Abstract
Background:
Optimal chemotherapy and the role for hematopoietic stem cell transplantation (HSCT) to treat mixed phenotype acute leukemia’s (MPAL) remain uncertain. A major limitation in interpreting available data is MPAL’s rarity and the use of definitions other than the current widely accepted criteria: the World Health Organization 2016 (WHO2016) classification.
Methods:
To assess the relative efficacy of chemotherapy type to treat pediatric MPAL, the Children’s Oncology Group (COG) Acute Leukemia of Ambiguous Lineage Task Force assembled a retrospective cohort of centrally-reviewed WHO2016 MPAL cases selected from banking studies for acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Patients were not treated on COG trials; treatment and outcome data were captured separately. We then integrated our findings with the available, mixed literature to develop a prospective trial in pediatric MPAL.
Results:
Central review confirmed 54 of 70 cases fulfilled WHO2016 criteria for MPAL. ALL induction regimens achieved remission in 72% (28/39) of cases versus 69% (9/13) for AML regimens. Five-year event-free and overall survival (EFS/OS) for the entire cohort were 72±8% and 77±7%. EFS and OS were 75±13% and 84±11% for those receiving ALL chemotherapy alone without HSCT (n=21).
Conclusion:
The results of the COG MPAL cohort and literature review suggest ALL chemotherapy without HSCT may be preferred initial therapy. We propose here a prospective trial within the COG to investigate this approach; AML chemotherapy and/or HSCT will be reserved for those with treatment failure assessed by minimal residual disease. Embedded biology studies will provide further insight into MPAL genomics.
Keywords: Pediatric Leukemia, Mixed-Phenotype Acute Leukemia, Biphenotypic Acute Leukemia, Acute Leukemia of Ambiguous Lineage, Hematopoietic Stem Cell Transplantation
Precis:
ALL-directed chemotherapy without hematopoietic stem cell transplantation may be sufficient therapy for most children with WHO2016-defined MPAL. The proposed prospective trial will investigate this approach and explore MPAL genomics and correlative biology.
INTRODUCTION
Mixed phenotype acute leukemia (MPAL) is uncommon, comprising 1–5% of newly diagnosed acute leukemia.1 MPAL exhibits a complex phenotype with concurrent representation of multiple leukemia lineages either as distinct populations of cells or as a single population expressing different features.2 In the absence of prospective clinical trials for MPAL, defining optimal therapy remains the most pressing knowledge gap. Chemotherapy approaches therefore vary and include treatment for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), or “hybrid” regimens blending elements of both.3 Likewise, there is great variation in the use and recommendations for hematopoietic stem cell transplantation (HSCT) in first complete remission (CR1).4–7
Past reports in MPAL suggest that contemporary ALL treatment regimens may be sufficient to cure many, if not most, pediatric patients with MPAL.3–6, 8–14 However, the majority of these case series lacked central review and, despite age-associated differences in survival, included only aggregate pediatric and adult data.1, 3,15 The most robust pediatric-only MPAL data stem from the iBFM AMBI2012 study.14 However, many of the analyses included MPAL diagnosed according to either the World Health Organization (WHO) or European Group for Immunological Characterization of Acute Leukemias (EGIL) classification systems. Within AMBI2012 and other cohorts, the varying classifications for MPAL, and even updated versions of the same classification, demarcate different populations and influence survival estimates.1, 2,14, 16–18 To best understand treatment effects within a uniformly-defined MPAL population, we therefore assembled a cohort strictly defined only by the most recent WHO classification (WHO2016).19
There is a critical need to move from retrospective studies to prospective trials for pediatric MPAL. The Children’s Oncology Group (COG) Acute Leukemia of Ambiguous Lineage (ALAL) Task Force was created in 2012 as a multi-disciplinary collaboration compromised of oncologists, pathologists, and transplant physicians to review treatment outcomes and develop a clinical trial framework. The Task Force developed the consensus investigational plan described below to serve as the basis for the COG’s planned prospective clinical trial in pediatric MPAL.
METHODS
Study Population
The cohort was assembled from children, adolescents, and young adults (1 to 30 years) diagnosed by their institutions with MPAL who enrolled between 2005 and 2018 on the consecutive COG ALL classification studies AALL03B1 (NCT00482352) or AALL08B1 (NCT01142427), both of which were open to MPAL. Patients who enrolled on the AML trial AAML0531 (NCT00372593) between 2006 and 2010 and were identified as MPAL by central review at time of diagnosis were also eligible for inclusion. Patients were not eligible for treatment on COG therapeutic trials and were treated according to provider discretion and institutional best care. All cases were reviewed centrally by two authors, B.L.W. (AALL03B1/ AALL08B1) and S.B.K. (AAML0531), using the diagnostic flow cytometry results and the WHO2016 criteria. Cases in which the diagnostic workup was insufficient to establish the diagnosis of WHO2016-defined MPAL underwent additional immunophenotyping using cryopreserved specimens (T.B.A., C.G.M.). Cases were classified according to MPAL phenotype (B/Myeloid, T/Myeloid, B/T/Myeloid, B/T) and blast clonality (Biphenotypic [Bi-P, one population] or bi/trilineal [Bi-L, two or more populations]). Cytogenetic findings were centrally reviewed and classified as adverse risk, favorable risk, or neutral using ALL and AML criteria from the source studies.20–22 All COG studies were approved by the respective Institutional Review Boards and informed consent was obtained and documented prior to participant enrollment. Leukemia cells obtained from pre-treatment bone marrow aspirates and/or peripheral blood at time of trial enrollment were cryopreserved and banked centrally with consent provided for future research.
