Abstract
Background:
There is inconsistency in the literature regarding the clinical effects of proton pump inhibitors (PPI) when added to dual antiplatelet therapy (DAPT) in subjects with coronary artery disease (CAD). We performed meta-analysis stratified by study design to explore these differences.
Methods and results:
39 studies [4 randomized controlled trials (RCTs) and 35 observational studies) were selected using MEDLINE, EMBASE and CENTRAL (Inception-January 2018). In 221,204 patients (PPI = 77,731 patients, no PPI = 143,473 patients), RCTs restricted analysis showed that PPI did not increase the risk of all-cause mortality (Risk Ratio (RR): 1.35, 95% Confidence Interval (CI), 0.56–3.23, P = 0.50, I2 = 0), cardiovascular mortality (RR: 0.94, 95% CI, 0.25–3.54, P = 0.92, I2 = 56), myocardial infarction (MI) (RR: 0.97, 95% CI, 0.62–1.51, P = 0.88, I2 = 0) or stroke (RR: 1.11, 95% CI, 0.25–5.04, P = 0.89, I2 = 26). However, PPI significantly reduced the risk of gastrointestinal (GI) bleeding (RR: 0.32, 95% CI, 0.20–0.52, P < 0.001, I2 = 0). Conversely, analysis of observational studies showed that PPI significantly increased the risk of all-cause mortality (RR: 1.25, 95% CI, 1.11–1.41, P < 0.001, I2 = 82), cardiovascular mortality (RR: 1.25,95% CI, 1.03–1.52, P = 0.02, I2 = 71), MI (RR: 1.30, 95% CI, 1.16–1.47, P < 0.001, I2 = 82) and stroke (RR: 1.60, 95% CI, 1.43–1.78, P < 0.001, I2 = 0), without reducing GI bleeding (RR: 0.74, 95% CI, 0.45–1.22, P = 0.24, I2 = 79).
Conclusion:
Meta-analysis of RCTs endorsed the use of PPI with DAPT for reducing GI bleeding without worsening cardiovascular outcomes. These findings oppose the negative observational data regarding effects of PPI with DAPT.
Keywords: Proton pump inhibitors, Dual antiplatelet therapy, Coronary artery disease, Meta- analysis
1. Introduction
Dual antiplatelet therapy (DAPT) reduces the risk of adverse cardiovascular events in patients with coronary artery disease (CAD) [1,2]. However, the addition of P2Y12 inhibitor to aspirin is associated with increased risk of significant bleeding [3,4]. The European Society of Cardiology (ESC) guidelines endorse PPI prescription (class I, Level: B) with DAPT for all CAD patients [5], whereas, the 2016 American College of Cardiology/American Heart Association focused update recommend the concomitant use of proton pump inhibitors (PPI) with DAPT in the following patients: (a) prior history of GI bleeding (Class I) and (b) higher risk of GI bleeding (i.e. advanced age, concomitant use of warfarin, steroids or non-steroidal inflammatory drugs (Class II a). The routine use of PPIs is not recommended for patients at low risk of GI bleeding (Class III: No Benefit) [6]. While there is paucity of randomized controlled trials (RCTs) on this subject, various meta-analyses and observational studies showed drug interaction and adverse cardiovascular outcomes with co-administration of PPI and DAPT [7-9]. Furthermore, these studies were also inconsistent regarding the protective effects of PPI on GI bleeding. We performed meta-analysis stratified according to study design to explore these clinical differences among RCTs and observational studies regarding use of PPI with DAPT in CAD.
2. Methods
Current meta-analysis was conducted and reported according to Cochrane Collaboration guidelines [10] and Preferred Reporting Item for Systematic Reviews and Meta-Analyses [11].
2.1. Data sources and searches
Electronic search was carried out by two authors (ANL and HR) using MEDLINE (Ovid SP, PubMed), EMBASE and CENTRAL data bases (Inception-January 2018). Following key search terms were used: “proton pump inhibitor” OR “omeprazole” OR “pantoprazole” OR “lansoprazole” OR “esomeprazole” OR “rabeprazole” OR “PPI” OR “dual antiplatelet therapy” OR “DAPT” OR “clopidogrel” AND “Acute coronary syndrome” OR “ACS” OR “percutaneous coronary intervention”. There was no restriction on article types, language, sample size, publication dates, co-morbidities or follow up duration. We also reviewed references contained in the relevant articles. All the citations were downloaded into End note X7 (Thompson ISI ResearchSoft, Philadelphia, Pennsylvania, USA) and duplicates were removed electronically and manually.
2.2. Study selection
Two authors (ANL and HR) screened the search results in a two steps process. Citations were screened at title and abstract level followed by full text screening based on prespecified inclusion criteria: [1] studies comparing PPI versus no PPI in patients with CAD receiving DAPT, [2] studies reporting at least one event for outcomes of interest in adult population (age ≥ 18 years) and [3] Full text articles. Studies were excluded if interaction of PPI was studied with single antiplatelet agent only or if DAPT was used for any other indication such as peripheral vascular disease or stroke.
