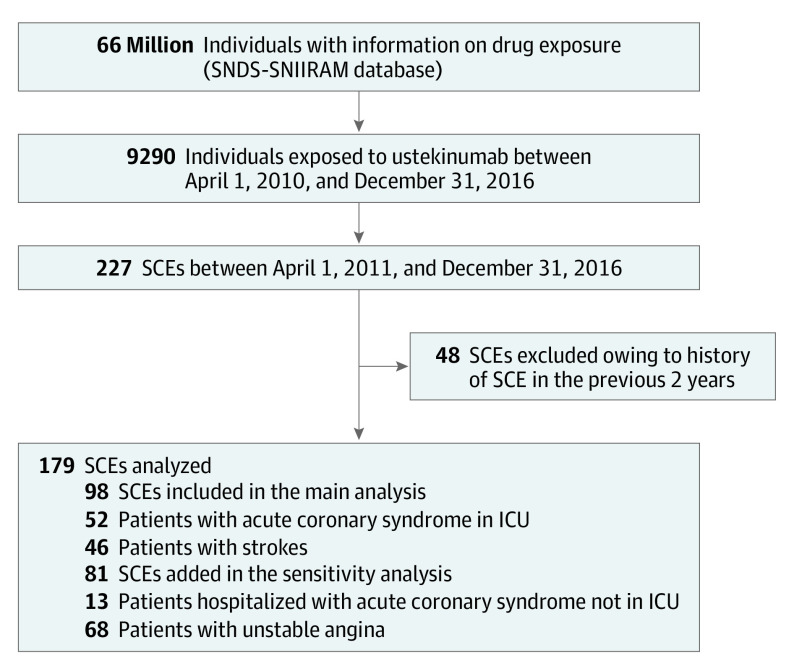
In this population, between April 2011 and December 2016, 98 patients who experienced SCEs (52 with acute coronary syndromes who were admitted to the ICU, including 2 leading to death, and 46 with strokes) met our criteria for inclusion in the main analysis. In addition, 13 patients with acute coronary syndrome who were hospitalized in non-ICU wards and 68 patients with unstable angina were added for the sensitivity analysis (Figure 2).
Figure 2. Study Flowchart.
ICU indicates intensive care unit; SCE, severe cardiovascular event; and SNDS-SNIIRAM, Système National des Données de Santé–Système National d’Information Inter-Régimes de l’Assurance Maladie.
The characteristics of the 98 patients included in the main analysis are described in Table 1. A total of 62 patients were men (63%), the median age was 57 years (interquartile range, 51-65 years), and 76 (78%) were at high cardiovascular risk. A total of 89 patients (91%) had psoriasis, 4 patients (4%) had Crohn disease, and 2 (2%) had both. The characteristics of the cases and time-controls are presented in eTable 5 in the Supplement.
Table 1. Characteristics of Cases Included in the Case-Time-Control Analysis.
| Characteristic | Cases, No. (%) (n = 98) |
|---|---|
| Event | |
| Acute coronary syndrome requiring hospitalization in ICU | 52 (53) |
| Stroke | 46 (47) |
| Sex | |
| Male | 62 (63) |
| Female | 36 (37) |
| Age, median (IQR), ya | 57 (51-65) |
| Indication | |
| Psoriasis | 89 (91) |
| Crohn disease | 4 (4) |
| Psoriasis and Crohn disease | 2 (2) |
| Undetermined | 3 (3) |
| Cardiovascular riskb | |
| Low | 22 (22) |
| High | 76 (78) |
| Previous cardiovascular event | 0 |
| Cardiovascular risk factorsb | |
| Agec | 62 (63) |
| Hypertension | 63 (64) |
| Dyslipidemia | 45 (46) |
| Tobacco use | 29 (30) |
| Diabetes | 25 (26) |
| Obesity | 16 (16) |
| No. of cardiovascular risk factorsb | |
| 0 | 4 (4) |
| 1 | 18 (18) |
| 2 | 30 (31) |
| 3 | 27 (28) |
| 4 | 14 (14) |
| 5 | 5 (5) |
| 6 | 0 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Assessed at the date of the event.
Measured in the year preceding the date of the event.