Statistical Analysis and Study Endpoints
Patient and diagnosis data were obtained from AALL03B1, AALL08B1 or AAML0531. A supplemental MPAL case report form captured additional information for this study. Treatment was classified as ALL, AML, or hybrid according to reported regimen. If only chemotherapy agents were described, ALL induction therapy was defined by use of steroid, vincristine, asparaginase, ± anthracycline, ± cyclophosphamide. AML induction therapy was defined by incorporation of an anthracycline and cytarabine. Hybrid therapy included combinations of all of the above. HSCT was classified according to donor type as a matched related donor, mismatched related donor, or matched unrelated donor. Cord blood transplants were not separately identified. Endpoints for analysis included morphologic complete remission rate at the end of induction (EOI CR), treatment-related mortality (TRM), event-free, and overall survival (EFS, OS). CR was defined as <5% blasts in the marrow without extramedullary disease as was the standard at the time for both ALL and AML. EFS was defined as the time from study enrollment to first event (relapse or remission death) or last contact. OS was the time from study enrollment to death or last contact. EFS and OS were estimated using the Kaplan-Meier method with standard errors of Peto et.al.23, 24 The log-rank test was used to compare survival curves. Cox proportional hazards regression was used to analyze the impact of starting therapy and HSCT on outcomes. Comparison of proportions between groups used the Fisher’s Exact test. All statistical tests were two-sided with significance set at a p-value <0.05. All analyses were performed using SAS software version 9.4. Graphics were generated with R Version 3.0.1.
RESULTS
Central Review
From the COG study databases, we identified 65 patients diagnosed by institutions as MPAL from 2005–2016 and with sufficient data for central review. An additional five cases initially identified by sites as lineage-specific acute leukemia (AML n=2, ALL n=3) were subsequently identified by central review to be MPAL. Following the entirety of the review process, only 54 of 70 (77%) cases were confirmed as WH02016-defined MPAL (CONSORT, Supplemental Figure 1). Even for patients diagnosed subsequent to publication of the narrower WHO2008 definition in 2009 (n=54), routine institutional flow cytometry remained insufficient to establish a diagnosis of MPAL in approximately 15% of patients.
Description of cohort
The majority of the cohort was less than ten years old at diagnosis and did not express adverse features for acute leukemia (Table 1). CNS involvement was present at diagnosis in a quarter of the cohort which is greater than expected for ALL but similar to that in AML populations. A small percentage fulfilled the WHO2016 subcategories of MPAL with KMT2A-R (4/54, 7.4%) or MPAL with t(9;22) (2/54, 3.7%). There were no significant differences between MPAL patients beginning with an ALL (72%, n=39/54) versus AML (24%, n=13/54) induction regimen. Treatment became markedly heterogeneous following induction (Table 2). Only two patients received a “hybrid” induction (Table 2) and both proceeded to HSCT. Both patients with t(9;22) MPAL were treated with tyrosine-kinase inhibitors. Patients receiving an ALL induction and continuing with ALL therapy (±HSCT) constituted the largest single treatment group in the cohort (25/54, 46.3%); all other therapy combinations each constituted <10% of the cohort. Sixteen patients underwent HSCT in 1st CR; five received a graft from a matched related donor and eleven from a matched unrelated donor or mismatched related donor. The median follow-up time for surviving patients in the cohort was 5.7 years (range 0.24–11.99 years).