2.3. Data extraction
Data abstraction was done by two authors (MSK and ANL) on study design, baseline characteristics of the participants, medical therapy, events, non-events, sample size and follow up duration on Microsoft Excel spreadsheets (Microsoft Corporation, Redmond, WA, USA). When available, data was extracted for intention to treat analysis. When possible, standard adjusted estimates were collected. Quality assessment of RCTs was appraised by Cochrane bias assessment tool [12](Supplement Table 1); while observational studies were evaluated using New-Castle Ottawa Scale [13]. We assessed eight domains in New-Castle Ottawa Scale and score of 6/8 was consistent with good quality data (Supplement Table 2).
2.4. Outcome measures
The primary outcome was all-cause mortality. The secondary outcomes were cardiovascular mortality, myocardial infarction(MI), stroke, and GI bleeding events. We used the definitions as reported in the included studies.
2.5. Statistical analysis
Meta-analysis was stratified according to study design (RCT and observational studies). Estimates were pooled using generic invariance weighted random effects model. Statistical heterogeneity was checked by Q statistics and quantified via I2 with value ≥75% was consistent with high degree of heterogeneity [14]. Outcomes were calculated as risk ratio (RR) and risk difference (RD) with 95% confidence interval (CI). Since both summary measures account for same data, forest plots are generated for RRs only. However, RDs are provided in Supplement Table 3. All analyses were conducted at 5% significance. Publication bias was assessed using Egger's regression test [15]. Comprehensive Meta-analysis software version 3.0 (Biostat, Englewood, NJ) was used for all the analyses.
3. Results
Initial search yielded 38,725 citations, 21,480 were duplicates and 15,830 were excluded at titles and abstract level screening and 1376 articles were removed based on prior inclusion/exclusion criteria. Ultimately, 39 studies (4 RCTs and 35 observational studies) were selected (Fig. 1). 15 studies recruited patients with acute coronary syndrome (ACS) while 24 studies had participants with mixed presentation (stable CAD and ACS). The pooled mean age was 65 ± 3 years, 72% were males, 25% had prior MI, 69% had hypertension and 33% had diabetes mellitus. Except for two studies [16,17], all studies used Clopidogrel as P2Y12 inhibitor. Whereas, different PPIs were used across all the studies. The pooled average follow-up duration was 15 months (Table 1).
Fig. 1.
Search strategy according to preferred reporting items of systematic reviews and meta-analyses.
Table 1.
baseline characteristics of the studies and participants.
| Studies (year/design) | Setting | Groups | N | Age (years) | Men (%) | Type of P2Y12 inhibitors |
Type of PPI | Prior MI (%) | DM (%) | HTN (%) | Smoking (%) | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brada [30] (2008/OS) | ACS | PPI | 705 | 64.3 ± 13.0 | 75 | Clopidogrel | NR | 59 | 31 | 53 | 58 | In-hospital |
| No PPI | 318 | 62.6 ± 11.4 | 79 | 27 | 34 | 61 | 62 | |||||
| Gao [25] (2009/RCT) | ACS | PPI | 114 | 58.2 ± 8.7 | NR | NR | Omeprazole | NR | NR | NR | NR | 0.5 |
