Key Points
Question
Among intracerebral hemorrhage (ICH) survivors with depression treated with selective serotonin reuptake inhibitors (SSRIs), what is the risk of recurrent cerebral bleeding vs decrease in severity of depressive symptoms?
Findings
In this cohort study, SSRIs were associated with both remission of post-ICH depression and higher risk of ICH recurrence. Selective serotonin reuptake inhibitor use is associated with a larger increase in recurrent ICH risk among patients with preexisting clinical, genetic, or neuroimaging risk factors for hemorrhagic stroke.
Meaning
Clinical history, genetics, and neuroimaging data may identify depressed survivors of ICH at higher risk of recurrent cerebral bleeding, for whom SSRI use warrants careful consideration.
Abstract
Importance
Selective serotonin reuptake inhibitors (SSRIs) are widely used to treat poststroke depression but are associated with increased incidence of first-ever intracerebral hemorrhage (ICH) in the general population. The decision to treat ICH survivors with SSRIs must therefore balance potential risks of ICH recurrence with presumed benefits on depressive symptoms.
Objective
To determine whether SSRI use among survivors of primary ICH was associated with ICH recurrence and decreased severity of depressive symptoms.
Design, Setting, and Participants
Longitudinal ICH cohort study at a tertiary care center enrolling from January 2006 to December 2017, with follow-up for a median of 53.2 months (interquartile range, 42.3-61.2 months). The study included 1279 consenting individuals (1049 White, 89 Black, 77 Hispanic, and 64 other race/ethnicity) of 1335 eligible patients presenting with primary ICH and who were discharged alive from initial hospitalization for stroke.
Main Outcomes and Measures
We conducted univariable and multivariable analyses for ICH recurrence risk and depression severity, including subset analyses for patients with 1 or more of the following characteristics associated with high ICH recurrence risk: (1) lobar ICH; (2) presence of the apolipoprotein ε2/ε4 gene variants; (3) prior history of ICH/TIA/ischemic stroke; and (4) Black or Hispanic race/ethnicity.
Results
Mean age of study participants was 71.3 years, with 602 women (47%); of the 1279 participants, 1049 were White, 89 were Black, 77 were Hispanic, and 64 were other race/ethnicity. SSRI exposure was associated with both ICH recurrence (subhazard ratio [SHR], 1.31; 95% CI, 1.08-1.59) and resolution of post-ICH depression (SHR, 1.53; 95% CI, 1.12 2.09). Among those individuals at high risk for recurrent ICH, SSRIs were associated with further elevation in risk for ICH recurrence (SHR, 1.79; 95% CI, 1.22-2.64) compared with all other survivors of ICH (SHR, 1.20; 95% CI, 1.01-1.42; P = .008 for comparison of effect sizes). The association of SSRI with reduced depressive symptoms did not differ between high those at high risk for recurrent ICH and all other ICH survivors.
Conclusions and Relevance
Selective serotonin reuptake inhibitor exposure after ICH is associated with both improvement in depressive symptoms and increased risk of recurrent hemorrhagic stroke. Clinical history, neuroimaging data, and genetic biomarkers may help to identify survivors of ICH more likely to safely tolerate SSRI use.
This study determines whether selective serotonin reuptake inhibitor use among survivors of primary intracerebral hemorrhage was associated with intracerebral hemorrhage recurrence and decreased severity of depressive symptoms.
Introduction
Intracerebral hemorrhage represents 10% to 15% of all strokes but accounts for almost half of stroke mortality worldwide.1 Survivors of ICH are at high risk for recurrent hemorrhagic stroke, representing an almost 100-fold increase in incidence compared with first-ever ICH risk.2,3 Prior studies identified several factors associated with recurrent ICH risk, including race/ethnicity, lobar hematoma location, apolipoprotein (APOE) gene variants ε2/ε4, and the presence of specific magnetic resonance imaging (MRI) findings (chiefly cerebral microbleeds and cortical superficial siderosis).4,5,6,7,8
Depression is a common occurrence following stroke, with a reported lifetime cumulative incidence as high as 55%, and is associated with poor long-term functional outcomes and increased mortality.9,10 Effective treatment of depression is therefore a crucial aspect of long-term care after stroke, especially for ICH survivors. Selective serotonin reuptake inhibitors (SSRIs) are generally considered first-line treatment agents for poststroke depression but are associated with increased risk of first-ever ICH, most likely owing to their antithrombotic effects.11 We have limited evidence on the association between SSRI use and recurrent ICH risk, while no study to date to our knowledge has evaluated their potential treatment effects on post-ICH depression. As a result, ICH survivors and their treating clinicians are left with limited insight into the potential risks and benefits of SSRI use after ICH.