Table 1:
Presenting features and initial therapy
| Characteristics of Cohort | Total Cohort | Initial Therapy† | p-value† | ||||
|---|---|---|---|---|---|---|---|
| ALL | AML | ||||||
| N | % | N | % | N | % | ||
| Overall | 54 | 100 | 39 | 75.0 | 13 | 25.0 | 0.0003 |
| Age | |||||||
| <10 years | 35 | 64.8 | 26 | 66.7 | 7 | 53.8 | 0.510 |
| ≥10 years | 19 | 35.2 | 13 | 33.3 | 6 | 46.2 | |
| Sex | |||||||
| Female | 26 | 48.1 | 21 | 53.8 | 4 | 30.8 | 0.205 |
| Male | 28 | 51.9 | 18 | 46.2 | 9 | 69.2 | |
| Presenting WBC | |||||||
| <100×103 /uL | 37 | 68.5 | 29 | 74.4 | 7 | 53.8 | 0.362 |
| ≥100×103 /uL | 8 | 14.8 | 5 | 12.8 | 3 | 23.1 | |
| Unknown | 9 | 16.7 | 5 | 12.8 | 3 | 23.1 | |
| CNS involvement at diagnosis | |||||||
| CNS negative | 30 | 55.6 | 23 | 59.0 | 6 | 46.1 | 0.748 |
| CNS positive‡ | 14 | 25.9 | 10 | 25.6 | 4 | 30.8 | |
| Unknown | 10 | 18.5 | 6 | 15.4 | 3 | 23.1 | |
| Cytogenetic abnormalities | |||||||
| Neutral | 36 | 66.7 | 26 | 66.7 | 8 | 61.5 | 0.087 |
| Favorable§ | 5 | 9.2 | 5 | 12.8 | 0 | 0.0 | |
| Unfavorable§ | 9 | 16.7 | 7 | 17.9 | 2 | 15.4 | |
| Not tested | 4 | 7.4 | 1 | 2.6 | 3 | 23.1 | |
| MPAL Phenotype | |||||||
| B/Myeloid | 33 | 61.1 | 26 | 66.7 | 7 | 53.8 | 0.517 |
| T/Myeloid | 19 | 35.2 | 11 | 28.2 | 6 | 46.2 | |
| B/T/Myeloid | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| B/T | 2 | 3.7 | 2 | 5.1 | 0 | 0.0 | |
| MPAL Lineage | |||||||
| Bi-Phenotypic | 25 | 46.3 | 19 | 48.7 | 6 | 46.2 | 0.441 |
| Bi- or Tri-Lineal | 25 | 46.3 | 18 | 46.2 | 5 | 38.5 | |
| Indeterminate | 4 | 7.4 | 2 | 5.1 | 2 | 15.4 | |
Does not include sparse data for hybrid induction (n=2/54, 3.7%). Fisher’s exact test.
Blasts present on cerebrospinal fluid cytospin or clinical involvement (e.g. cranial nerve findings).
Favorable (Trisomies 4&10 n=4, chromosomes >50, n=1), Unfavorable (KMT2A gene rearrangement n=4, BCR-ABL n=2, intrachromosomal amplification of chromosome 21 n=0, chromosomes <44 and/or DNA index <0.81 n=3, recurrent AML genetic findings n=0).
Table 2:
Heterogeneity of frontline therapy for pediatric MPAL
| Induction Therapy | Post-induction Therapy, n (%)† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL only | ALL +HSCT |
AML only | AML +HSCT |
Hybrid only | Hybrid +HSCT | HSCT only | Missing§§ | Total | |
| ALL | 21 (38.9) | 4 (7.4) | 0 (0.0) | 2 (3.7) | 4 (7.4) | 0 (0.0) | 3 (5.6) | 5 (9.3) | 39 (72.2) |
| AML | 2 (3.7) | 0 (0.0) | 4 (7.4) | 3 (5.6) | 0 (0.0) | 0 (0.0) | 2 (3.7) | 2 (3.7) | 13 (24.1) |
| Hybrid‡ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.7) | 0 (0.0) | 0 (0.0) | 2 (3.7) |
| Total | 23 (42.6) | 4 (7.4) | 4 (7.4) | 5 (9.3) | 4 (7.4) | 2 (3.7) | 5 (9.3) | 7 (13.0) | 54 (100) |
percent of total cohort.
Hybrid= components of AML + ALL therapy (n=2).
Regimen #1= prednisone, thioguanine, cytarabine, daunorubicin, vincristine, asparaginase); Regimen #2= cytarabine, daunorubicin, etoposide, prednisone, vincristine, asparaginase, ifosfamide, etoposide);
Missing= no data available.
Treatment Outcomes
Rates of morphologic EOI CR were similar for patients who received an ALL induction (71.8%, 28/39) as compared to AML (69.2%, 9/13) (p=1.00); both patients with a hybrid induction achieved CR (Table 3). Patients with MPAL expressing T-lineage (T/Myeloid, B/T) had a trend toward a lower CR rate than B/Myeloid (61.9% [13/21] versus 78.8% [26/33], p=0.074). No demographic or treatment variables present at diagnosis were associated with EOI CR.
Table 3:
Complete remission rates at end of induction by starting therapy and MPAL phenotype
|
Characteristic |
CR | No CR | p-value† | |||
|---|---|---|---|---|---|---|
| N | %† | N | %† | |||
| Starting therapy | ALL | 28 | 71.8 | 11 | 28.2 | 1.000 |
| AML | 9 | 69.2 | 4 | 30.8 | ||
| Hybrid | 2 | 100.0 | 0 | 0.0 | ||
| MPAL Phenotype‡ | B/Myeloid | 26 | 78.8 | 7 | 21.2 | 0.074 |
| T/Myeloid | 13 | 68.4 | 6 | 31.6 | ||
| B/T | 0 | 0.0 | 2 | 100.0 | ||
Fisher’s exact test. % each characteristic.
No patients in the cohort were B/T/Myeloid.
CR= complete remission (leukemia blasts <5% in the bone marrow without extramedullary disease).