| No PPI | 123 | 57.5 ± 9.2 | NR | NR | NR | NR | NR | |||||
| Ho [19](2009/OS) | ACS | PPI | 5244 | 67.7 ± 11.4 | 98.4 | Clopidogrel | Omeprazole, Rabeprazole | 26.4 | 45.5 | NR | NR | 17.3 |
| No PPI | 2961 | 65.7 ± 11.7 | 98.9 | 20.1 | 38 | NR | NR | |||||
| O'Donoghue (PRINCIPLE-TIMI 44/TRITON-TIMI 38) [17](2009/OS) | Mixed | PPI | 28/2257 | 63.1/62 | 67.9/70.3 | Clopidogrel | Pantoprazole, Omeprazole, Esomeprazole, Lansoprazole, Rabeprazole | 39.3/17.2 | 39.3/24.2 | 78.6/65.9 | 32.1/36.7 | 6 |
| No PPI | 71/4538 | 64.1/60 | 71.3/74.7 | 23.9/18.1 | 23.9/22.5 | 77.5/63.5 | 9.9/38.7 | |||||
| PPI | 25/2272 | 61.8/61 | 72.0/73.0 | Prasugrel | 52.0/17.6 | 52.0/23.5 | 92.0/64.6 | 24.0/38.5 | ||||
| No PPI | 77/4541 | 64.7/60 | 71.4/76.0 | 23.4/18.2 | 23.4/23.0 | 83.1/63.9 | 15.6/38.3 | |||||
| Bhatt [27] (2010/RCT) | Mixed | PPI | 1876 | 68.5 | 66.9 | Clopidogrel | Omeprazole | 30.5 | 31.7 | 80.1 | 12.5 | 3.5 |
| No PPI | 1885 | 68.7 | 69.5 | 28.5 | 28.6 | 81.4 | 14.1 | |||||
| Charlot [31](2010/OS) | ACS | PPI | 6753 | 67.5 ± 12.5 | 61.8 | Clopidogrel | Pantoprazole, Lansoprazole, Omeprazole, Esomeprazole, Rabeprazole | NR | 5.8 | NR | NR | 12 |
| No PPI | 17,949 | 64.1 ± 12.5 | 71.3 | NR | 3.7 | NR | NR | |||||
| Evanchan [32] (2010/OS) | ACS | PPI | 1369 | 63.5 | NR | Clopidogrel | Esomeprazole, Pantoprazole, Omeprazole, Lansoprazole | - | 46 | 61 | NR | 12 |
| No PPI | 4425 | 62.9 | NR | - | 36 | 64 | NR | |||||
| Gaglia [33 ] (2010/OS) | Mixed | PPI | 318 | 63.8 ± 11.6 | 61.9 | Clopidogrel | Esomeprazole, Omeprazole, Lansoprazole, Rabeprazole | 28.1 | 36.3 | 78.5 | 13.8 | 12 |
| No PPI | 502 | 63.7 ± 11.6 | 64.1 | 23.7 | 33.1 | 74.8 | 18.9 | |||||
| Gaspar [34] (2010/OS) | ACS | PPI | 274 | 65 ± 13 | 73.7 | Clopidogrel | Lansoprazole Omeprazole, Rabeprazole | 20.1 | 25.5 | 67.5 | 34.7 | 6 |
| No PPI | 528 | 61 ± 13 | 76.7 | 20.1 | 27.1 | 61.4 | 43 | |||||
| Gupta [18] (2010/OS) | Mixed | PPI | 72 | 61.7 ± 1.2 | NR | Clopidogrel | Rabeprazole, Omeprazole, Lansoprazole | NR | 36 | 76 | 25 | 50 |
| No PPI | 243 | 62.0 ± 0.7 | NR | NR | 30 | 68 | 33 | |||||
| Huzdik [35] (2010/OS) | Mixed | PPI | 18 | 62.8 ± 9.4 | 83.3 | Clopidogrel | Omeprazole | 76.5 | 44.4 | 72.2 | NR | 12 |
| No PPI | 20 | 60.5 ± 11.8 | 65 | 70 | 30 | 70 | NR | |||||
| Kreutz [36] (2010/OS) | Mixed | PPI | 6828 | 67.5 ± 10.4 | 62 | Clopidgrel | Esomeprazole, Omeprazole, Pantoprazole, Lansoprazole | NR | 25.9 | 50.6 | NR | 12 |
| No PPI | 9862 | 65.2 ± 10.6 | 73.9 | NR | 22.7 | 46.5 | NR | |||||
| Ray [37] (2010/OS) | Mixed | PPI | 7593 | 60.8 ± 11.3 | 45.6 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole, Rabeprazole | NR | NR | NR | NR | 12 |
| No PPI | 13,003 | 60.4 ± 11.2 | 53.1 | NR | NR | NR | NR | |||||
| Sarasoff [38] (2010/OS) | Mixed | PPI | 698 | 68.7 ± 11.1 | 62.9 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole, Rabeprazole | 36.1 | 28.8 | 62.9 | 16.5 | 1 |
| No PPI | 2640 | 66.3 ± 10.8 | 66.1 | 29.5 | 25 | 66.1 | 17.2 | |||||
| Tentzeris [39] (2010/OS) | Mixed | PPI | 691 | 64.1 ± 12.4 | 65.4 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole, Rabeprazole | 18.7 | 18.7 | 73.7 | 27.9 | 7.8 |
| No PPI | 519 | 64.4 ± 11.9 | 72.6 | 19.7 | 26 | 78.2 | 23.1 | |||||