Therefore, the aim of this study was to assess associations between SSRI use and (1) ICH recurrence and (2) reduction in severity of post-ICH depressive symptoms. We also sought to determine whether these associations are modified by patient characteristics (demographics, race/ethnicity, and medical history), ICH characteristics (location, size, and associated functional impairment), and personal genetic information (APOE genotype) associated with higher risk of ICH recurrence. We postulate that more precise estimates of individual risks would better inform clinical decision-making regarding depression treatment with SSRIs after ICH.
Methods
Study Participants and Eligibility Criteria
We analyzed data for ICH survivors consecutively enrolled in the single-center Massachusetts General Hospital ICH study.2,6 Participants were adults (age ≥18 years) presenting between January 2006 and December 2017 and diagnosed as having primary (ie, spontaneous) ICH. All ICH diagnoses were confirmed on computed tomography (CT) scan obtained within 24 hours of symptoms onset. Individuals presenting with secondary ICH (ie, due to trauma, transformation of ischemic infarct, infection, demyelinating lesion, intracranial tumor, ruptured aneurysm, or other vascular abnormalities) were ineligible. Study protocols and procedures were approved by the institutional review board at Massachusetts General Hospital. Written informed consent was obtained from all study participants or their surrogates.
Participants’ Enrollment and Baseline Data Collection
Eligible individuals (or their surrogates) underwent a structured research interview to collect demographic and medical history data, supplemented via semiautomated review of medical records and billing codes.2,12 We specifically sought to determine whether participants had pre-ICH history of depression or other mood disorders, defined as either (1) self-reported or informant-reported prior diagnosis of any mood disorder; or (2) history of any mood disorders (by Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria) based on review of medical records and International Classification of Diseases, Ninth Revision (ICD-9)/International Classification of Diseases, Tenth Revision (ICD-10)–based billing codes. We determined hematoma location and presence of intraventricular hemorrhage (IVH) on arrival CT scans, based on consensus review by study staff.12 Intracerebral hemorrhage location was defined as lobar (selective involvement of the cortex and/or subcortical white matter), nonlobar (selective involvement of thalamus, basal ganglia, cerebellum, or brainstem), or multiple locations. We used an established semiautomated software algorithm to determine ICH volume.2 We determined APOE genotype on DNA extracted from blood samples, as previously described.13 Research staff in charge of genotyping and neuroimaging data analysis were blinded to clinical data.
Longitudinal Follow-up
We contacted participants and/or their surrogates by telephone at 3 and 6 months after index ICH and every 6 months thereafter.12 We gathered information and reviewed medical records pertaining to recurrent stroke, death, and changes in medication regimens. We also administered the following scales: (1) modified Rankin Scale (mRS); (2) the Katz and Lawton questionnaires for Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs)14,15; and (3) the 4-item version of the Geriatric Depression Scale (GDS-4).16 Study staff augmented telephone-based follow-up data with semiautomated review of longitudinal electronic medical records.2
Exposures and Outcomes of Interest
Age at index ICH was analyzed as a continuous variable. Race/ethnicity was analyzed as a categorical variable, with White patients as the reference group owing to their numerical preponderance. Educational level was dichotomized using as cutoff at least 12 years of education. Lobar vs nonlobar ICH location was analyzed as a dichotomous variable. Intracerebral hemorrhage volume was analyzed as a continuous variable. We analyzed mRS as an ordinal variable. We used 2 separate ordinal variables capturing scores for ADLs and IADLs questionnaires. We included in the SSRI category the following medications: fluoxetine, sertraline, paroxetine, escitalopram, fluvoxamine, citalopram, volazodone, and vortioxetine. We did not include other agents with serotoninergic properties, such as serotonin and norepinephrine reuptake inhibitors and tricyclic antidepressants. We focused on recurrent ICH and severity of depressive symptoms as outcomes of interest. Recurrent ICH events were first identified via telephone calls and medical records, then confirmed by study staff after consensus review of pertinent imaging information. We analyzed the associations of SSRI with clinical remission of depressive symptoms, defined as participants meeting both of the following criteria: (1) documented evidence in medical records or billing information of remission of depressive symptoms and (2) GDS-4 score dropping to less than 2.16 Study staff conducting outcome ascertainments were blinded to all clinical data.