Five-year EFS and OS rates for the entire cohort were 72±8% and 77±7%, respectively. For patients starting treatment with an ALL chemotherapy regimen (n=39), EFS and OS were 73±10% and 78±9%; for those beginning with AML therapy (n=13), EFS and OS were 62±14% and 69±14% (EFS p=0.626, OS p= 0.548) (Figure 1). TRM was uncommon and not significantly different between these two groups (ALL, n=2/39 [5%] versus AML, n=1/13 [8%], p=0.731). A subset of patients successfully proceeded to HSCT in CR1 per provider discretion. Five-year EFS rates for HSCT in CR1 (n=16) compared to no HSCT (n=38) were 80±12% versus 68±10 (p=0.225) and five-year OS rates were 80±12% versus 75±9% (p=0.721), respectively (Figure 2). Multivariable analysis including initial therapy (ALL versus AML) and HSCT (yes/no), showed no significant association with EFS or OS (Supplemental Table 1). EOI CR was associated with significantly greater OS but not EFS (Supplemental Figure 2). Neither MPAL phenotype nor clonality was associated with differences in EFS or OS (Figure 3, Supplemental Figure 3). For patients treated with ALL chemotherapy alone without HSCT in CR1 (n=21), EFS and OS rates were 75±13% and 84±11% (Supplemental Figure 4).
Figure 1: Association of starting therapy regimen and survival.
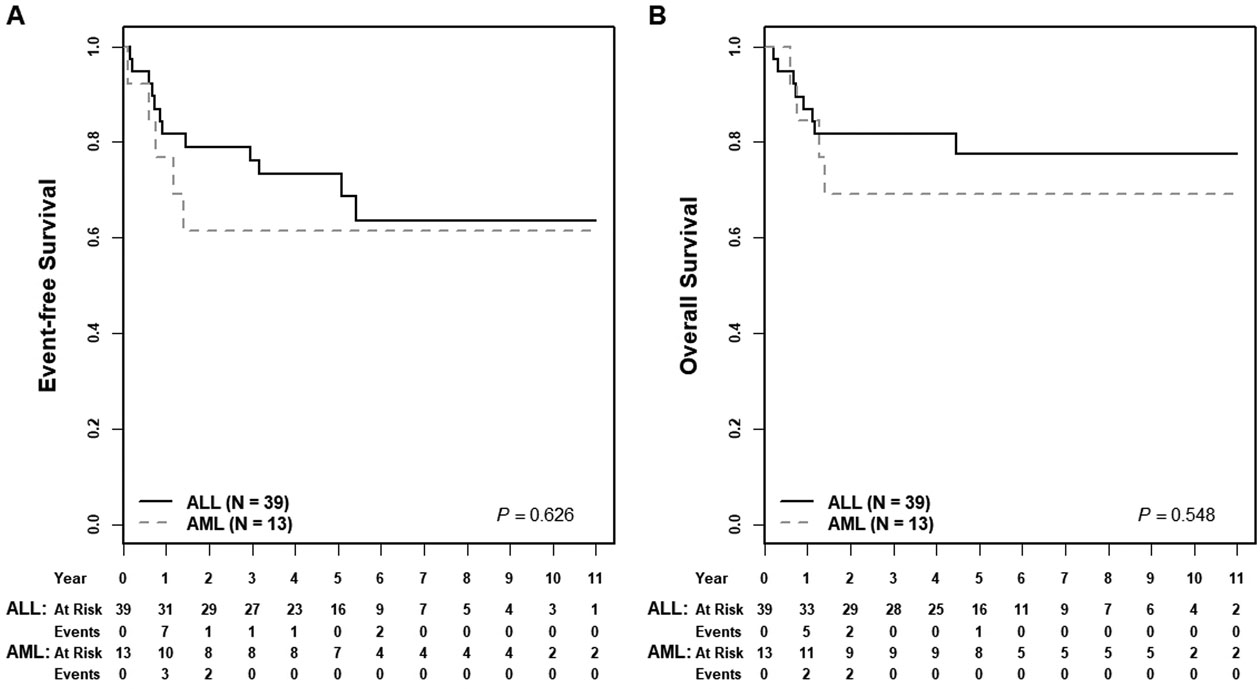
(A) Five-year EFS rates for starting therapy with an ALL compared to an AML regimen were 73±10% versus 62±14% (p=0.626); (B) five-year OS rates were 78±9% versus 69±14% (p=0.548).
Figure 2: Association of hematopoietic stem cell transplantation and survival.