| Yasu [40] (2010/OS) | Mixed | PPI | 103 | 69.0 ± 9.6 | 67 | Clopidogrel | Rabeprazole | 32 | 35 | 64.1 | 24.3 | 13 |
| No PPI | 188 | 67.4 ± 10.1 | 72.4 | 17.6 | 39.7 | 64.8 | 27.1 | |||||
| Ziaris [41] (2010/OS) | Mixed | PPI | 340 | 62.1 ± 10.5 | 82.4 | Clopidogrel | Omeprazole | 17.1 | 30 | 50.9 | 49.7 | 12 |
| No PPI | 248 | 61.7 ± 10.8 | 81.9 | 17.7 | 26.2 | 46.4 | 50.8 | |||||
| Banerjee [42] (2011/OS) | ACS | PPI | 867 | 64.5 ± 10.3 | 98.2 | Clopidogrel | NR | 24.9 | 51.4 | 92.4 | 40 | 72 |
| No PPI | 3678 | 63.8 ± 9.9 | 98.3 | 17.6 | 45.1 | 88.9 | 39.5 | |||||
| Yi-hong [43] (2011/RCT) | ACS | PPI | 86 | 62.1 ± 10.6 | 72.1 | Clopidogrel | Omeprazole | NR | NR | NR | NR | 1 |
| No PPI | 86 | 61.8 ± 11.2 | 73.3 | NR | NR | NR | NR | |||||
| Harjai [44] (2011/OS) | Mixed | PPI | 751 | 66 ± 11 | 62 | Clopidogrel | Omeprazole, Esomeprazole | 22 | 30 | 73 | 21 | 6 |
| No PPI | 1902 | 64 ± 12 | 72 | 21 | 27 | 65 | 26 | |||||
| Nakayama [45] (2011/OS) | Mixed | PPI | 280 | 68.4 ± 9.9 | 79 | Clopidogrel | Lansoprazole Omeprazole, Rabeprazole | 16 | 37 | 89 | 65 | 30 |
| No PPI | 284 | 66.9 ± 9.9 | 75 | 21 | 45 | 89 | 61 | |||||
| Rossini [46] (2011/OS) | Mixed | PPI | 1158 | 64 ± 11 | 75.6 | Clopidogrel | Lansoprazole Omeprazole, Pantoprazole | 24.9 | 27.1 | 63.6 | 49.3 | 12 |
| No PPI | 170 | 63 ± 11 | 81.2 | 29 | 28 | 65.2 | 49.7 | |||||
| Simon [47] (2011/OS) | ACS | PPI | 1453 | 64 ± 13 | 73 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole | 10 | 30 | 52 | 35 | 12 |
| No PPI | 900 | 65 ± 14 | 72 | 14 | 35 | 53 | 33 | |||||
| Aihara [48] (2012/OS) | Mixed | PPI | 500 | 68 ± 11 | 72.6 | Clopidogrel | Lansoprazole Omeprazole, Rabeprazole | 15.8 | 40.8 | 71.2 | 44.6 | 12 |
| No PPI | 500 | 69 ± 10 | 75.8 | 16.8 | 39.4 | 69 | 43.2 | |||||
| Bhurke [49] (2012/OS) | ACS | PPI | 2674 | 61.3 ± 11.7 | 70 | Clopidogrel | Pantoprazole, Lansoprazole, Omeprazole, Esomeprazole, Rabeprazole | NR | 29.1 | NR | NR | 9 |
| No PPI | 2674 | 61.3 ± 11.7 | 70.1 | NR | 28.6 | NR | NR | |||||
| Burkard [50] (2012/OS) | Mixed | PPI | 109 | 66.5 ± 10.5 | 68.8 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole | 22 | 29.6 | 72.5 | 24.8 | 36 |
| No PPI | 692 | 63.3 ± 11.3 | 79.9 | 27.9 | 17.2 | 65 | 29.8 | |||||
| Chitose [51] (2012/OS) | Mixed | PPI | 331 | 70.3 ± 11.0 | 71.6 | Clopidogrel, Ticlopidine | NR | 25.4 | 35.3 | 77.9 | 23.9 | 18 |
| No PPI | 939 | 68.9 ± 10.9 | 72.2 | 22.9 | 33.7 | 79 | 26.2 | |||||
| Douglas [52] (2012/OS) | ACS | PPI | 9111 | 71 | 58 | Clopidogrel | Lansoprazole Omeprazole, Esomeprazole | NR | 34 | NR | 69 | 10 |
| No PPI | 15,360 | 68 | 65 | NR | 29 | NR | 69 | |||||
| Goodman [16] (Clopidogrel/Ticagrelor) (2012/OS) | ACS | PPI | 6539 | 63 | 72.4 | Clopidogrel/Ticagrelor | Pantoprazole, Lansoprazole, Omeprazole, Esomeprazole, Rabeprazole | 20.8 | 25.8 | 65.6 | 36.2 | 12 |
| No PPI | 12,062 | 62 | 71.2 | 20.5 | 24.7 | 65.4 | 35.7 | |||||
| Ng [26] (2012/RCT) | ACS | PPI | 163 | 64.3 ± 13.8 | 77.3 | Clopidogrel | Esomeprazole | NR | NR | NR | 19.6 | 6 |
| No PPI | 148 | 63.1 ± 13.2 | 72.3 | NR | NR | NR | 18.9 | |||||
| Hauptle [53] (2012/OS) | ACS | PPI | 87 | 68 ± 9 | 70.1 | Clopidogrel | NR | NR | 12.6 | 79.3 | 29.9 | 12 |
