Key Points
Question
Does low-dose hydrocortisone decrease treatment failure in patients with COVID-19–related acute respiratory failure?
Findings
In this randomized clinical trial that included 149 patients and was terminated early following the recommendation of the data and safety monitoring board, there was no significant difference in the rate of treatment failure (defined as death or persistent respiratory support with mechanical ventilation or high-flow oxygen therapy) on day 21 between the hydrocortisone and placebo groups (42.1% vs 50.7%, respectively).
Meaning
Low-dose hydrocortisone did not significantly reduce treatment failure in patients with COVID-19–related acute respiratory failure; however, the study was stopped early and was therefore likely underpowered.
Abstract
Importance
Coronavirus disease 2019 (COVID-19) is associated with severe lung damage. Corticosteroids are a possible therapeutic option.
Objective
To determine the effect of hydrocortisone on treatment failure on day 21 in critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and acute respiratory failure.
Design, Setting, and Participants
Multicenter randomized double-blind sequential trial conducted in France, with interim analyses planned every 50 patients. Patients admitted to the intensive care unit (ICU) for COVID-19–related acute respiratory failure were enrolled from March 7 to June 1, 2020, with last follow-up on June 29, 2020. The study intended to enroll 290 patients but was stopped early following the recommendation of the data and safety monitoring board.
Interventions
Patients were randomized to receive low-dose hydrocortisone (n = 76) or placebo (n = 73).
Main Outcomes and Measures
The primary outcome, treatment failure on day 21, was defined as death or persistent dependency on mechanical ventilation or high-flow oxygen therapy. Prespecified secondary outcomes included the need for tracheal intubation (among patients not intubated at baseline); cumulative incidences (until day 21) of prone position sessions, extracorporeal membrane oxygenation, and inhaled nitric oxide; Pao2:Fio2 ratio measured daily from day 1 to day 7, then on days 14 and 21; and the proportion of patients with secondary infections during their ICU stay.
Results
The study was stopped after 149 patients (mean age, 62.2 years; 30.2% women; 81.2% mechanically ventilated) were enrolled. One hundred forty-eight patients (99.3%) completed the study, and there were 69 treatment failure events, including 11 deaths in the hydrocortisone group and 20 deaths in the placebo group. The primary outcome, treatment failure on day 21, occurred in 32 of 76 patients (42.1%) in the hydrocortisone group compared with 37 of 73 (50.7%) in the placebo group (difference of proportions, –8.6% [95.48% CI, –24.9% to 7.7%]; P = .29). Of the 4 prespecified secondary outcomes, none showed a significant difference. No serious adverse events were related to the study treatment.
Conclusions and Relevance
In this study of critically ill patients with COVID-19 and acute respiratory failure, low-dose hydrocortisone, compared with placebo, did not significantly reduce treatment failure (defined as death or persistent respiratory support) at day 21. However, the study was stopped early and likely was underpowered to find a statistically and clinically important difference in the primary outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT02517489
This randomized clinical trial compares the effect of low-dose hydrocortisone vs placebo on treatment failure (death or persistent respiratory support dependency) at 21 days in critically ill patients with COVID-19 and acute respiratory failure in France.
Introduction
As of August 17, 2020, more than 20 million people worldwide have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and nearly 800 000 people have died of coronavirus disease 2019 (COVID-19).1 Acute respiratory failure is a major cause of intensive care unit (ICU) admission for patients with COVID-19.2,3 In the absence of specific intervention, the treatment of COVID-19 relies on relieving symptoms and organ support. The potential shorter recovery associated with remdesivir, an antiviral drug, was not observed in the subgroup of critically ill patients.4 Until recently, no drug had been shown to improve survival.
Although the pathophysiology of COVID-19 remains incompletely understood, organ damage, especially diffuse lung injury, results from both the direct cytotoxicity of the virus and dysregulated immune response. The importance of a cytokine storm has been discussed5,6 and debated7,8; regardless, it is clear that excessive inflammation plays a role in the development of pulmonary disease.9 Immunomodulatory drugs, such as corticosteroids, are therefore being investigated as therapeutic options for COVID-19.10
The efficacy and safety of corticosteroids in patients with viral pneumonia remains largely uncertain because of a scarcity of randomized trials and inconclusive observational studies.11 At the onset of the pandemic, there was equipoise regarding use of corticosteroids for severe COVID-19.12 Corticosteroids may impair immune defenses and hamper viral clearance, potentially leading to subsequent excess mortality such as has been suggested in patients with severe influenza.13 Yet one observational study reported that methylprednisolone was associated with a 25% relative reduction in short-term mortality among patients with COVID-19–related acute respiratory distress syndrome.14 Recently, an open-label randomized trial found that dexamethasone improved day-28 survival in patients hospitalized with COVID-19.15 The purpose of this study was to evaluate the effect of hydrocortisone for the treatment of ICU patients with COVID-19–related acute respiratory failure.