Over 50 years for men and 60 years for women.
Risk of SCE Associated With Ustekinumab Initiation
Across the whole study population, the OR for the case-time-control analysis was 2.41 (95% CI, 0.83-7.01) (Table 2). Because of the heterogeneity of the ORs obtained from patients with high vs low cardiovascular risk, the main analysis was stratified according to cardiovascular risk factors, as prespecified in our study protocol. Among patients at high cardiovascular risk, we observed a significantly increased risk of SCE in the first 6 months after ustekinumab initiation (OR, 4.17; 95% CI, 1.19-14.59), but this risk was not observed among patients at low cardiovascular risk (OR, 0.30; 95% CI, 0.03-3.13). The detailed results for the overall population and for the subgroups with high or low cardiovascular risk are presented in Table 2. The results for the case-crossover and the case-time-control analyses are presented separately in eTable 6 in the Supplement.
Table 2. Risk for Severe Cardiovascular Events Among Patients Exposed to Ustekinumab Treatment Initiationa.
| Population | Total No. | Ustekinumab initiation, No. | OR (95% CI)d | |
|---|---|---|---|---|
| Risk periodb | Reference periodc | |||
| Overall population | 98 | 16 | 6 | 2.41 (0.83-7.01) |
| Cardiovascular risk | ||||
| Lowe | 22 | 2 | 2 | 0.30 (0.03-3.13) |
| Highf | 76 | 14 | 4 | 4.17 (1.19-14.59) |
Abbreviation: OR, odds ratio.
The main analysis was stratified according to the cardiovascular risk (P = .05 for interaction).
Up to 6 months preceding the risk period.
The 6- to 12-month period preceding the event.
Association between ustekinumab initiation and cardiovascular events, controlling for time trends in the prescribing patterns of ustekinumab (see Methods).
Fewer than 2 cardiovascular risk factors and no history of severe cardiovascular events.
More than 2 cardiovascular risk factors or a history of severe cardiovascular events.
Sensitivity Analyses
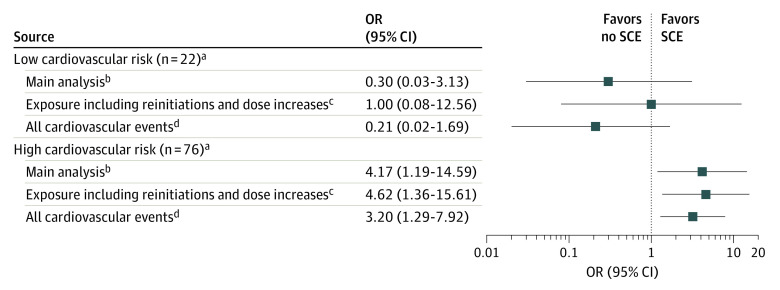
Two sensitivity analyses were conducted. First, we widened the definition of triggering circumstances by adding the following to initiation: reinitiation (ie, later initiation of another course of ustekinumab) and dose increase. The OR for SCE in the overall population was 3.41 (95% CI, 1.15-10.15). Among patients at high cardiovascular risk, the OR was 4.62 (95% CI, 1.36-15.61), and among patients at low cardiovascular risk, the OR was 1.00 (95% CI, 0.08-12.56).
Second, we expanded the definition of SCE by adding patients with unstable angina and those hospitalized in non-ICU wards with acute coronary syndrome to those with stroke and those hospitalized in the ICU with acute coronary syndrome (n = 179). The OR for SCE in the overall population was 1.75 (95% CI, 0.86-3.56). Among patients at high cardiovascular risk, the OR was 3.20 (95% CI, 1.29-7.92), and among patients at low cardiovascular risk, the OR was 0.21 (95% CI, 0.02-1.69).
The results from the main and sensitivity analyses according to cardiovascular risk are shown in Figure 3. For the subpopulation with low cardiovascular risk, no association between ustekinumab initiation and SCE was observed.
Figure 3. Association Between Ustekinumab Treatment Initiation and Severe Cardiovascular Events (SCEs) According to Cardiovascular Risk.