| No PPI | 631 | 64 ± 11 | 74.3 | NR | 15.8 | 62.1 | 34.5 | |||||
| Lin [54] (2012/OS) | ACS | PPI | 5173 | 68.3 ± 11.4 | 66.2 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole, Rabeprazole | NR | 35.1 | 54.1 | NR | 12 |
| No PPI | 31,926 | 65.4 ± 12.4 | 71.6 | NR | 33 | 55.7 | NR | |||||
| Macaione [55] (2012/OS) | ACS | PPI | 121 | 63.7 ± 10.6 | 80.2 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Esomeprazole | 16.5 | 41.3 | 70.2 | 37.2 | 36 |
| No PPI | 55 | 65.8 ± 8.8 | 87.3 | 14.5 | 49.1 | 81.8 | 27.3 | |||||
| Ortolani [56] (2012/OS) | ACS | PPI | 3519 | 69 ± 12 | 69 | Clopidogrel | Pantoprazole, Lansoprazole Omeprazole, Rabeprazole | 20 | 24 | 63 | NR | 12 |
| No PPI | 377 | 63 ± 12 | 77 | 15 | 20 | 57 | NR | |||||
| Jiang [57] (2013/OS) | Mixed | PPI | 1570 | 72.4 ± 12.3 | 66 | Clopidogrel | Lansoprazole Omeprazole, Esomeprazole | NR | NR | NR | 19.2 | 12 |
| No PPI | 1110 | 69.5 ± 13.8 | 65 | NR | NR | NR | 15.6 | |||||
| Zou [58] (2014/OS) | ACS | PPI | 6188 | 66.2 ± 10.2 | 73.5 | Clopidogrel | Pantoprazole, Omeprazole, Esomeprazole | 17.3 | 25.8 | 71.3 | 32.2 | 12 |
| No PPI | 1465 | 65.7 ± 10.6 | 73.9 | 19.8 | 23.6 | 70.4 | 31 | |||||
| Hsieh [59] (2015/OS) | Mixed | PPI | 670 | 68.3 ± 10.7 | 63.6 | Clopidogrel | NR | 31.8 | 100 | NR | NR | 12 |
| No PPI | 5933 | 66.5 ± 10.5 | 66.4 | 31.3 | 100 | NR | NR | |||||
| Weisz [60] (2015/OS) | Mixed | PPI | 2697 | 64.4 ± 10.5 | 70.1 | Clopidogrel | NR | 28.6 | 34.8 | 83.7 | 22.7 | 24 |
| No PPI | 5885 | 63.2 ± 11.0 | 75.9 | 23.7 | 31.4 | 77.8 | 22.6 | |||||
| Gargiulo [61 ] (2016/OS) | Mixed | PPI | 738 | 71.2 | 72.5 | Clopidogrel | Pantoprazole, Lansoprazole | 27 | 23.3 | 72.5 | 22.6 | 24 |
| No PPI | 1232 | 68.1 | 79.2 | 26.1 | 24.8 | 71.3 | 24.4 |
ACS = Acute Coronary Syndrome, DM = Diabetes Mellitus, HTN = Hypertension, MI = Myocardial Infarction, NR = Not Reported, OS = Observational Studies, PPI = Proton Pump Inhibitors, RCT = Randomized Controlled Trial, Mixed = Both ACS and non-ACS presentations.
A total of 221,204 patients (PPI = 77,731 patients and no PPI = 143,473 patients) participated in meta-analysis. In the RCTs restricted analysis, PPI did not increase all-cause mortality (RR: 1.35, 95% CI, 0.56–3.23, P = 0.50, I2 = 0; Fig. 2), cardiovascular mortality (RR: 0.94, 95% CI, 0.25–3.54, P = 0.92, I2 = 56; Fig. 3), MI (RR: 0.97, 95% CI, 0.62–1.51, P = 0.88, I2 = 0; Fig. 4) or stroke (RR: 1.11, 95% CI, 0.25–5.4, P = 0.89, I2 = 26; Fig. 5). While, PPI significantly reduced the risk of GI bleeding (RR: 0.32, 95% CI, 0.20–0.52, P < 0.001, I2 = 0; Fig. 6). Conversely, analysis of the observational studies showed that PPI was associated with significant increase in risk of all-cause mortality (RR: 1.25, 95% CI, 1.11–1.41, P < 0.001, I2 = 82; Fig. 2), cardiovascular mortality (RR: 1.25, 95% CI, 1.03–1.52, P = 0.02, i2 = 71; Fig. 3), MI (RR: 1.30, 95% CI, 1.16–1.47, P < 0.001, I2 = 82; Fig. 4) and stroke (RR: 1.60,95% CI, 1.43–1.78, P < 0.001, I2 = 0; Fig. 5), without providing meaningful protection against GI bleeding (RR: 0.74, 95% CI, 0.45–1.22, P = 0.24, I2 = 79; Fig. 6). Egger's regression test did not detect publication bias [Intercept: −0.56, 95% CI, −1.89, 0.76, P (2-tailed) =0.39].