Statistical Methods
Categorical variables were compared using Fisher exact test (2-sided) and continuous variables using the Mann-Whitney rank sum or unpaired t test. We conducted separate analyses for recurrent ICH and remission of depressive symptoms. We implemented univariable (log-rank) and multivariable (Fine and Gray competing risk regression) survival analysis models for each outcome. Survival models for ICH recurrence had inception at time of enrollment, while models for improvement in depressive symptoms had inception at time of depression diagnosis. All models included SSRI use as time-varying exposures and death as a competing outcome. All univariable analyses used the log-rank test, with single-variable Cox models used to generate univariable point estimates and confidence intervals for effect size. All variables with P less than .20 for association with the dependent outcome variable were included in multivariable analyses. After variable selection, we generated a minimal model by backward elimination of nonsignificant variables (P > .05). Selective serotonin reuptake inhibitor use was the only variable prespecified for forced inclusion in all models, regardless of significance. The proportional hazard assumption was tested using graphic checks and Schoenfeld residuals-based tests. Multicollinearity was assessed by computing variance inflation factors for all predictors and removing all variables with variance inflation factors greater than 5 (none required removal as part of the analyses presented in the Results section).
We prespecified several additional analyses for all outcomes of interest. First, we sought to minimize confounding by indication by performing additional multivariable modeling using propensity score matching. We used the balanced, parallel, variable ratio (1:n) nearest neighbor approach to create clusters of matched patients for analyses.17 Clusters were defined by pairing individuals taking SSRI with nearest-neighbor individuals not taking SSRI based on the following prespecified predictors of interest: (1) all identified risk factors for ICH recurrence (other than SSRI use); (2) all factors associated with post-ICH depression remission (other than SSRI use); and (3) all variables associated (at P < .10) with SSRI use during follow-up in our previously described logistic regression models. All clusters with identical propensity parameters were then collapsed to form strata for subsequent multivariate analyses of primary outcomes. Because SSRI use was incorporated in our analyses as a time-varying covariate (see previous paragraphs), propensity score matching was also computed separately for each analysis interval, in a time-varying fashion.18 Second, we compared the effects of different agents in the SSRI class. Third, we separately analyzed use of SSRI at low dose (at or less than half of maximum recommended dosing) vs high dose (greater than half of maximum recommended dosing). Both individual SSRI agents and dosing were handled as time-varying exposures (thus modeling dose changes and switches between different SSRIs). Fourth, we repeated all analyses in subsets of participants at higher risk for repeat ICH: (1) lobar ICH cases (vs nonlobar ICH cases); (2) patients with history of prior ICH (vs those without); (3) patients who were black or Hispanic (vs White); and (4) APOE gene ε2 or ε4 carriers (vs noncarriers).
We adjusted for multiple testing burden using the false discovery rate (FDR) method, and 2-sided significance was set at P less than .05 (after FDR adjustment).19 All analyses were performed using the R software (the R Foundation for Statistical Computing), version 3.2.6.
Results
Study Participants and Follow-up Information
A total of 1279 survivors of ICH were included in the present study (Table 1.) We lost 77 of 1279 participants to follow-up (annual loss-to-follow-up rate of 1.3%). Median follow-up time to recurrent ICH was 53.2 months (interquartile range [IQR], 42.3-61.2 months). During follow-up, we identified 128 individuals who experienced recurrent ICH, for an annual rate of 4.2% (95% CI, 3.4%-5.5%). During follow-up, 766 participants (60%) were diagnosed as having depression; of these, 282 (22% of all study participants) were diagnosed at 3 months after ICH, while the remaining 484 (39% of all study participants) were diagnosed at 6 months or beyond. We followed up survivors of ICH diagnosed as having depression for a median of 26.3 months (IQR, 18.4-35.5 months), with resolution of depression in 418 (55%) in this subgroup.
Table 1. Participant Characteristicsa.
| Variable | No. (%) |
|---|---|
| No. of individuals | 1279 |
| Demographic | |
| Age at enrollment, mean (SD), y | 71.3 (11.9) |
| Female | 602 (47) |
| Race/ethnicity | |
| White | 1049 (82) |
| Black | 89 (7) |
| Hispanic | 77 (6) |
| Other | 64 (5) |
| Education (≥12 y) | 789 (61) |
| Medical history | |
| Hypertension | 984 (77) |
| Ischemic heart disease | 245 (19) |
| Atrial fibrillation | 235 (18) |
| Diabetes | 253 (20) |
| Prior ICH | 76 (6) |
| Prior ischemic stroke/TIA | 114 (9) |
| Pre-ICH depression | 239 (19) |
| Acute ICH characteristics | |
| ICH location | |
| Lobar | 601 (47) |
| Nonlobar | 627 (49) |
| Multiple locations | 51 (4) |
| ICH volume, median (IQR) | 16.5 (3.9-25.2) |
| IVH extension | 397 (31) |
| Admission GCS score, median (IQR) | 14 (9-15) |
| Discharge mRS score, median (IQR) | 4 (3-5) |
| Genetic information | |
| APOE ε2 (≥1 copy) | 190 (15) |
| APOE ε4 (≥1 copy) | 266 (21) |
| Post-ICH medication use | |
| SSRIs | 281 (22) |
| Antiplatelets | 125 (10) |
| Oral anticoagulants | 166 (13) |
Abbreviations: ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IQR, interquartile range; IVH, intraventricular hemorrhage; mRS, modified Rankin Scale; SSRI, selective serotonin reuptake inhibitors; TIA, transient ischemic attack.