Methods
Ethical and Regulatory Issues
The ethics committee (Comité de Protection des Personnes Ouest 1, France) as well as the French regulatory agency approved this trial, as an adaptation of the design of a parent trial, focused on the group of patients with SARS-CoV-2 infection (see below). The ClinicalTrials.gov website was updated as soon as ethical and regulatory approvals were obtained. Each patient or surrogate provided either written or oral informed consent prior to inclusion. If the patient could not consent and no surrogate was available, the ethics committee authorized emergency inclusion in the study, in which case deferred consent was obtained as soon as possible from the patient or surrogate.
Design
The present trial was embedded in the ongoing Community-Acquired Pneumonia: Evaluation of Corticosteroids (CAPE COD) trial. Methodological issues relating to this original approach have been described elsewhere.16 The protocol, including the statistical analysis plan, is presented in Supplement 1 and Supplement 2. Briefly, the CAPE COD trial was designed to determine the superiority of low-dose hydrocortisone compared with placebo in reducing mortality on day 28 in ICU patients with community-acquired pneumonia. When the COVID-19 outbreak developed, it was rapidly recognized that the benefits and risks of corticosteroids needed to be assessed, particularly in severe forms of the disease; the design of the ongoing trial allowed the inclusion of patients with COVID-19; there was a unique opportunity to assess the efficacy and safety of corticosteroids in a trial of high methodological standard, albeit in an unprecedented pandemic context (eg, centers trained in the trial procedures and already active, availability of the drug and the placebo in a form guaranteeing double-blinding, electronic case report form in place, only minor amendments required to obtain regulatory authorizations); and the methodology had to be adapted to this pandemic context.
The Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease (CAPE COVID) trial was therefore embedded within the parent trial. Because all participating centers were exclusively admitting only patients with COVID-19 during the initial phase of the pandemic, inclusion of patients with pneumonia of other origin were discontinued. Patients with COVID-19 had been included in the parent trial, from March 7, 2020. The use of a different primary outcome from the parent trial (ie, better suited to the epidemic emergency) and a sequential mode analysis for patients with COVID-19, including those who had already been enrolled, was approved by the ethics committee on March 30 and by the regulatory agency on April 9, 2020. By this time, 26 patients with COVID-19 had been included in the parent trial. This embedded trial was planned as a placebo-controlled group sequential design using the Lan and DeMets approach,17 with a planned interim analysis every 50 patients. Thus, the first analysis could be performed for the first 50 patients according to the method approved for the subtrial devoted to COVID-19.
Participants
Patients aged at least 18 years admitted to 1 of the 9 participating French ICUs for acute respiratory failure could be included if they had a biologically confirmed (reverse transcriptase–polymerase chain reaction) or suspected (suggestive chest computed tomography scan result in the absence of any other cause of pneumonia) COVID-19. The experimental treatment had to be administered within 24 hours of the onset of the first severity criterion (see below) or within 48 hours for patients referred from another hospital. One of 4 severity criteria had to be present: need for mechanical ventilation with a positive end-expiratory pressure (PEEP) of 5 cm H20 or more; a ratio of Pao2 to fraction of inspired oxygen (Fio2) less than 300 on high-flow oxygen therapy with an Fio2 value of at least 50%; for patients receiving oxygen through a reservoir mask, a Pao2:Fio2 ratio less than 300, estimated using prespecified charts; or a Pulmonary Severity Index18 greater than 130. Patients receiving vasopressors to correct hypotension related to sedative drugs and mechanical ventilation at high PEEP levels could be included. Principal exclusion criteria were septic shock and do-not-intubate orders.
Randomization and Allocation Concealment
Randomization was centralized and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer-generated random number using a blocking schema; the range of block sizes remains confidential until the completion of the parent trial. Randomization was stratified by center and by use of mechanical ventilation at the time of inclusion.
Treatments and Blinding
Patients received a continuous intravenous infusion of hydrocortisone at an initial dose of 200 mg/d or its placebo (saline). Both hydrocortisone and placebo were provided in industrially prepared packaging (Serb Specialty Pharmaceuticals). Treatment was continued at 200 mg/d until day 7 and then decreased to 100 mg/d for 4 days and 50 mg/d for 3 days, for a total of 14 days. If the patient’s respiratory and general status had sufficiently improved by day 4, a short treatment regimen was used (200 mg/d for 4 days, followed by 100 mg/d for 2 days and then 50 mg/d for the next 2 days, for a total of 8 days). All of the following criteria had to be present to consider this adaptive scheme: patient breathing spontaneously; Pao2:Fio2 ratio greater than 200; Sequential Organ Failure Assessment (SOFA)19 score on day 4 less than or equal to SOFA score on day 1; and strong probability of being discharged from the ICU (including intermediate-care units) before day 14, according to the physician of record. In all cases, treatment was stopped on ICU discharge. Patients otherwise received standard care for acute respiratory failure.20 Since no antiviral treatment improved survival or clinically relevant parameters, adjunctive treatments could be administered at the discretion of the patients’ primary physicians.