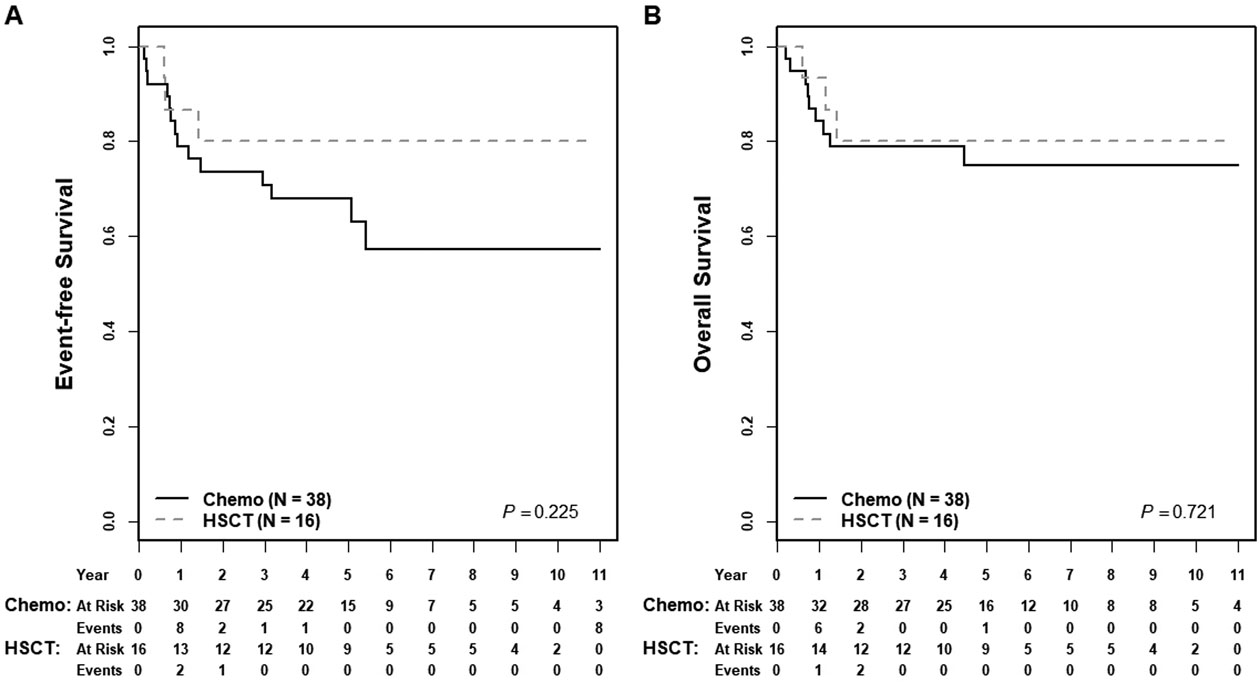
(A) Five-year EFS rates for patients receiving HSCT in CR1 compared to no HSCT were 80±12% versus 68±10% (p=0.225); (B) five-year OS rates were 80±12% versus 75±9% (p=0.721).
Figure 3: Association of MPAL phenotype and survival.
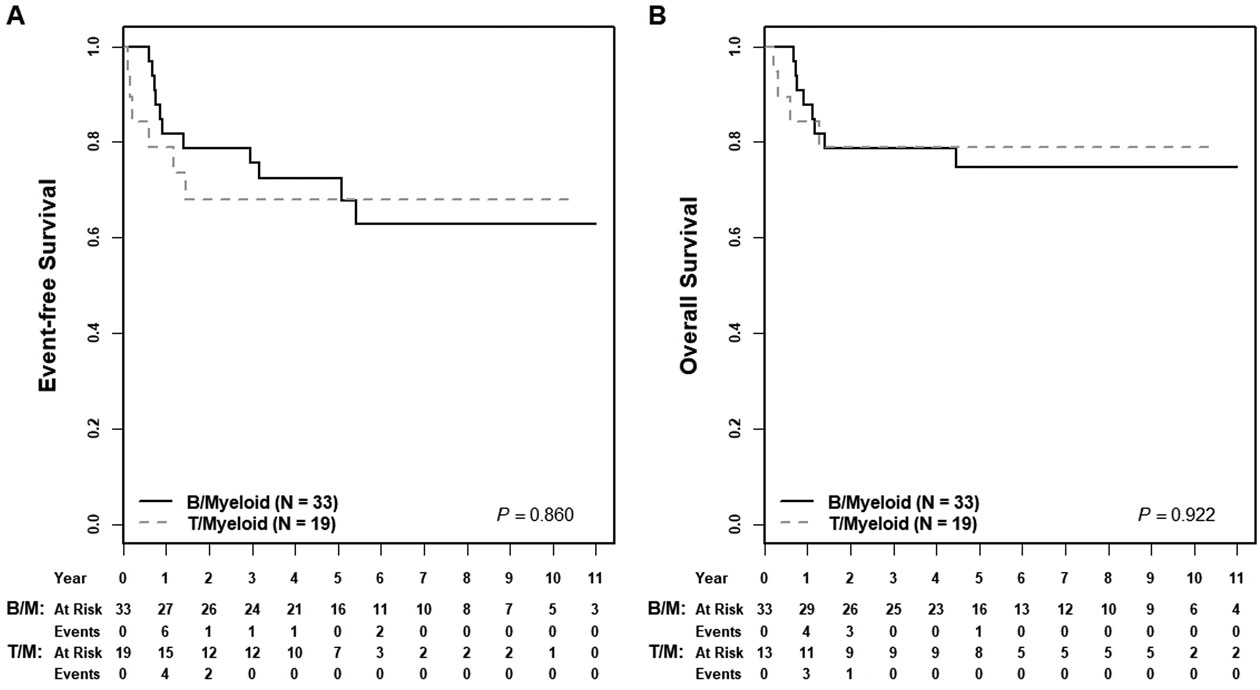
(A) Five-year EFS rates for B/Myeloid compared to T/Myeloid MPAL were 73±10% versus 68±15% (p=0.860); five-year OS rates were 75±9% versus 80±12% (p=0.922).