Fig. 2.
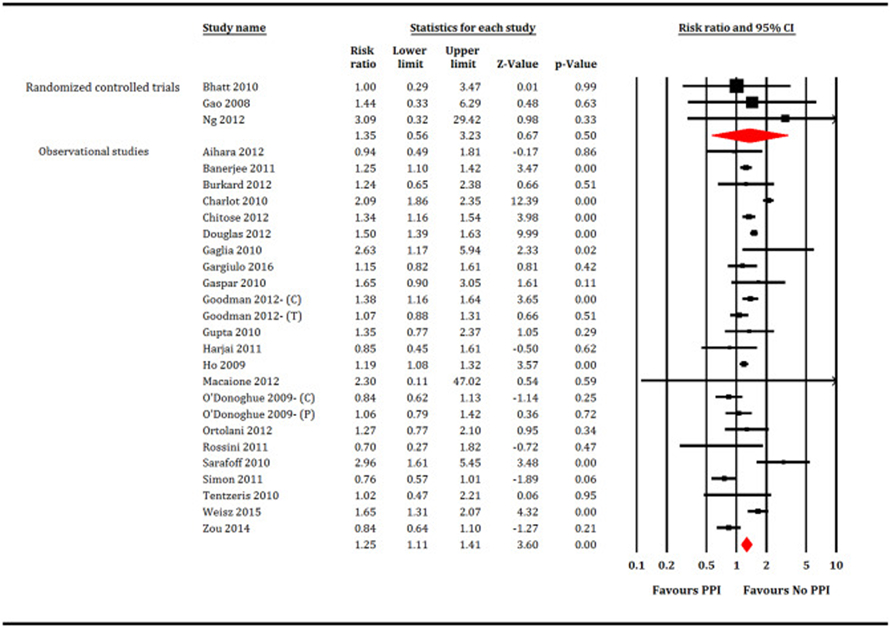
Forest plot showing comparison between proton pump inhibitors (PPI) versus no PPI for all-cause mortality. C = Clopidogrel, T = Ticagrelor, P = Prasugrel.
Fig. 3.
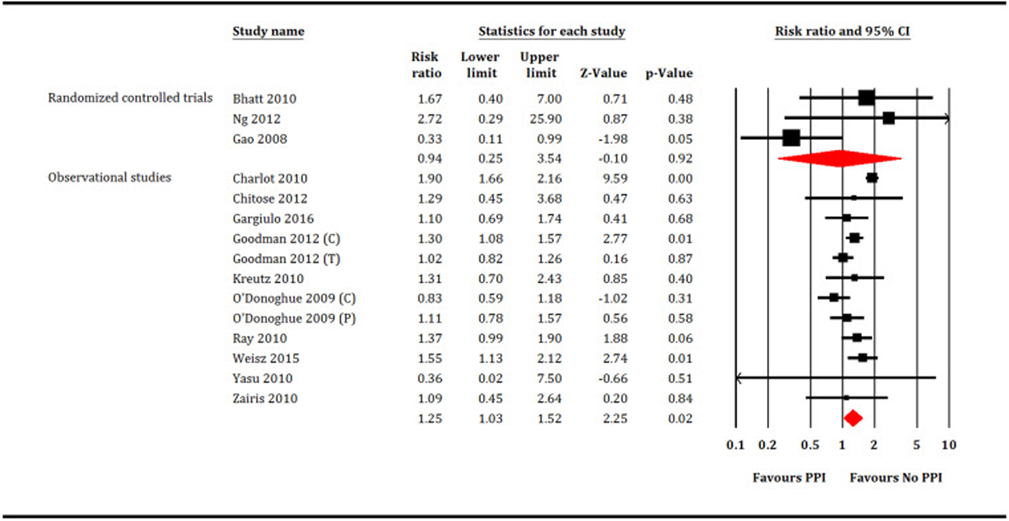
Forest plot showing comparison between proton pump inhibitors (PPI) versus no PPI for cardiovascular mortality. C = Clopidogrel, T = Ticagrelor, P = Prasugrel.
Fig. 4.