Outcome Measures and Data Collection
The primary outcome was treatment failure on day 21, defined as death or persistent dependency on mechanical ventilation or high-flow oxygen therapy.
Prespecified secondary outcomes included the use of tracheal intubation (for patients not intubated at inclusion); the use of prone position (with the number of sessions), extracorporeal membrane oxygenation or inhaled nitric oxide (with the number of days the treatment was used); the Pao2:Fio2 ratio recorded daily from day 1 to day 7 and then on days 14 and 21; and the proportion of patients with and the number of episodes of nosocomial infections recorded during the ICU stay. Because some patients were still hospitalized in the ICU when the data were analyzed, nosocomial infections were recorded up to day 28 (which was a post hoc decision). The diagnosis of nosocomial infection was made by the clinician in charge and provided that an antibiotic therapy had been prescribed.
Death on day 21 and status on day 21 (determined using a 5-item scale: death, presence in the ICU on mechanical ventilation, high-flow or low-flow oxygen therapy, ICU discharge) were post hoc outcomes.
Apart from death, the adverse events expected in this context (such as the need for intubation in a patient breathing spontaneously at baseline) were only reported if the clinician thought they might be related to the study treatment.
Sample Size
The event rate, defined as treatment failure on day 21 (ie, death or persistent dependency on mechanical ventilation or high-flow oxygen therapy), was assumed to be 30% in the control group, acknowledging a high level of uncertainty owing to the unprecedented nature of COVID-19. The trial was designed to test the superiority of hydrocortisone over placebo with an assumed event rate of 15% in the hydrocortisone group,21 with 80% power and a 5% 2-sided type I error rate. Because of the sequential nature of the design, with 6 analyses (5 interim and a final one), the maximal required sample size was 290.
Statistical Analysis
Patients were analyzed according to their randomization group. For the primary analysis, missing data were considered treatment failure. No imputation was made for secondary outcomes. All performed statistical tests were 2-sided. P ≤ .0452 was considered a significant difference in the primary outcome because of the interim analyses, and P ≤ .05 as indicating statistical significance for secondary outcomes. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Categorical variables were summarized as frequencies and percentages and continuous variables as medians (interquartile ranges). Treatment failure on day 21 was reported as proportions in each group and compared using a 2-proportion z test based on normal approximation. Difference of proportions was also estimated with its 95% confidence interval.
A sensitivity analysis was performed on patients without missing data. Cumulative incidence of patients with at least 1 prone-position session, and incidence of patients experiencing secondary infections during their ICU stay, were estimated using a competing-risk approach,22 with death and end of ICU stay as competing events. For competing-risk models, proportionality assumptions were studied including an interaction term with the time in Fine and Gray models; results of these tests were not significant. Given the limited number of events, analyses of extracorporeal membrane oxygenation and inhaled nitric oxide were only descriptive. Evolution of Pao2:Fio2 ratio was analyzed using a mixed linear model. The status on day 21 was analyzed using a Fisher exact test. For death on day 21, difference of proportion was estimated with its 95% confidence interval and compared between the 2 groups using a 2-proportion z test.
Data were analyzed with SAS version 9.4 (SAS Institute Inc), and R version 3.3.1 (R Foundation for Statistical Computing) was used for the statistical analyses.