DISCUSSION
Due to the rarity of pediatric MPAL, reports limited to children and adolescents are sparse.4, 8–10, 13 Similar to the AMBI2012 registry,14 the strength of the cohort presented here is the use of central review of immunophenotyping, supplemented as necessary with additional characterization from residual banked samples. This ensured all cases met the WHO2016 definition without potential “contamination” by non-MPAL leukemia (i.e. ALL or AML). In the resulting cohort of strictly defined WHO2016 MPAL, irrespective of treatment approach, children achieved a 5-year OS of ~70% or greater. Survival for children with MPAL continues to exceed that of adults, where less than half are expected to survive.1, 3, 15 Although a selected cohort, this series demonstrated durable remissions are possible for a subset of MPAL receiving ALL chemotherapy without HSCT consolidation. The initial favorable response to ALL induction therapy observed in most patients supports the chemo-sensitivity of many, albeit not all, MPAL to lymphoid-directed therapy.
While adding therapy and outcome data to the limited literature for a pediatric, verified MPAL cohort using the newest WHO2016 definition is significant, we have reached the limit of treatment insights possible from retrospective studies alone. The COG will therefore prospectively study uniform, contemporary ALL therapy for pediatric patients with WHO2016-defined MPAL as a stratum on the frontline high-risk ALL trial AALL1732. Review of this retrospective COG cohort and associated published data served as the foundation for the following consensus investigative framework to answer key questions for MPAL therapy.
What are the diagnostic considerations for trial eligibility in MPAL?
The primary options for MPAL classification remain the WHO and EGIL criteria; these two systems result in overlapping but distinct populations.16, 18, 25 The EGIL system is considered easier to apply in a standardized fashion as the WHO 2008 and 2016 classifications incorporate the concept of subjective “strength” of antigen positivity (negative, low, strong) to delineate lineage specificity.19, 25 Inherent variability within this approach is evident from the rate of review failure in the COG cohort as well as in recent MPAL reports.11, 12, 18 However, the WHO definition is widely accepted internationally and its reliance on strongly-expressed antigens specific to lineage differentiation is considered most likely to delineate a “true” MPAL population.25 In consideration for trial eligibility, the Task Force therefore supported implementing a strict interpretation of the WHO MPAL classification albeit with mandatory integrated central review. Prospective trials in MPAL should aim to capture comprehensive data at diagnosis for morphology, immunophenotype, and immunohistochemistry to enable continued refinement of the WHO criteria.
Understanding the phenotypic heterogeneity of MPAL in the context of underlying biology will advance disease classification and potentially refine therapy selection. Recent studies exploring the biology of MPAL demonstrated overlap with lineage-specific leukemia, such as T/Myeloid MPAL with ETP, B/Myeloid with ZNF384-driven B-ALL, and a high prevalence of “ALL-like” epigenetic signatures.26, 27 The COG will prospectively explore MPAL genomics and immunophenotyping in the context of uniform ALL therapy as a step toward the overarching goal for biology-directed MPAL classification and treatment.
What is the optimal chemotherapy approach to pediatric MPAL?
The COG MPAL cohort described here, the iBFM AMBI2012 registry study,14 and the few pediatric case series for MPAL3, 9–11 all suggest beginning with ALL therapy results in similar or greater OS than AML regimens. A recent meta-analysis of MPAL patients also reported a survival benefit for starting with ALL therapy, although patient-level data revealed equivalent OS with AML therapy.1 To avoid the acute and long-term morbidity associated with AML treatment, the Task Force therefore recommended selecting ALL-directed chemotherapy regimens as starting therapy within the context of a clinical trial for de novo pediatric MPAL. The overlapping biology of many MPALs with ALL as described above also suggests potential sensitivity to ALL therapy.26 The MPAL stratum on the COG frontline ALL trial AALL1732 will test this strategy using COG “high risk” ALL therapy from time of diagnosis for all MPAL patients irrespective of phenotype or blast populations. This notably differs from the iBFM consortium proposal to restrict ALL therapy to the subset of MPAL with favorable outcomes from the AMBI2012 registry.14 Once a regimen backbone and expected EFS/OS are established, holistic examination of available international data will potentially support future collaborative trials to refine therapy selection and/or risk stratification.
What is the role for HSCT in the treatment of MPAL?