OR indicates odds ratio.
aLow risk if fewer than 2 cardiovascular risk factors and no history of SCE; high risk if more than 2 cardiovascular risk factors or a history of SCE.
bExposure: ustekinumab initiation; event: hospitalized in intensive care unit with acute coronary syndrome and stroke; 6-month periods.
cExposure: ustekinumab initiation, reinitiation (after 6 months without ustekinumab prescription), and dose increases; event: hospitalized in intensive care unit with acute coronary syndrome and stroke; 6-month periods.
dExposure: ustekinumab initiation; event: acute coronary syndrome, stroke, and unstable angina; 6-month periods.
Discussion
In this population-based study including 9290 patients exposed to ustekinumab, we observed an excess of patients with acute coronary syndrome and stroke within the 6 months after treatment initiation among patients at high cardiovascular risk. This association was not observed among patients at low cardiovascular risk. On account of our study design, which addresses the general question of why SCE is occurring during a specific period (rather than the more classic question of why SCE is occurring to a specific patient), our results do not mean that more SCEs are observed among patients with a higher baseline cardiovascular risk but that the 6-month period after ustekinumab initiation is associated with a higher risk for SCE in this subpopulation.
We chose to focus on early events for 2 reasons. First, a meta-analysis of randomized clinical trials reported a possible excess of SCEs as early as 2011 among adults exposed to anti–IL-12/23p40 antibodies (ustekinumab or briakinumab) during the first months of treatment.15 The decision to stop the development of briakinumab left ustekinumab as the only anti–IL-12/23p40 antibody on the market. Although no recommendation has been explicitly released by the European or US drug agencies, the association between anti–IL-12/23p40 antibody use and atherosclerotic cardiovascular disease has been a subject of controversy.15,28,29,30,31,32 Second, there is an immunologic substratum supporting the short-term triggering of SCE in the first weeks or months of treatment with anti–IL-12/23p40 antibodies, based on the hypothesis of a therapeutically induced acute change in the immunologic environment of atherosclerotic plaques.1,2,17,18 Ustekinumab inhibits the p40 subunit shared by IL-12, a helper T cell subtype 1 (TH1) inducer, and IL-23, which maintains TH17 cell homeostasis. Several experimental studies have shown that IL-12 and associated TH1 responses are proatherogenic.33 In contrast, the role of the TH17 pathway, associated with IL-17A, remains ambiguous. Interleukin 17A could have a proatherogenic effect by promoting plaque growth, while a protective effect could also result from the stabilization of atherosclerotic plaques.16,17,34,35 Interleukin 17A may be associated with 2 mechanisms accounting for the latter: the stimulation of type I collagen production by smooth muscle cells in the fibrous caps and a reduction of inflammatory cells within the vascular lesions through a decrease in the endothelial expression of vascular cell adhesion molecule 1.1,17 A stabilizing association of IL-17A with atherosclerotic plaque was also suggested by a large human study reporting that high IL-17A plasma levels after myocardial infarction were associated with a lower recurrence rate.36 The anti–IL-12/23p40 antibody ustekinumab, by the inhibition of the TH17 pathway, may therefore induce atherosclerotic plaque rupture and atherothrombotic events, including stroke and acute coronary syndrome.
Strengths and Limitations
Our study has several strengths. First, we used a design specifically suited to detecting a putative triggering association with early events. Previous work on the cardiovascular risk of ustekinumab, based on cohort studies, did not identify an increased risk in the medium or long term.37,38,39 However, our focus on a triggering association of treatment initiation with ustekinumab raises a new question, which required a different study design. Another major advantage of self-controlled designs vs cohort studies is the absence of indication bias and of measured or unmeasured time-invariant confounders because individuals serve as their own controls.25 Treatments such as antilipidemics, antihypertensives, or antidiabetics may act as time-dependent confounders because discontinuing a cardiovascular treatment may be associated with triggering an SCE. We confirmed that no patient initiating ustekinumab in the risk or reference period discontinued one of these treatments in the 12 months before SCE. We selected a 6-month duration for the risk period to include the time lapse observed for an SCE in clinical trials15 and to allow enough time for the pathologic immune changes that could promote SCEs.40 Second, we included sensitivity analyses in which we broadened the definition of an SCE and the types of triggers (adding reinitiation and dose increase). Consistent results were observed in each stratum of cardiovascular risk. Third, our study framework comprised all patients exposed to ustekinumab, and data on every SCE were collected. Although we ended up with a relatively small number of SCEs observed within our study window, the exhaustiveness of the initial population of 66 million individuals yielded maximum power. The absence of attrition bias is another major strength for our study.