Data and Safety Monitoring Board and Trial Suspension
The data and safety monitoring board (DSMB) met when the primary end point was collected for the first 50 and 100 patients and each time recommended further inclusions. When enrollment slowed at the end of the first wave of the epidemic in France, the DSMB agreed to meet on June 30, 2020, to analyze the results of the first 149 patients, which the Lan and DeMets approach allows because of its flexibility. On June 30, 2020, the DSMB recommended suspension of inclusions pending publication of the results of the RECOVERY trial and possible changes in treatment recommendations. The sponsor decided to discontinue the study on July 3, 2020, considering that it would be unethical to resume a corticosteroid vs placebo trial, and that the results should be published and included in the prospective meta-analysis conducted by the World Health Organization.23
Results
Trial Flow and Baseline Characteristics of Participants
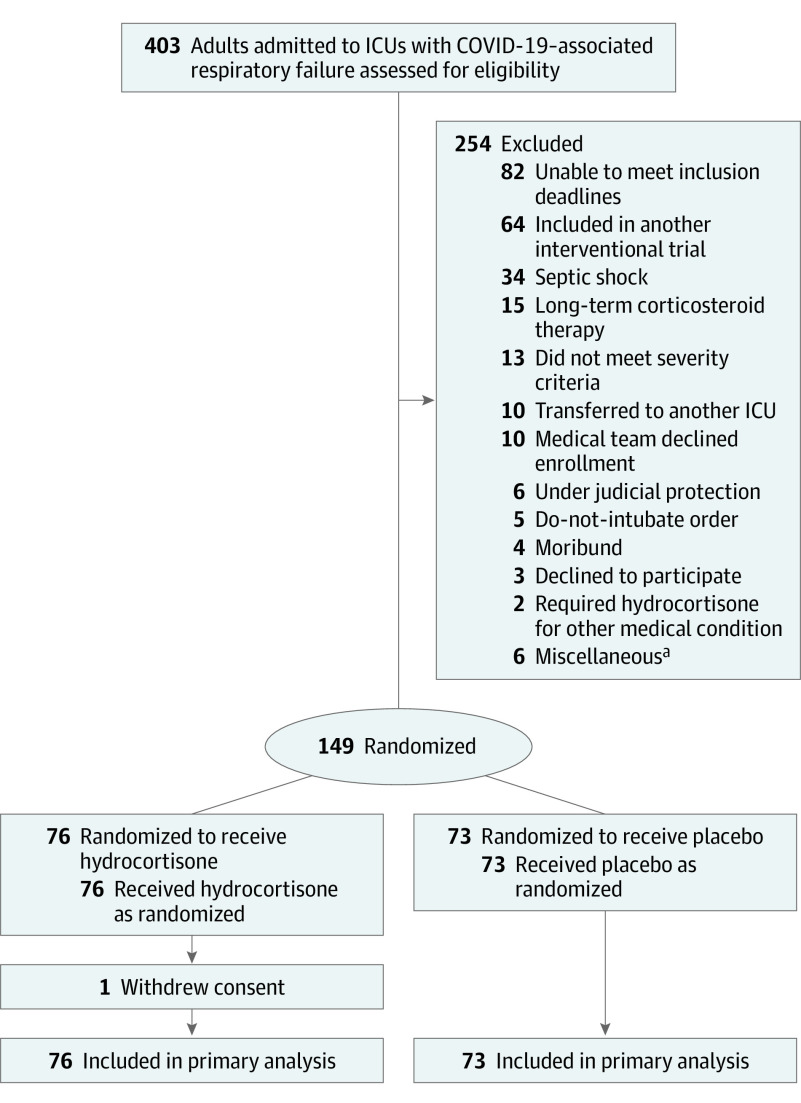
Between March 7 and June 1, 2020, 149 patients were enrolled, of whom 76 were randomized to the hydrocortisone group and 73 to the placebo group (Figure 1). The mean age was 62.2 years and 30.2% were women (Table 1; eTable in Supplement 3). One patient withdrew consent; and for the primary outcome this patient was considered to have experienced treatment failure on day 21. Results of SARS-CoV-2 reverse transcriptase–polymerase chain reaction testing were positive in 96.6% of patients. Median durations of symptoms prior to randomization were 9 days in the hydrocortisone group and 10 days in the placebo group. All patients were hypoxemic, and 121 of 149 (81.2%) were mechanically ventilated at baseline. No patient was included solely based on the Pulmonary Severity Index. Vasopressors were administered in 18 of 76 patients (23.7%) in the hydrocortisone group and 13 of 73 patients (17.8%) in the placebo group.
Figure 1. Study Flow of the CAPE COVID Trial.
Randomization was stratified by center and use of mechanical ventilation at the time of inclusion. CAPE COVID indicates Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
aOne patient had aspiration of gastric content associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Five patients had hospital-acquired SARS-CoV-2 infection, which was misinterpreted as a criterion of noninclusion.
Table 1. Baseline Patient Characteristics in the CAPE COVID Trial.