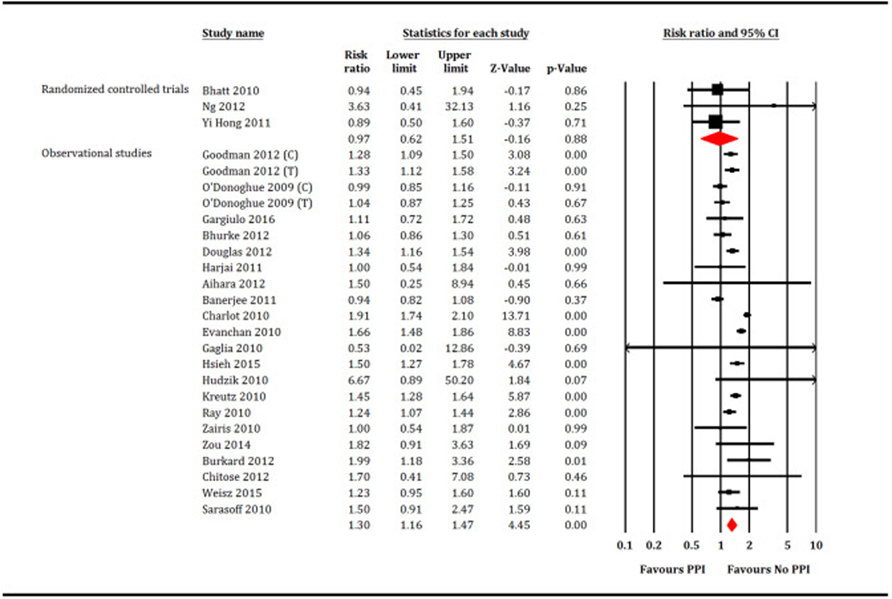
Forest plot showing comparison between proton pump inhibitors (PPI) versus no PPI for myocardial infarction. C = Clopidogrel, T = Ticagrelor, P = Prasugrel.
Fig. 5.
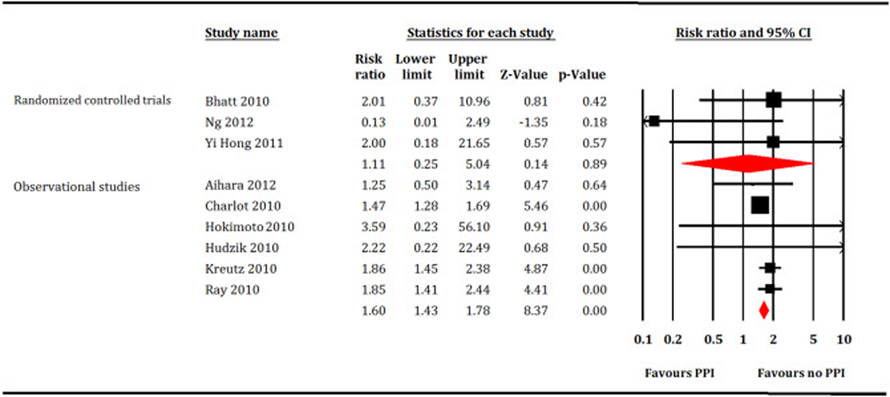
Forest plot showing comparison between proton pump inhibitors (PPI) versus no PPI for stroke.
Fig. 6.
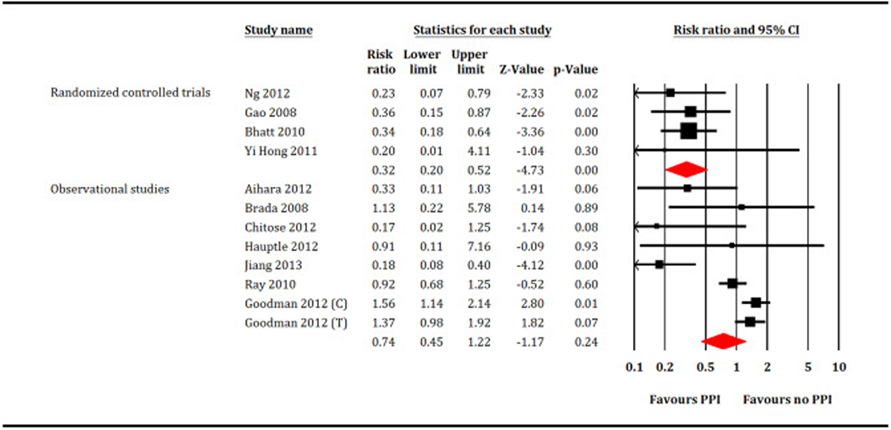
Forest plot showing comparison between proton pump inhibitors (PPI) versus no PPI for gastrointestinal bleeding. C = Clopidogrel, T = Ticagrelor.
4. Discussion
In this review of 39 studies enrolling 221,204 subjects with CAD requiring DAPT, meta-analysis of RCTs suggested that over an average follow up duration of one year, PPI prevented 36 GI bleeding events per 1000 patients compared with no PPI. This benefit was achieved without increasing the risk cardiovascular events or mortality. Conversely, analysis of observational studies suggested that the use of PPI was associated with significant risk of mortality and cardiovascular outcomes without providing protection against GI bleeding.