Several adult MPAL cohorts support a potential benefit for HSCT in first CR with poor survival from chemotherapy alone.5,8,28,29 HSCT in first remission for adult MPAL patients is therefore recommended.7 In contrast, multiple pediatric studies suggest no benefit for HSCT in CR1, particularly in those with favorable features.4,8,9,13 In our study, no clear benefit was evident for OS from HSCT in CR1. A subset of patients with early favorable responses to an ALL induction who continued with ALL chemotherapy without HSCT in CR1 achieved a 5-year OS of >80%. However, for patients with refractory disease where chemotherapy alone is insufficient, HSCT may be beneficial. In the AMBI2012 registry, there was similarly no significant benefit for HSCT in CR1 overall, but a trend for improved EFS from HSCT was present in those with EOI MRD ≥5%.14 Patients proceeding to HSCT should be at least in morphologic CR prior to HSCT.28 Recent data suggest irradiation-based myeloablative HSCT conditioning regimens likely offer a survival advantage over other preparatory regimens and should be considered where age-appropriate.29 Contrary to recommendations for adult MPAL, the Task Force therefore recommends reserving HSCT consolidation only for patients poorly responsive to ALL therapy who achieve at least a morphologic CR prior to HSCT. Future studies are required to assess the impact of pre-HSCT MRD on post-transplant survival.
What is the role for MRD to identify early treatment failure?
In our series, approximately one in four patients was eventually failed by ALL therapy. MRD offers potential to identify these patients early in their course. Interpretation of MRD is complicated by MPAL’s variable phenotype and potential sub-clonal emergence from selection pressure in the context of ALL therapy. Both the AMBI2012 registry and a separate multi-institution pediatric series utilized a central review process14, 30 and demonstrated excellent OS of ~90% in patients with pediatric MPAL treated with ALL therapy and achieving MRD <0.01% prior to the end of consolidation (EOC). In the AMBI2012 registry, EOI MRD ≥5% was also associated with a particularly poor 5-year EFS of <50%.14 The Task Force therefore recommended that only patients with EOI MRD <5% and EOC MRD <0.01% continue with ALL therapy; survival for patients meeting these criteria will be monitored carefully to assess adequacy of ALL chemotherapy alone. Patients who exceed these thresholds may benefit from early intensification of therapy with AML chemotherapy and/or HSCT consolidation at the discretion of the treating provider. Survival for those with MPAL, KMT2A-R or MPAL, t(9;22) require additional scrutiny in future trials to better integrate MPAL biology and MRD stratification. The Task Force also acknowledged the possibility, that akin to T-ALL or ETP,31, 32 patients with T/Myeloid or B/T MPAL with EOI MRD ≥5% may yet respond to ALL therapy. Data for this small subset was too sparse to justify an exception on the clinical trial to continue with ALL therapy and requires further study. Thus, the COG trial will treat all MPAL uniformly with ALL therapy and incorporate MRD to identify treatment failure.
Conclusion
Addressing the knowledge gap for pediatric MPAL therapy will require prospective clinical trials. The upcoming COG high-risk ALL trial AALL1732 will implement the above investigative framework to provide MRD-stratified, uniform ALL therapy without HSCT consolidation to a strictly-diagnosed de novo MPAL cohort. Incorporation of embedded studies for immunophenotyping, genomics, and correlative biology will improve classification and lead to innovative treatment strategies. The consensus investigative plan and proposed trial will establish a treatment baseline to support rational, stepwise investigation into new approaches for diagnosis and/or therapy to improve outcomes for children with this orphan disease.
Supplementary Material
Funding & Acknowledgment:
Supported by the Children’s Oncology Group via the National Cancer Institute (NCI) awards U10 CA98543, U10 CA98413, U10 CA180886, U24 CA114766, U24 CA196173 and U10 CA180899, and an NCI outstanding Investigator Award R35-CA197695 (to C.G.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was received from the St. Baldrick’s Foundation, The Henry Schueler 41&9 Foundation (to C.G.M.), and Cookies 4 Kids (to H.I.). MLL is the UCSF Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. EAR is a KiDS of NYU Foundation Professor at NYU Langone Health. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia. C.G.M. is the William E. Evans Endowed Chair at St. Jude Children’s Research Hospital.
Footnotes
Potential conflicts of interest: SPH (Amgen [stock ownership], Merck [stock ownership], Novartis [consulting fees]. PAZM (ImmunoGen [employment, stock ownership]). The other authors report no potential competing interests.