Significance testing in univariable analyses via the log-rank test.
SSRI Use and Associated Factors
We identified 135 survivors of ICH receiving SSRI therapy at time of ICH; of these, 111 continued receiving SSRI after ICH without treatment interruption. An additional 170 participants started receiving SSRI after ICH, for a total of 281 ICH survivors (21.9%) prescribed SSRIs during follow-up. Median time to SSRI initiation was 10.8 months (IQR, 3.4-18.9 months). Median duration of therapy during follow-up was 26.8 months (IQR, 20.3-37.9). A total of 157 survivors of ICH (55.9%) were prescribed 1 SSRI agent, 68 (24.2%) were prescribed 2, and 56 (19.9%) were prescribed at least 3 separate SSRI agents. Among SSRI users, a total of 233 (82.9%) received high dosing at least once during follow-up. Pre-ICH history of depression, prior history of stroke/TIA, and higher mRS were independently associated with higher likelihood of receiving SSRIs (Table 2).
Table 2. Factors Associated With SSRI Use After Intracerebral Hemorrhage.
| Variable | SSRI users (n = 281) | No SSRI exposure (n = 998) | Univariable P value | Multivariable | |
|---|---|---|---|---|---|
| OR (95% CI) | P value | ||||
| Education, ≥12 y, No. (%) | 187 (67) | 602 (60) | .059 | 1.74 (0.93-3.25) | .09 |
| History of pre-ICH depression, No. (%) | 101 (36) | 138 (14) | <.001 | 3.49 (1.89-8.15) | .005 |
| Discharge mRS score, median (IQR)a | 4 (3-5) | 4 (3-4) | .004 | 1.83 (1.20-2.80) | .007 |
| ICH volume, median (IQR)b | 18.5 (4.1-26.8) | 14.2 (3.5-21.8) | .001 | 1.35 (0.93-1.95) | .15 |
| Prior ICH, No. (%) | 36 (13) | 40 (4) | <.001 | 3.18 (1.54-6.55) | .002 |
| Prior ischemic stroke or TIA, No. (%) | 34 (12) | 80 (8) | .007 | 1.69 (1.11-2.57) | .02 |
Abbreviations: ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale, OR, odds ratio; SSRI, selective serotonin reuptake inhibitor; TIA, transient ischemic attack.
Effect size calculated for 1-point increase in mRS score.
Effect size calculated for 10-cm3 increase in ICH volume.
SSRI Use, ICH Recurrence, and Post-ICH Depression Remission
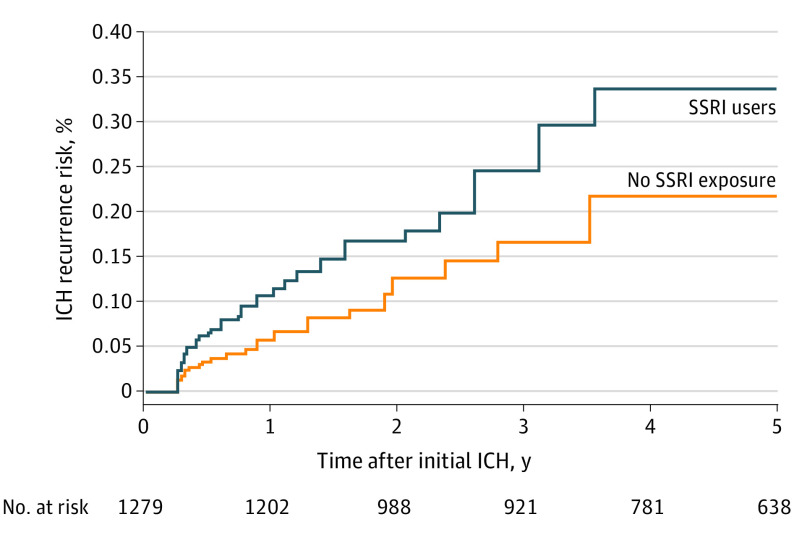
SSRIs use during follow-up was associated with increased risk of ICH recurrence in univariable analyses (Table 3). Other risk factors for ICH recurrence included non-White race/ethnicity, lower educational attainment, prior history of ICH, lobar ICH location, and the APOE ε2 / ε4 alleles. Figure 1 presents cumulative incidence functions for recurrent ICH risk based on SSRI exposure. In multivariable analyses, SSRI use was an independent risk factor for recurrent ICH (Table 3). Selective serotonin reuptake inhibitor use was also associated with increased likelihood of remission of depressive symptoms in both univariable and multivariable analyses (Table 3).