This study also had some limitations. First, we did not include cardiovascular death resulting from a nonatherothrombotic mechanism (n = 12; mostly heart failure) in our outcome because our hypothesis of a trigger was specific to a destabilization of atherosclerotic plaques. In addition, we could not identify cardiovascular deaths occurring outside the hospital because the causes of death were not yet recorded in the SNDS database. Including all-cause deaths may have resulted in a dilution of the OR because of a misclassification bias owing to a majority of noncardiovascular deaths. Second, we used drug dispensations as a proxy for drug intake. This approximation is widely acknowledged and used in pharmacoepidemiologic studies. Third, clinical and biological data regarding the cardiovascular risk factors may have led to a misclassification bias between the high vs low cardiovascular risk strata. Tobacco use and obesity are known to be underreported in hospital discharge coding. Dietary factors and physical activity are not reported at all. In addition, dyslipidemia was considered only when it involved issues of statins or fibrates, and it is possible that the triggering risk is higher among patients with untreated dyslipidemia.41 However, we believe that our criteria for defining patients’ cardiovascular risk profiles were sufficiently discriminant to identify 2 distinct subpopulations according to this risk. Both the triggering association suggested among patients at high cardiovascular risk and the absence of any association among the patients at low risk are in line with the immunologic hypothesis because only those in the first group are liable to have atherosclerotic plaques likely to be destabilized. Fourth, one may suggest that the incidence of SCEs during the period up to 6 months after ustekinumab initiation is hypothetically the same as would occur without ustekinumab; with the additional hypothesis that ustekinumab treatment would decrease the risk of SCE during the 6- to 12-month period, this may lead to an OR greater than 1. However, the first hypothesis would not be compatible with the randomized clinical trials reporting more SCEs among patients exposed to anti–IL-12/23p40 antibodies than to placebo in the first months after the initiation, and the second one remains to be substantiated. Fifth, the observational design does not allow for proving causality.
The observation of an association between ustekinumab initiation and SCE among patients with cardiovascular risk factors suggests the need for caution regarding the prescription of ustekinumab in this population. The risk seems to concern patients with psoriasis rather than Crohn disease, probably because they are older and have a higher preexisting cardiovascular risk. In addition to the detection of classic cardiovascular risk factors, imaging of atherosclerotic plaques could be considered when determining the individual cardiovascular risk for each patient. Novel strategies used to screen for high-risk coronary plaques, such as coronary computed tomography angiography or fluorine 18–labeled fluorodeoxyglucose positron emission tomography or computed tomography, could help improve the detection of patients at higher risk.42 Our data do not rule out the hypothesis of a dual association of anti–IL-12/23p40 therapy with atherosclerotic disease (eg, a short-term destabilization in a subset of patients) and a beneficial or neutral association with atherosclerosis development in the longer term.
New biologics targeting the TH17 pathway (anti–IL-17 therapies: secukinumab, ixekizumab, and brodalumab; anti–IL-23p19 therapies: guselkumab, tildrakizumab, and risankizumab) have already been marketed or are in the premarketing phases for psoriasis and should be carefully monitored with respect to cardiovascular events, with a specific focus on the period after treatment initiation.35 Physicians should be aware that randomized clinical trials do not fully identify this risk because of the small sample sizes and the small number of comorbidities compared with real-world settings. Thus, caution regarding patients at high cardiovascular risk should be exercised when prescribing these treatments, and recommendations for early, active management of cardiovascular comorbidities among patients with psoriasis should be reinforced.