| Characteristic | No. (%) | |
|---|---|---|
| Hydrocortisone (n = 76) | Placebo (n = 73) | |
| Demographics and past medical history | ||
| Sex | ||
| Women | 22 (28.9) | 23 (31.5) |
| Men | 54 (71.1) | 50 (68.5) |
| Age, median (IQR), y | 63.1 (51.5-70.8) | 66.3 (53.5-72.7) |
| Never smoker, No./total (%) | 57/75 (76.0) | 57/72 (79.2) |
| COPD or asthma | 7 (9.2) | 4 (5.4) |
| Diabetes | 13 (17.1) | 14 (19.2) |
| Immunosuppression | 6 (7.9) | 3 (4.1) |
| BMI, median (IQR)a | 27.5 (25.3-32.4) [n = 59] | 28.4 (26.0-31.2) [n = 61] |
| Clinical data at inclusion, median (IQR) | ||
| Symptom duration, d | 9.0 (7.0-11.5) [n = 76] | 10.0 (8.0-12.0) [n = 72] |
| Heart rate, bpm | 85.0 (68.0-100.0) [n = 55] | 81.0 (72.0-100.0) [n = 57] |
| Systolic blood pressure, mm Hg | 112.0 (104.0-133.0) | 126.5 (111.0-145.0) |
| Temperature, °C | 37.7 (36.8-38.6) [n = 66] | 37.8 (36.9-38.6) [n = 66] |
| Laboratory values at inclusion, median (IQR)b | ||
| RT-PCR–positive | 72 (94.7) | 72 (98.6) |
| C-reactive protein, mg/L | 154.0 (113.0-271.0) [n = 57] | 185.0 (119.0-237.0) [n = 53] |
| Procalcitonin, ng/mL | 0.4 (0.2-0.7) [n = 52] | 0.4 (0.2-0.8) [n = 46] |
| Lymphocytes, ×109/L | 0.9 (0.5-1.4) [n = 65] | 0.7 (0.6-1.3) [n = 57] |
| Lactate, mg/dL | 9.9 (8.1-12.6) [n = 73] | 9.9 (8.1-14.4) [n = 64] |
| Arterial pH | 7.4 (7.3-7.5) [n = 75] | 7.4 (7.3-7.5) [n = 72] |
| Paco2, mm Hg | 39.0 (34.0-47.0) | 38.9 (34.0-45.4) |
| Pao2:Fio2 | 130.0 (96.7-188.0) [n = 75] | 133.0 (89.8-174.8) [n = 72] |
| Respiratory support at inclusion | ||
| Mechanical ventilation | 62 (81.6) | 59 (80.8) |
| Noninvasive ventilation, No./total (%) | 2/62 (3.2) | 2/59 (3.4) |
| Positive end-expiratory pressure, median (IQR), cm H2O | 10.0 (8.0-12.0) | 10.0 (8.0-12.0) |
| Fio2, median (IQR) | 95.0 (60.0-100.0) | 90.0 (60.0-100.0) |
| High-flow oxygen therapy, No. (%) | 10 (13.2) | 9 (12.3) |
| Nonrebreathing mask with a reservoir bag, No. (%) | 4 (5.3) | 5 (6.8) |
| Scores, median (IQR) | ||
| Pneumonia severity indexc | 101.0 (82.0-121.0) [n = 43] | 102.0 (80.0-120.0) [n = 51] |
| Simplified Acute Physiology Score IId | 32.5 (25.0-38.5) | 32.0 (27.0-39.0) |
| Sequential Organ Failure Assessmente | 6.0 (4.0-8.0) [n = 74] | 6.0 (4.0-7.5) [n = 72] |
| Concomitant therapy, No. (%) | ||
| ≥1 | 44 (57.9) | 47 (64.4) |
| Hydroxychloroquine | 11 (14.5) | 8 (11.0) |
| Hydroxychloroquine + azithromycin | 23 (30.3) | 28 (38.4) |
| Ritonavir-lopinavir | 10 (13.2) | 11 (15.1) |
| Eculizumab | 3 (3.9) | 2 (2.7) |
| Remdesivir | 2 (2.6) | 3 (4.1) |
| Tocilizumab | 1 (1.3) | 2 (2.7) |
Abbreviations: BMI, body mass index; CAPE COVID, Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; Fio2, fraction of inspired oxygen; IQR, interquartile range; RT-PCR, reverse transcriptase–polymerase chain reaction.
SI conversion factor: To convert lactate values to mmol/L, multiply by 0.111.
Calculated as weight in kilograms divided by height in meters squared.
Reported values are those designated in the original parent trial. Additional data available at inclusion are reported in eTable 1 in Supplement 3.
Calculated at inclusion. The index18 classifies pneumonia into 5 classes of increasing severity; the median value observed corresponds to class IV, with a mortality of 9.3% in community-acquired pneumonia (but this score has not been specifically validated in COVID-19).
Calculated during the first 24 hours of intensive care unit (ICU) stay. The score24 is an overall severity score for ICU patients. For a patient population, the relationship between mortality and score is sinusoidal. The median score observed corresponds to a predicted mortality of 13% and the first and third quartiles to a predicted mortality of 6% and 23%, respectively.
Calculated at inclusion. The assessment19 evaluates from 1 to 4 for each organ the severity of neurologic, cardiovascular, respiratory, kidney, hematologic, and hepatic dysfunctions. The evolution of the score during hospitalization is a better prognostic parameter than an isolated value. In patients with severe acute respiratory failure, a median score of 6 probably corresponds to moderate impairment of other functions.
The median duration of study treatments was 10.5 days (interquartile range, 6.0-14.0) for hydrocortisone and 12.8 days (interquartile range, 8.0 to 13.0) for placebo (P = .25).