Table 3. Univariable and Multivariable Analyses of Risk for Outcomes of Interest Following Intracerebral Hemorrhage.
| Variable | Univariable P value | Multivariablea | |
|---|---|---|---|
| SHR (95% CI) | P value | ||
| Outcome | |||
| ICH recurrence | |||
| Race/ethnicity (White) | .01 | 0.59 (0.37-0.94) | .03 |
| Education, ≥12 y | .02 | 0.69 (0.51-0.93) | .02 |
| Prior ICH | <.001 | 3.11 (1.48-6.52) | .003 |
| ICH location (lobar) | .002 | 2.19 (1.25-3.83) | .007 |
| APOE ε2/ε4 (≥1 copy) | .047 | 1.35 (1.02-2.87) | .04 |
| SSRI use | .008 | 1.31 (1.08-3.59) | .006 |
| Depression remission | |||
| Education, ≥12 y | .048 | 1.37 (1.04-1.78) | .03 |
| Prior ICH | .01 | 0.43 (0.23-0.79) | .008 |
| Prior ischemic stroke or TIA | .04 | 0.64 (0.43-0.95) | .03 |
| Pre-ICH depression | .03 | 0.68 (0.52-0.89) | .005 |
| ICH location (lobar) | .02 | 0.59 (0.50-0.93) | .02 |
| ICH volume (per 10-cm3 increase) | .047 | 0.85 (0.67-1.08) | .19 |
| Discharge mRS (per 1 point increase) | .008 | 0.79 (0.66-0.95) | .02 |
| APOE ε2/ε4 (≥1 copy) | .11 | 0.81 (0.67- 0.98) | .04 |
| SSRI use | .04 | 1.53 (1.12-2.09) | .009 |
Abbreviations: ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, Modified Rankin Scale; SHR, subhazard ratio; SSRI, selective serotonin reuptake inhibitor; TIA, transient ischemic attack.
Multivariable analyses adjusted for all variables listed.
Figure 1. Intracerebral Hemorrhage (ICH) Recurrence Risk and Selective Serotonin Reuptake Inhibitor (SSRI) Use.
Cumulative incidence functions for ICH recurrence during follow-up, based on exposure to SSRIs. Numbers below the graph (marked n) indicated total study sample size retained at each time point during follow-up.
Sensitivity Analyses
We matched 281 ICH survivors receiving SSRIs with 281 not receiving SSRIs for the following characteristics: race/ethnicity, education (< or ≥12 years), prior ICH, prior ischemic stroke or transient ischemic attack, pre-ICH depression, and ICH location (lobar vs nonlobar), APOE genotype (ε2/ε4 carries vs noncarriers), and discharge mRS (0-2 vs 3-5). After propensity matching, SSRI use was still associated with both ICH recurrence (subhazard ratio [SHR], 1.30; 95% CI, 1.02-1.66, P = .046) and resolution of post-ICH depression (SHR, 1.55; 95% CI, 1.05-2.29; P = .04).
We found that no individual SSRI had significantly different effects for association with either ICH recurrence or post-ICH depression remission. Use of high-dose SSRIs was associated with higher ICH recurrence risk (SHR, 1.61; 95% CI, 1.15-2.25), with a larger effect size (comparison P = .02) than low-dose SSRI use (SHR, 1.25; 95% CI, 1.01-1.55). In contrast, while high SSRI dosing was associated with depression remission (SHR, 1.55; 95% CI, 1.07-2.24), we found no statistically significant difference (P = .32) when comparing effect sizes with low SSRI dosing (SHR, 1.42; 95% CI, 1.03-1.96).