Conclusions
Our results from real-world data suggest that ustekinumab treatment may be associated with triggering acute coronary syndrome and stroke within the first 6 months of treatment initiation among patients at high cardiovascular risk. A close collaboration between cardiologists and biologic prescribers could be beneficial to evaluate the risk of SCEs for patients who are receiving ustekinumab. Further investigations should help explore the underlying physiopathological mechanisms. In an epidemiologic perspective, the same systematic strategy could be used to screen for early SCE among patients exposed to other newly developed biotherapies blocking the IL-17 and IL-23 pathway.
eAppendix. Construction of Covariates
eTable 1. Algorithm for Identifying Cardiovascular Risk Factors
eTable 2. Algorithm for Identifying Indication of Ustekinumab
eTable 3. Characteristics of Patients Exposed to Ustekinumab
eTable 4. Incidence Rates of Major Adverse Cardiovascular Events (MACEs) During the First 12 Months Following Ustekinumab Initiation
eTable 5. Characteristics of Cases and Time-Controls
eTable 6. Risk of Major Adverse Cardiovascular Events Triggered by Ustekinumab Initiation
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204-212. doi: 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 2.Caiazzo G, Fabbrocini G, Di Caprio R, et al. Psoriasis, cardiovascular events, and biologics: lights and shadows. Front Immunol. 2018;9:1668. doi: 10.3389/fimmu.2018.01668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. 2019;124(3):437-450. doi: 10.1161/CIRCRESAHA.118.313129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721-728. doi: 10.1093/cvr/cvz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorokin AV, Kotani K, Elnabawi YA, et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis. Circ Res. 2018;123(11):1244-1254. doi: 10.1161/CIRCRESAHA.118.313608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahil SK, Capon F, Barker JN. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin Immunopathol. 2016;38(1):11-27. doi: 10.1007/s00281-015-0539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. doi: 10.1161/CIRCIMAGING.117.007394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). J Invest Dermatol. 2020;140(1):85-93. doi: 10.1016/j.jid.2019.07.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iskandar IYK, Ashcroft DM, Warren RB, et al. Comparative effectiveness of biological therapies on improvements in quality of life in patients with psoriasis. Br J Dermatol. 2017;177(5):1410-1421. doi: 10.1111/bjd.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535. doi: 10.1002/14651858.CD011535.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Minno MND, Iervolino S, Peluso R, Scarpa R, Di Minno G; CaRRDs Study Group . Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol. 2011;31(3):705-712. doi: 10.1161/ATVBAHA.110.214585 [DOI] [PubMed] [Google Scholar]
- 12.Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76(1):81-90. doi: 10.1016/j.jaad.2016.07.042 [DOI] [PubMed] [Google Scholar]
- 13.Low ASL, Symmons DPM, Lunt M, et al. ; British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) and the BSRBR Control Centre Consortium . Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(4):654-660. doi: 10.1136/annrheumdis-2016-209784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canault M, Peiretti F, Mueller C, et al. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis. 2004;172(2):211-218. doi: 10.1016/j.atherosclerosis.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Ryan C, Leonardi CL, Krueger JG, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306(8):864-871. doi: 10.1001/jama.2011.1211 [DOI] [PubMed] [Google Scholar]
- 16.Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206(10):2067-2077. doi: 10.1084/jem.20090545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisterå A, Robertson A-KL, Andersson J, et al. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17–dependent pathway. Sci Transl Med. 2013;5(196):196ra100. doi: 10.1126/scitranslmed.3006133 [DOI] [PubMed] [Google Scholar]
- 18.Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35(2):258-264. doi: 10.1161/ATVBAHA.114.303567 [DOI] [PubMed] [Google Scholar]
- 19.Fatkhullina AR, Peshkova IO, Dzutsev A, et al. An interleukin-23–interleukin-22 axis regulates intestinal microbial homeostasis to protect from diet-induced atherosclerosis. Immunity. 2018;49(5):943-957. doi: 10.1016/j.immuni.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: From the Système National D’information Interrégimes de l’Assurance Maladie (SNIIRAM) to the Système National des Données de Santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149-S167. doi: 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 21.Suissa S. The case-time-control design: further assumptions and conditions. Epidemiology. 1998;9(4):441-445. doi: 10.1097/00001648-199807000-00016 [DOI] [PubMed] [Google Scholar]