References
- 1.Maruffi M, Sposto R, Oberley MJ, Kysh L, Orgel E. Therapy for children and adults with mixed phenotype acute leukemia: a systematic review and meta-analysis. Leukemia. 2018;32: 1515–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg OK, Arber DA. Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia. 2010;24: 1844–1851. [DOI] [PubMed] [Google Scholar]
- 3.Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117: 3163–3171. [DOI] [PubMed] [Google Scholar]
- 4.Park JA, Ghim TT, Bae K, et al. Stem cell transplant in the treatment of childhood biphenotypic acute leukemia. Pediatr Blood Cancer. 2009;53: 444–452. [DOI] [PubMed] [Google Scholar]
- 5.Tian H, Xu Y, Liu L, et al. Comparison of outcomes in mixed phenotype acute leukemia patients treated with chemotherapy and stem cell transplantation versus chemotherapy alone. Leuk Res. 2016;45: 40–46. [DOI] [PubMed] [Google Scholar]
- 6.Munker R, Brazauskas R, Wang HL, et al. Allogeneic Hematopoietic Cell Transplantation for Patients with Mixed Phenotype Acute Leukemia. Biol Blood Marrow Transplant. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125: 2477–2485. [DOI] [PubMed] [Google Scholar]
- 8.Gerr H, Zimmermann M, Schrappe M, et al. Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol. 2010;149: 84–92. [DOI] [PubMed] [Google Scholar]
- 9.Al-Seraihy AS, Owaidah TM, Ayas M, et al. Clinical characteristics and outcome of children with biphenotypic acute leukemia. Haematologica. 2009;94: 1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachir F, Zerrouk J, Howard SC, et al. Outcomes in patients with mixed phenotype acute leukemia in Morocco. J Pediatr Hematol Oncol. 2014;36: e392–397. [DOI] [PubMed] [Google Scholar]
- 11.Oberley MJ, Li S, Orgel E, Phei Wee C, Hagiya A, O’Gorman MRG. Clinical Significance of Isolated Myeloperoxidase Expression in Pediatric B-Lymphoblastic Leukemia. Am J Clin Pathol. 2017;147: 374–381. [DOI] [PubMed] [Google Scholar]
- 12.Raikar SS, Park SI, Leong T, et al. Isolated myeloperoxidase expression in pediatric B/myeloid mixed phenotype acute leukemia is linked with better survival. Blood. 2018;131: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113: 5083–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hrusak O, de Haas V, Stancikova J, et al. International cooperative study identifies treatment strategy in childhood ambiguous lineage leukemia. Blood. 2018;132: 264–276. [DOI] [PubMed] [Google Scholar]
- 15.Shi R, Munker R. Survival of patients with mixed phenotype acute leukemias: A large population-based study. Leuk Res. 2015;39: 606–616. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg OK, Seetharam M, Ren L, Alizadeh A, Arber DA. Mixed phenotype acute leukemia: A study of 61 cases using World Health Organization and European Group for the Immunological Classification of Leukaemias criteria. Am J Clin Pathol. 2014;142: 803–808. [DOI] [PubMed] [Google Scholar]
- 17.Pomerantz A, Rodriguez-Rodriguez S, Demichelis-Gomez R, et al. Mixed-phenotype acute leukemia: suboptimal treatment when the 2008/2016 WHO classification is used. Blood Res. 2016;51: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Ancker W, Terwijn M, Westers TM, et al. Acute leukemias of ambiguous lineage: diagnostic consequences of the WHO2008 classification. Leukemia. 2010;24: 1392–1396. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute: Risk-Group Classification of Patients With Newly Diagnosed Acute Lymphoblastic Leukemia (COG-AALL03B1). Availabkle online https://clinicaltrials.gov/show/NCT00482352 (Last Accessed March 3, 2017).
- 21.National Cancer Institute: Risk-Based Classification System of Patients With Newly Diagnosed Acute Lymphoblastic Leukemia (COG-AALL08B1). Available online https://clinicaltrials.gov/show/NCT01142427 (Last accessed March 3, 2017).
- 22.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab Ozogamicin in Children and Adolescents With De Novo Acute Myeloid Leukemia Improves Event-Free Survival by Reducing Relapse Risk: Results From the Randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2014;32: 3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53: 457–481. [Google Scholar]
- 24.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dworzak MN, Buldini B, Gaipa G, et al. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94: 82–93. [DOI] [PubMed] [Google Scholar]
- 26.Alexander TB, Gu Z, Iacobucci I, et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature. 2018;562: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Wang F, Morita K, et al. Integrative genomic analysis of adult mixed phenotype acute leukemia delineates lineage associated molecular subtypes. Nature Communications. 2018;9: 2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu H, Saitoh T, Machida S, et al. Allogeneic hematopoietic stem cell transplantation for adult patients with mixed phenotype acute leukemia: results of a matched-pair analysis. Eur J Haematol. 2015;95: 455–460. [DOI] [PubMed] [Google Scholar]
- 29.Munker R, Labopin M, Esteve J, Schmid C, Mohty M, Nagler A. Mixed phenotype acute leukemia: outcomes with allogeneic stem cell transplantation. A retrospective study from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017;102: 2134–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberley MJ, Raikar SS, Malvar J, et al. Minimal Residual Disease Risk-Stratification in Pediatric Mixed Phenotype Acute Leukemia: Results of a Multi-Center Cohort Study. Blood. 2018;132: 558–558. [Google Scholar]
- 31.Parekh C, Gaynon PS, Abdel-Azim H. End of induction minimal residual disease alone is not a useful determinant for risk stratified therapy in pediatric T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62: 2040–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118: 2077–2084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