Primary Outcome
Treatment failure on day 21 occurred in 32 of 76 patients (42.1%) in the hydrocortisone group compared with 37 of 73 (50.7%) in the placebo group (difference of proportions, –8.6% [95.48% CI, –24.9% to 7.7%]; P = .29). (Table 2, eFigure 1 in Supplement 3).
Table 2. Treatment Failures, Secondary Outcomes, and Post Hoc Analyses in the CAPE COVID Trial.
| No. (%) | Difference in proportions,% (CI)a | P value | ||
|---|---|---|---|---|
| Hydrocortisone (n = 76) | Placebo (n = 73) | |||
| Primary outcome | ||||
| Treatment failure on day 21 (death or persistent dependence of mechanical ventilation or high-flow oxygen therapy) | 32 (42.1) | 37 (50.7) | −8.6 (−24.9 to 7.7) | .29 |
| Secondary outcomes | ||||
| Endotracheal intubation (for patients noninvasively ventilated at inclusion) | 8/16 (50.0) | 12/16 (75.0) | ||
| Prone position | ||||
| No. (%) | 36 (47.4) | 39 (53.4) | HR, 0.85 (0.55 to 1.32) | .47 |
| No. of sessions per patient, median (IQR) | 2.0 (1.0-3.0) | 2.0 (2.0-4.0) | ||
| Extracorporeal membrane oxygenation | ||||
| No. (%) | 2 (2.7) | 2 (2.7) | ||
| Inhaled nitric oxide | ||||
| No. (%) | 5 (6.7) | 11 (15.0) | ||
| Duration, median (IQR), d | 3.0 (1.0 to 5.0) | 2.0 (1.0 to 8.0) | ||
| Nosocomial infections on day 28b | ||||
| No. (%) | 28 (37.7) | 30 (41.1) | HR, 0.81 (0.49 to 1.35) | .42 |
| Post hoc outcomes | ||||
| Status on day 21 (5-item scale) | ||||
| Deathc | 11 (14.7) | 20 (27.4) | −12.7 (−25.7 to 0.3) | .057 |
| Mechanical ventilation | 17 (22.7) | 17 (23.3) | ||
| High-flow oxygen therapy | 3 (4.0) | 0 | ||
| Low-flow oxygen therapy | 1 (1.3) | 4 (5.5) | ||
| Discharged from ICUd | 43 (57.3) | 32 (43.8) | ||
Abbreviations: CAPE COVID, Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease; HR, hazard ratio.
For the primary outcome, the single missing outcome in the hydrocortisone group was presumed to be a treatment failure. The confidence interval is 95.48% for the primary outcome (owing to the sequential design with multiple analyses) and 95% for secondary outcomes. No statistical test was used when it was clear that the number of events was too few and a test was unnecessary. The incidence of patients with at least 1 prone-position session and the incidence of patients with at least 1 nosocomial infection used a competing risk approach, with the patient who withdrew consent censored on the last reported date. For nosocomial infections, data were censored on day 28 as a post hoc analysis.
Nosocomial infections were defined when they were diagnosed by the clinician in charge and antibiotic treatment was prescribed.
Death on day 21 was both a component of status on day 21 (ordinal variable analyzed by a Fisher exact test, P = .06) and a categorical variable analyzed by a 2-proportion z test.
Patients discharged alive from the ICU were transferred to the floor.
Secondary Outcomes
Of the 16 patients in each group who did not require invasive mechanical ventilation at baseline, 8 (50%) in the hydrocortisone group and 12 (75%) in the placebo group required subsequent intubation. A total of 137 of 149 patients (92%) were intubated, either before inclusion or during treatment. There was no significant between-group difference in rates of prone positioning (36/76 patients [47.4%] in the hydrocortisone group vs 39/73 [53.4%] in the placebo group; hazard ratio, 0.85 [95% CI, 0.55 to 1.32]; P = .47) (Table 2; eFigure 2 in Supplement 3). Too few patients were treated with extracorporeal membrane oxygenation or inhaled nitric oxide to allow statistical testing.
Daily evolution of Pa02:Fio2 ratio during the first week and on days 14 and 21 did not significantly differ between the groups (P = .37) (eFigure 3 in Supplement 3).
On day 28, 58 patients (38.9%) had at least 1 episode of nosocomial infection, 28 of 75 (37.3%) in the hydrocortisone group vs 30 of 73 (41.1%) in the placebo group, for a total of 90 infections (40 vs 50). At least 1 episode of ventilator-associated pneumonia occurred in 22 of 75 patients (29.0%) in the hydrocortisone group, vs 20 of 73 patients (27.4%) in the placebo group. The proportions of bacteremia were 6.6% in the hydrocortisone group and 11.0% in the placebo group. Figure 2 shows the cumulative incidence of nosocomial infections.
