Dear Editor,
Shah et al. observed an awfully high prevalence (53.5%) of pulmonary embolism (PE) among 30 intensive care unit (ICU) patients with coronavirus disease 2019 (COVID-19) in Oxford, UK [1]. Although several studies have focused on this cardiovascular complication of PE in COVID-19 patients, the prevalence of PE varies from study to study [2–4]. Therefore, we explored the pooled prevalence of PE in COVID-19 patients by a quantitative meta-analysis. Details of our study are shown in Supplementary file 1.
PubMed, EMBASE and Web of Science were reviewed up to August 12th, 2020 to identify relevant studies. Studies reporting the prevalence of confirmed PE in COVID-19 patients and with the sample size ≥ 30 were included. The pooled prevalence and corresponding 95% confidence interval (CI) were used to assess the combined effects. An additional analysis comparing the prevalence of PE in COVID-19 patients admitted to ICU and non-ICU was conducted. Heterogeneity between studies was estimated with I2 statistic and Cochran’s Q (reported as χ2 and P values) [5]. Subgroup analysis and meta-regression analysis were conducted by country, study design, sample size, quality score, PE diagnosis and prevalence of prophylactic anticoagulation to explore possible sources of heterogeneity.
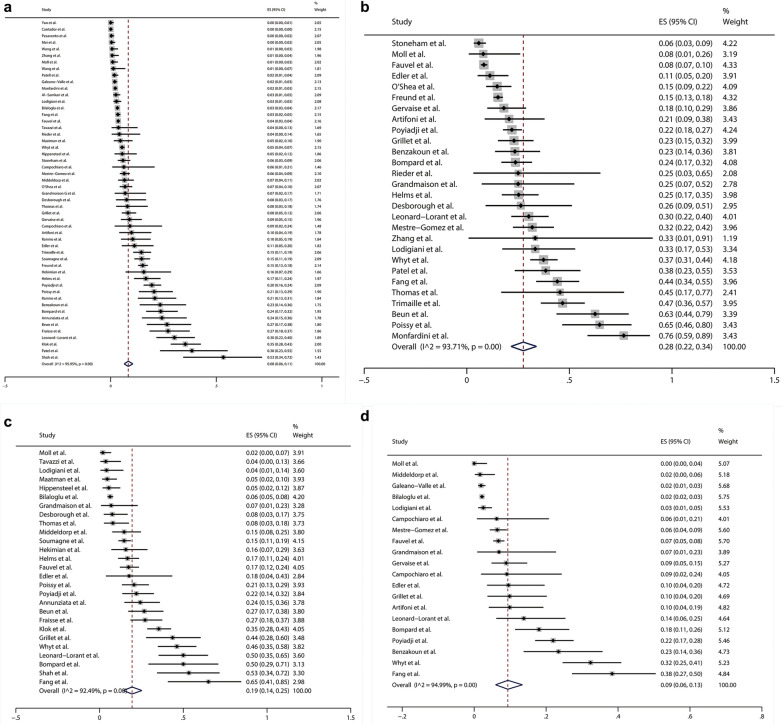
Among 1981 potentially related studies, 49 articles (52 studies) comprising 20,523 COVID-19 patients were enrolled in this meta-analysis after rigorous screening (Suppl. File 2, Fig. S1). The principal characteristics and details about the PE diagnosis of the included studies were shown in Supplementary file 2, Table S4 and Table S5. The pooled prevalence of PE in COVID-19 patients was 8% (95% CI 6–11%; χ2 = 1259.68, P < 0.01; I2 = 95.95%; random-effects model) (Fig. 1a). Due to the obvious heterogeneity, we performed subgroup analysis and meta-regression. None of these factors we explored further was significantly correlated with the inter-study heterogeneity on subgroup analysis (Suppl. File 2, Table S6). However, the results of meta-regression indicated that sample size (P = 0.019) and the proportion of patients undergoing PE diagnosis (P < 0.001) might be potential sources of heterogeneity (Suppl. File 2, Table S6). The pooled prevalence of PE in patients undergoing PE diagnosis was 28% (95% CI 22–34%; χ2 = 429.11, P < 0.01; I2 = 93.71%; random-effects model) on the basis of 28 studies consisting of 4387 patients undergoing PE diagnosis (Fig. 1b). The significantly higher pooled prevalence of PE was observed in COVID-19 patients admitted to ICU (19%, 95% CI 14–25%; χ2 = 346.07, P < 0.01; I2 = 92.49%) compared with those admitted to non-ICU (9%, 95% CI 6–13%; χ2 = 379.37, P < 0.01; I2 = 94.99%) (Fig. 1c, d). The Begg’s test (P = 0.002) and Egger’s test (P < 0.001) suggested that potential publication bias existed within our analysis.
Fig. 1.
a Forest plot of the pooled prevalence and its 95% confidence interval (CI) for pulmonary embolism (PE) among coronavirus disease 2019 (COVID-19) patients showed that there was a relatively high prevalence of PE (8%, 95% CI 6–11%; χ2 = 1259.68, P < 0.01; I2 = 95.95%) in COVID-19 patients; b Forest plot of the pooled prevalence and its 95% CI for PE among the patients undergoing PE diagnosis showed that the pooled prevalence of PE in patients undergoing PE diagnosis was as high as 28% (95% CI 22–34%; χ2 = 429.11, P < 0.01; I2 = 93.71%); c The pooled prevalence of PE (19%, 95% CI 14–25%; χ2 = 346.07, P < 0.01; I2 = 92.49%) in COVID-19 patients admitted to intensive care unit (ICU) was significantly higher than (d) The pooled prevalence of PE (9%, 95% CI 6–13%; χ2 = 379.37, P < 0.01; I2 = 94.99%) in COVID-19 patients admitted to non-ICU
In summary, it is needed to pay more attention to the relatively high prevalence of PE in COVID-19 patients, especially in ICU wards. Future studies that will explore the detection method considering high infectivity of COVID-19 and antithrombotic treatment balancing the risk of thrombosis and the risk of bleeding are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Ying Wang, Xuan Liang, Wenwei Xiao, Peihua Zhang and Jian Wu (All are from Department of Epidemiology, College of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis.
Author contributions
LS, HY, and YW conceptualized the study. LS and JX extracted the data. LS, JX, and YW analyzed the data. LS, GD, HY, and YW contributed to the methodology. LS, HY, and YW wrote and reviewed the manuscript. All the authors approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Number 81973105), the National Science and Technology Major Projects of China (Grant Number 2018ZX10301407) and Joint Construction Project of Henan Medical Science and Technology Research Plan (Grant Number LHGJ20190679). The funders have no role in the data collection, data analysis, preparation of manuscript and decision to submission.
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no any potential conflict of interest regarding this submitted manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haiyan Yang, Email: yhy@zzu.edu.cn.
Yadong Wang, Email: wangyd76@163.com.
References
- 1.Shah A, Frost JN, Aaron L, Donovan K, Drakesmith H, Collaborators Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24:320. doi: 10.1186/s13054-020-03051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pesavento R, Ceccato D, Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non-critically ill patients with Covid-19: the Padua province experience. J Thromb Haemost. 2020 doi: 10.1111/jth.15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hekimian G, Lebreton G, Brechot N, Luyt CE, Schmidt M, Combes A. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020;24:274. doi: 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang C, Garzillo G, Batohi B, et al. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: relationship with pulmonary parenchymal disease. Clin Radiol. 2020 doi: 10.1016/j.crad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.