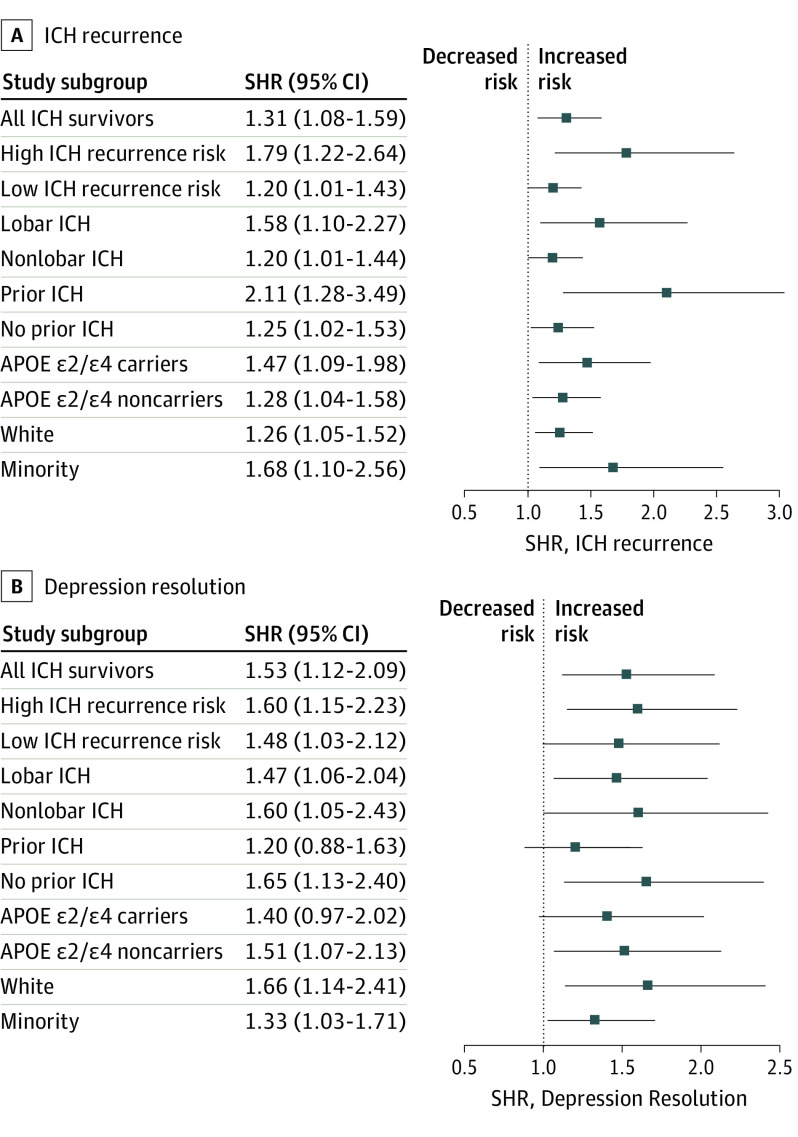
We then examined associations between SSRI use and outcomes of interest among ICH survivors at high vs low risk of recurrent hemorrhagic stroke. High-risk patient categories included: (1) lobar ICH survivors (vs nonlobar ICH survivors); (2) ICH survivors with history of prior ICH (vs those without); (3) APOE ε2 and ε4 carriers (vs noncarriers with the ε3ε3 genotype); (4) Black and Hispanic ICH survivors (vs White ICH survivors). Intracerebral hemorrhage recurrence rates in the high-risk group were 6.1% yearly (95% CI, 5.4%-7.0%) with SSRI exposure and 3.8% yearly (95% CI, 2.9%-4.7%) without SSRI exposure. In comparison, recurrence rates in the low-risk group were 2.9% yearly (95% CI, 2.4%-3.5%) with SSRI exposure and 2.3% yearly (95% CI, 2.0%-2.9%) without SSRI exposure. In multivariable analyses, SSRI use was associated with ICH recurrence risk with a larger effect among those with at least 1 high-risk characteristic (SHR, 1.79; 95% CI, 1.22-2.64), when compared with those with no high-risk characteristics (SHR, 1.20; 95% CI, 1.01-1.42; P = .008 for comparison of effect sizes).
Figure 2A summarizes association results for SSRI use and ICH recurrence among individuals at high vs low risk or recurrent cerebral bleeding. Among all high-risk vs low-risk comparisons, only patients with prior ICH showed a stronger association between SSRI use and ICH recurrence (P = .008 for comparison of effect sizes). In contrast, the association of SSRIs with depression remission did not differ significantly (P = .47) comparing participants at high risk for ICH recurrence vs low-risk participants. Figure 2B summarizes subgroup association analyses for SSRI use and depression remission, none of which showed significant heterogeneity of effects (all P > .20).