Figure 2. Nosocomial Infections Cumulative Incidence.
Cumulative proportion of patients who have had at least 1 nosocomial infection. Nosocomial infections were defined when they were diagnosed by the clinician in charge and antibiotic treatment was prescribed. All patients were observed to death or 28 days (the patient who withdrew consent being censored on the last reported date). HR indicates hazard ratio.
Post Hoc Outcomes
The status on day 21 did not significantly differ between both groups (P = .06) (Table 2; eFigure 1 in Supplement 3). The proportion of patients still ventilated at day 21 was 17 of 75 (22.7%) in the hydrocortisone group vs 17 of 73 (23.3%) in the placebo group. Additionally, 4 of 75 patients were treated with high-flow oxygen therapy in the hydrocortisone group, vs 0 of 73 in the placebo group. In the hydrocortisone group, 43 of 75 patients (57.3%) were discharged from the ICU on day 21, vs 32 of 73 (43.8%) in the placebo group. The proportion of patients who died did not significantly differ between both groups (11/75 [14.7%] in the hydrocortisone group vs 20/73 [27.4%] in the placebo group; difference of proportion, –12.7% [95% CI, –25.7% to 0.3%]; P = .06).
Serious Adverse Events
Apart from deaths, 3 serious adverse events were reported, all in the hydrocortisone group: 1 episode of cerebral vasculitis possibly related to SARS-CoV-2, 1 episode of cardiac arrest related to a pulmonary embolism, and 1 episode of intra-abdominal hemorrhage related to anticoagulant therapy for pulmonary embolism. No serious adverse events were attributed to the study treatment.
Discussion
In this randomized clinical trial that was terminated early, hydrocortisone, compared with placebo, did not significantly reduce the rate of treatment failure, defined as death or persistent dependency on mechanical ventilation or high-flow oxygen therapy, on day 21 among critically ill patients with COVID-19. In addition, hydrocortisone, compared with placebo, did not significantly reduce the proportion of patients receiving mechanical ventilation on day 21.
The primary end point was deemed to be relevant both at the individual level and at the population level, by combining a clinically robust criterion (mortality) with criteria indicative of constraint resources utilization in a pandemic context. This outcome was also consistent with outcomes used in trials of corticosteroids in non-ICU patients with community-acquired pneumonia, namely speeding recovery and shortening hospital stays.25 The failure rate was initially estimated to be 30% in the control group, with substantial uncertainty at the beginning of the epidemic. The observed rate of the primary outcome in the placebo group was much higher than expected (50.7% cases vs 30.0%).
The trial was terminated prematurely after the press release of the dexamethasone trial. According to those findings, dexamethasone may reduce mortality on day 28 in mechanically ventilated patients and, to a lesser extent, in oxygen-dependent patients.15 The DSMB therefore recommended stopping the trial after 149 patients of the planned maximum of 290 had been enrolled. This trial is therefore likely underpowered. The observed difference in the post hoc outcome of proportion of deaths at day 21 was not statistically significant; however, the finding was consistent with the reduced mortality observed with dexamethasone in the subgroup of mechanically ventilated patients.15 A dose of 6 mg of dexamethasone is approximatively equivalent to 160 mg of hydrocortisone, very close to the initial daily dose used in this trial.
In severe community-acquired pneumonia, meta-analysis of the few available randomized trials suggest a reduction in mortality in patients treated with corticosteroids26; however, these findings need to be confirmed. In previous outbreaks of coronavirus pneumonia, the lack of high-quality trials precluded any conclusions regarding the use of corticosteroids in severe acute respiratory syndrome (SARS)27 or Middle East respiratory syndrome (MERS).28 In these reports, increased rates of adverse effects with corticosteroids, related to the use of high doses, have been observed.12 Clearance of viral RNA may be decreased by corticosteroids in SARS29 and MERS,28 but this effect has not been proven for COVID-19, and its clinical relevance is uncertain. In influenza pneumonia, despite the absence of randomized trials and conflicting results from observational studies, it has been suggested that corticosteroids may increase the risk of death.12 In patients with COVID-19, the risk of worsening the viral diffusion in the body, worsening the cytotoxic effect of the virus, or both is uncertain. However, the observed numerically lower rate of deaths in hydrocortisone-treated patients in this trial is reassuring in this regard. Most of the patients were included more than 1 week after the onset of their symptoms. It is possible that the peak of viral excretion occurs earlier in the course of COVID-19 and that the deterioration leading to ICU hospitalization is related to the deregulation of the pulmonary inflammatory response. The favorable effect of dexamethasone was more likely in patients treated after 7 days from onset of symptoms compared with those treated earlier.15
In this trial, hydrocortisone therapy was not associated with an increase in the rate of secondary infections, a concerning risk with corticosteroids,30 especially in mechanically ventilated patients with ventilator-associated pneumonia.