- 22.European Commission Stelara (ustekinumab): annexe 1: résumé des caractéristiques du produit. Accessed August 5, 2020. https://ec.europa.eu/health/documents/community-register/2020/20200120147051/anx_147051_fr.pdf
- 23.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144-153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 24.Consiglio GP, Burden AM, Maclure M, McCarthy L, Cadarette SM. Case-crossover study design in pharmacoepidemiology: systematic review and recommendations. Pharmacoepidemiol Drug Saf. 2013;22(11):1146-1153. doi: 10.1002/pds.3508 [DOI] [PubMed] [Google Scholar]
- 25.Suissa S. The case-time-control design. Epidemiology. 1995;6(3):248-253. doi: 10.1097/00001648-199505000-00010 [DOI] [PubMed] [Google Scholar]
- 26.Greenland S. Basic problems in interaction assessment. Environ Health Perspect. 1993;101(suppl 4):59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press; 1996. [Google Scholar]
- 28.Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti–IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27(5):622-627. doi: 10.1111/j.1468-3083.2012.04500.x [DOI] [PubMed] [Google Scholar]
- 29.Dommasch ED, Troxel AB, Gelfand JM. Major cardiovascular events associated with anti–IL 12/23 agents: a tale of two meta-analyses. J Am Acad Dermatol. 2013;68(5):863-865. doi: 10.1016/j.jaad.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC. Association of anti–IL-12/23 biologic agents ustekinumab and briakinumab with major adverse cardiovascular events. J Eur Acad Dermatol Venereol. 2013;27(12):1586-1587. doi: 10.1111/jdv.12126 [DOI] [PubMed] [Google Scholar]
- 31.Reich K, Langley RG, Lebwohl M, et al. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol. 2011;164(4):862-872. doi: 10.1111/j.1365-2133.2011.10257.x [DOI] [PubMed] [Google Scholar]
- 32.Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017;176(4):890-901. doi: 10.1111/bjd.14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114(10):1640-1660. doi: 10.1161/CIRCRESAHA.114.302761 [DOI] [PubMed] [Google Scholar]
- 34.Danzaki K, Matsui Y, Ikesue M, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(2):273-280. doi: 10.1161/ATVBAHA.111.229997 [DOI] [PubMed] [Google Scholar]
- 35.Allam G, Abdel-Moneim A, Gaber AM. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed Pharmacother. 2018;106:1412-1418. doi: 10.1016/j.biopha.2018.07.110 [DOI] [PubMed] [Google Scholar]
- 36.Simon T, Taleb S, Danchin N, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J. 2013;34(8):570-577. doi: 10.1093/eurheartj/ehs263 [DOI] [PubMed] [Google Scholar]
- 37.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128-1134. doi: 10.1111/jdv.12768 [DOI] [PubMed] [Google Scholar]
- 38.Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from the German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307(10):875-883. doi: 10.1007/s00403-015-1593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol. 2019;155(6):700-707. doi: 10.1001/jamadermatol.2019.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang PS, Schneeweiss S, Glynn RJ, Mogun H, Avorn J. Use of the case-crossover design to study prolonged drug exposures and insidious outcomes. Ann Epidemiol. 2004;14(4):296-303. doi: 10.1016/j.annepidem.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 41.Bots ML, Palmer MK, Dogan S, et al. ; METEOR Study Group . Intensive lipid lowering may reduce progression of carotid atherosclerosis within 12 months of treatment: the METEOR study. J Intern Med. 2009;265(6):698-707. doi: 10.1111/j.1365-2796.2009.02073.x [DOI] [PubMed] [Google Scholar]
- 42.Joshi AA, Lerman JB, Dey AK, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol. 2018;3(10):949-956. doi: 10.1001/jamacardio.2018.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Construction of Covariates
eTable 1. Algorithm for Identifying Cardiovascular Risk Factors
eTable 2. Algorithm for Identifying Indication of Ustekinumab
eTable 3. Characteristics of Patients Exposed to Ustekinumab
eTable 4. Incidence Rates of Major Adverse Cardiovascular Events (MACEs) During the First 12 Months Following Ustekinumab Initiation
eTable 5. Characteristics of Cases and Time-Controls
eTable 6. Risk of Major Adverse Cardiovascular Events Triggered by Ustekinumab Initiation