Figure 2. Associations Between Selective Serotonin Reuptake Inhibitor (SSRI) Use, Intracerebral Hemorrhage (ICH) Recurrence, and Post-ICH Depression Resolution Among Participants at High vs Low ICH Recurrence Risk.
Point estimates and confidence intervals of hazard ratios for associations between SSRI exposure and ICH recurrence risk (A) or resolution of post-ICH depression (B) in subsets of patients at high vs low risk for recurrent ICH. Dashed vertical line indicates the point estimate for the effect of SSRI exposure in the entire study for graphical comparison purposes. SHR indicates subhazard ratio.
Discussion
In a single-center observational cohort study, we demonstrated an association between SSRI use and increased risk of recurrent hemorrhage after primary ICH. We also found that SSRI use was associated with increased likelihood of post-ICH depression remission. The association between SSRI use and ICH recurrence had larger effect size within subgroups at higher risk for recurrent hemorrhagic stroke (carriers of the APOE ε2/ε4 alleles, patients with lobar ICH, patients with prior ICH, and minority participants). In contrast, the association between SSRI prescription and depression remission did not vary substantially based on underlying ICH recurrence risk.
Our findings linking SSRI exposure with recurrent ICH risk are consistent with existing evidence demonstrating an association between SSRI use and risk of first-ever ICH.11 These associations are presumed to reflect the known antithrombotic effects of SSRIs. Additional potential mechanisms linking SSRIs and ICH recurrence include worsening of hypertension and increased production of inflammatory cytokines with chronic use.20 Prior studies directly investigating the use of SSRI after a first ICH have been limited to date. A longitudinal study leveraging a nationwide administrative database in Denmark previously found no association between SSRI exposure and increase in intracranial hemorrhage recurrence risk, in contrast with our findings.21 This study had several notable differences from our analysis, chiefly: (1) use of billing and coding information rather than neuroimaging to confirm recurrent ICH events; (2) limited access to individual-level information on ICH recurrence risk; and (3) lack of capture of fatal ICH cases that did not result in hospitalization. It is worth mentioning we did demonstrate larger effect sizes for higher SSRI doses, supporting our hypothesis of a direct biologic association between their use and recurrent ICH.
We also found that that APOE ε2/ε4 carriers and survivors of lobar ICH are more likely to have persistent depressive symptoms following ICH. The APOE genotype and ICH location (lobar vs nonlobar) are established markers for the subtype of small-vessel disease most likely to account for the index ICH event.22 Specifically, APOE ε2/ε4 and lobar ICH location are consistently associated with cerebral amyloid angiopathy (CAA), thus suggesting a preferential etiopathogenetic role for this small-vessel disorder in depression after ICH.8,23 A number of biological mechanisms may account for these findings. Cerebral amyloid angiopathy is known to preferentially affect lobar cerebral regions (in comparison with hypertensive small vessel disease), likely resulting in widespread disruptions of cortical networks underpinning development of mood symptoms.24 Patients with CAA are also known to harbor comorbid Alzheimer disease, caused by concomitant parenchymal amyloidosis and independently associated with depression risk.25
Limitations
Our study has some limitations. First and foremost, SSRI prescription and use were based on decisions made by individual health care clinicians in a nonrandomized, nonblinded fashion. As a result, our analyses may be susceptible to inherent bias, especially indication and severity bias. Of specific concern, previously discussed CAA markers (ie, APOE ε2/ε4 variants and lobar ICH location) were associated with both persistent depressive symptoms (thus increasing likelihood of being prescribed SSRIs and duration of therapy) and increased ICH recurrence risk. However, we did identify similar associations between SSRI use and ICH recurrence among participants with and without CAA-related markers, suggesting that the increased risk of recurrence associated with SSRIs was not driven solely by confounding with underlying CAA. In addition, after propensity score matching for CAA-related markers, SSRIs were still associated with ICH recurrence risk. Ultimately, randomized clinical trials of SSRI for treatment of post-ICH depression will be required to definitively address the possibility of inherent bias. We also did not have information on patient compliance with antidepressive treatments, thus having to rely on prescription data alone. However, we would expect decreased SSRI exposure from medication non-compliance to reduce statistical power for our analysis, biasing us toward the null hypothesis rather than generating spurious associations with ICH recurrence or post-ICH depression. Finally, despite the overall size of our study, we had limited statistical power for analyses in patient subgroups of interest. Our approach also has a number of strengths. We were able to assemble extensive individual-level data for participating ICH survivors using validated standardized methods and with limited loss to follow-up, thus allowing us to explore the role of clinical, imaging, and genetic data in treatment of post-ICH depression.