Limitations
This study has several limitations. First, the trial was stopped early and lacked power. Second, while this study was embedded within an existing trial, it had not been planned to record certain data relevant to COVID-19, such as the prevalence of hypertension. Third, the COVID-19 pandemic context has not, to date, allowed for the capture and analysis of all the data provided for in the parent protocol. Fourth, diagnosis of nosocomial infections was not adjudicated; however, the double-blind nature of the trial suggests that the comparison of the rate of secondary infections between the 2 groups may still be valid.
Conclusions
In this study of critically ill patients with COVID-19 and acute respiratory failure, low-dose hydrocortisone, compared with placebo, did not significantly reduce treatment failure (defined as death or persistent respiratory support) at day 21. However, the study was stopped early and likely was underpowered to find a statistically and clinically important difference in the primary outcome.
Study Protocol
Statistical Analysis Plan
eAppendix.
eTable. Baseline Characteristics of the 149 Patients: Additional Data
eFigure 1. Primary Outcome and Post hoc Analysis of Treatment Failure at 21 Days
eAppendix 2. Prone Position Cumulative Incidence and PaO2:FiO2 Ratios Evolution
eFigure 2. Prone Position Cumulative Incidence
eFigure 3. Change Over Time of PaO2:FiO2 Ratios
Members of the CRICS-TriGGERSep Network
Data Sharing Statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.COVID-19 dashboard. Johns Hopkins Center for Systems Science and Engineering. Accessed August 17, 2020. https://coronavirus.jhu.edu/map.html
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members . Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. Published online May 22, 2020. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473-474. doi: 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 7.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. Published online June 30, 2020. doi: 10.1001/jamainternmed.2020.3313 [DOI] [PubMed] [Google Scholar]
- 8.Guillon A, Hiemstra PS, Si-Tahar M. Pulmonary immune responses against SARS-CoV-2 infection: harmful or not? Intensive Care Med. Published online July 17, 2020. doi: 10.1007/s00134-020-06170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouan Y, Guillon A, Gonzalez L, et al. . Functional alteration of innate T cells in critically ill patients with COVID-19. J Exp Med. Published online May 6, 2020. Accessed August 18, 2020. https://www.medrxiv.org/content/10.1101/2020.05.03.20089300v1.full.pdf [Google Scholar]
- 10.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease (COVID-19): a review. JAMA. 2020;323(18):1824-1836. doi: 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 11.Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46(2):315-328. doi: 10.1007/s00134-020-05943-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. doi: 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno G, Rodríguez A, Reyes LF, et al. ; GETGAG Study Group . Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44(9):1470-1482. doi: 10.1007/s00134-018-5332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Y, et al. . Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1-11. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. Published online July 17, 2020. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 16.Dequin PF, Le Gouge A, Tavernier E, Giraudeau B, Zohar S. Embedding a COVID-19 group sequential clinical trial within an ongoing trial: lessons from an unusual experience. Stat Biopharmaceut Res. Published online July 23, 2020. doi: 10.1080/19466315.2020.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659-663. doi: 10.2307/2336502. [DOI] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM, et al. . A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243-250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 20.Papazian L, Aubron C, Brochard L, et al. . Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres A, Sibila O, Ferrer M, et al. . Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677-686. doi: 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. JASA 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.Sterne JAC, Diaz J, Villar J, et al. ; WHO COVID-19 Management and Characterization Working Group . Corticosteroid therapy for critically ill patients with COVID-19: a structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21(1):734. doi: 10.1186/s13063-020-04641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957-2963. doi: 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 25.Blum CA, Nigro N, Briel M, et al. . Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385(9977):1511-1518. doi: 10.1016/S0140-6736(14)62447-8 [DOI] [PubMed] [Google Scholar]
- 26.Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. . Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(7):519-528. doi: 10.7326/M15-0715 [DOI] [PubMed] [Google Scholar]
- 27.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabi YM, Mandourah Y, Al-Hameed F, et al. ; Saudi Critical Care Trial Group . Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197(6):757-767. doi: 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 29.Lee N, Allen Chan KC, Hui DS, et al. . Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. doi: 10.1016/j.jcv.2004.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13-e20. doi: 10.1016/j.jinf.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eAppendix.
eTable. Baseline Characteristics of the 149 Patients: Additional Data
eFigure 1. Primary Outcome and Post hoc Analysis of Treatment Failure at 21 Days
eAppendix 2. Prone Position Cumulative Incidence and PaO2:FiO2 Ratios Evolution
eFigure 2. Prone Position Cumulative Incidence
eFigure 3. Change Over Time of PaO2:FiO2 Ratios
Members of the CRICS-TriGGERSep Network
Data Sharing Statement