Conclusions
In conclusion, we demonstrated that SSRI use following primary ICH is associated with increased risk of recurrence hemorrhage and with higher likelihood of depression remission. Among individuals at high risk for recurrent ICH, use of SSRI was associated with a greater increase in repeated hemorrhagic stroke risk. Future studies will be required to confirm that clinical, neuroimaging, and genetic markers of ICH recurrence risk can assist in selecting patients most likely to receive net benefit from SSRI treatment of depression after primary ICH.
References
- 1.Thrift AG, Thayabaranathan T, Howard G, et al. . Global stroke statistics. Int J Stroke. 2017;12(1):13-32. [DOI] [PubMed] [Google Scholar]
- 2.Biffi A, Anderson CD, Battey TW, et al. . Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314(9):904-912. doi: 10.1001/jama.2015.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(6):660-667. doi: 10.1136/jnnp-2013-306476 [DOI] [PubMed] [Google Scholar]
- 4.Boulouis G, Charidimou A, Pasi M, et al. . Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci. 2017;380:64-67. doi: 10.1016/j.jns.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Etten ES, Gurol ME, van der Grond J, et al. . Recurrent hemorrhage risk and mortality in hereditary and sporadic cerebral amyloid angiopathy. Neurology. 2016;87(14):1482-1487. doi: 10.1212/WNL.0000000000003181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. . Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018;91(1):e37-e44. doi: 10.1212/WNL.0000000000005729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biffi A, Sonni A, Anderson CD, et al. ; International Stroke Genetics Consortium . Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934-943. doi: 10.1002/ana.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marini S, Crawford K, Morotti A, et al. ; International Stroke Genetics Consortium . Association of apolipoprotein E with intracerebral hemorrhage risk by race/ethnicity: a meta-analysis. JAMA Neurol. 2019;76(4):480-491. doi: 10.1001/jamaneurol.2018.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towfighi A, Ovbiagele B, El Husseini N, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research . Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(2):e30-e43. doi: 10.1161/STR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 10.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017-1025. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MP, Ziff OJ, Banerjee G, Ambler G, Werring DJ. The impact of selective serotonin reuptake inhibitors on the risk of intracranial haemorrhage: a systematic review and meta-analysis. Eur Stroke J. 2019;4(2):144-152. doi: 10.1177/2396987319827211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biffi A, Bailey D, Anderson CD, et al. . Risk factors associated with early vs delayed dementia after intracerebral hemorrhage. JAMA Neurol. 2016;73(8):969-976. doi: 10.1001/jamaneurol.2016.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raffeld MR, Biffi A, Battey TW, et al. . APOE ε4 and lipid levels affect risk of recurrent nonlobar intracerebral hemorrhage. Neurology. 2015;85(4):349-356. doi: 10.1212/WNL.0000000000001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727. doi: 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 15.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 16.Pocklington C, Gilbody S, Manea L, McMillan D. The diagnostic accuracy of brief versions of the Geriatric Depression Scale: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2016;31(8):837-857. doi: 10.1002/gps.4407 [DOI] [PubMed] [Google Scholar]
- 17.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69-80. doi: 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Liu Q, Shepherd BE. Performance of time-dependent propensity scores: a pharmacoepidemiology case study. Pharmacoepidemiol Drug Saf. 2015;24(1):98-106. doi: 10.1002/pds.3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsueh HM, Chen JJ, Kodell RL. Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat. 2003;13(4):675-689. doi: 10.1081/BIP-120024202 [DOI] [PubMed] [Google Scholar]
- 20.Nezafati MH, Eshraghi A, Vojdanparast M, Abtahi S, Nezafati P. Selective serotonin reuptake inhibitors and cardiovascular events: a systematic review. J Res Med Sci. 2016;21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt LB, Goertz S, Wohlfahrt J, Melbye M, Munch TN. Recurrent intracerebral hemorrhage: associations with comorbidities and medicine with antithrombotic effects. PLoS One. 2016;11(11):e0166223. doi: 10.1371/journal.pone.0166223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19(1):3-10. doi: 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charidimou A, Zonneveld HI, Shams S, et al. . APOE and cortical superficial siderosis in CAA: meta-analysis and potential mechanisms. Neurology. 2019;93(4):e358-e371. doi: 10.1212/WNL.0000000000007818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brakowski J, Spinelli S, Dörig N, et al. . Resting state brain network function in major depression: depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res. 2017;92:147-159. doi: 10.1016/j.jpsychires.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184-190. doi: 10.1016/j.maturitas.2014.05.009 [DOI] [PubMed] [Google Scholar]