Several observational studies have shown that PPIs interfere with the efficacy of DAPT and subsequently may cause adverse cardiovascular outcomes [8,16,18,19]. The interaction between omeprazole and clopidogrel is considered vigorous due to the inhibitory effect of PPI on CYP2C19 isoenzyme [20,21]. Clopidogrel is a prodrug and requires conversion to its active metabolite for inhibition of platelet aggregation. While the conversion from prodrug to active metabolite requires CYP2C19 enzymes, there are genetic variations in CYP2C19 enzymes leading to poor metabolism of the drug in certain patients. Subsequently these patients have reduced efficacy of Clopidogrel. Similarly, Omeprazole and Esomeprazole are inhibitors of CYP2C19 and they can significantly reduce the efficacy of Clopidogrel by preventing conversion to its active metabolite. Therefore, FDA precautions against use of Omeprazole and Esomeprazole use in patients taking Clopidogrel [22].
There is observational data suggesting that the blunting effect of PPI could be expanded to ticagrelor and prasugrel. In a post hoc analysis of PRINCIPLE (Prasugrel In Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation)-TIMI 44 and TRITON (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel)-TIMI 38 trials, there was modest attenuation of the in vitro antiplatelet effects of prasugrel and clopidogrel in the setting of PPI therapy. Another hypothesis for PPI related enhanced cardiovascular risk is that PPI use is a marker for high risk of cardiovascular complications rather than a cause of cardiovascular complications [16,23]. In post hoc analysis of the PLATO (Platelet Inhibition and Patient Outcomes) trial the use of PPI was independently associated with higher risk of cardiovascular events for both clopidogrel and ticagrelor [16]. Similar observation was made in the post-hoc analysis of CREDO (Clopidogrel for Reduction of Events During Observation) and CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) where PPI use was associated with worse cardiovascular outcomes in both clopidogrel (Estimated hazard ratio (EHR): 1.67, 95% CI 1.06 to 2.64) and placebo arms (HER: 1.56, 95% CI 1.06 to 2.30) [24].
Despite this negative interaction between PPI and antiplatelet therapy shown by observational data, this effect was not translated into any significant clinical impression in RCTs. Gao and colleagues [25] reported that early use of omeprazole in acute MI not only reduced the incidence of GI bleeding compared with control (5.3% versus 14.6%, P = 0.017) but also had protective effect on all-cause mortality (3.5% versus 10.6%, P = 0.035). In another trial by Ng et al. [26], omeprazole was superior to famotidine in reducing the risk of GI bleeding (HR: 0.212, P = 0.008) without increasing the risk of cardiovascular outcomes (P = 0.77). In the largest COGENT study (Clopidogrel and the Optimization of Gastrointestinal Events Trial) [27], the addition of omeprazole to DAPT significantly reduced the risk of major GI bleeding without increasing the risk of cardiovascular events, though with broader confidence interval around HRs and limited statistical power. Our meta-analysis is in consensus with these findings and highlights the importance of potential bias introduced by observational studies which results in conflicting outcomes.
We compare our results with prior meta-analyses. Cardoso et al. [28] (39 studies and 214,851 patients) reported 60% relative risk reduction in GI bleeding with PPI (odds ratio (OR): 0.40, 95% CI, 0.22–0.74) but at the cost of increased risk of cardiovascular events. However, Cardoso’s meta-analysis had certain limitations. First, authors combined RCTs and observational studies together in their pooled analysis. This strategy has the potential to generate higher risk of selection and attrition biases. Second, the analysis was primarily focused on interaction of PPI with single clopidogrel therapy and a subgroup analysis on DAPT was limited by various confounders due to mixing of RCTs and propensity matched score studies. Third, post hoc analyses of various RCTs were treated as RCTs which is issue of standardized and comprehensive reporting because post hoc analyses generally do not fill the criteria of a RCT [29]. Another systematic review by Melloni and colleagues (35 studies) was in consensus with our findings [9]. However, authors focused on observational studies only and lacked separate examination of RCTs. Hence, comparison of the effect sizes among RCTs and observational studies could not be performed. Furthermore, key endpoint of GI bleeding was not included. Similar issues related to study design [7,8] or lack of assessment of important endpoints [7] were noticed in other meta-analyses.
That said, the current meta-analysis has certain limitations. First, RCTs data is dominated by the COGENT study population which contributes ~ 84% of RCTs cohort [27]. The COGENT suffered from premature study termination, abbreviated follow up duration and had a high-risk population. Furthermore, due to low event rate and limited follow up duration, COGENT lacked statistical power to detect cardiovascular harm. Second, studies had heterogeneities with regards to clinical presentation, drugs and dosages, procedural techniques, definition of the endpoints and follow up duration which could not be compensated due to lack of access to individual patient data. Therefore, certain outcomes such as stent thrombosis or coronary revascularization could not be assessed. Finally, this review predominantly generates the evidence regarding clopidogrel based DAPT.
In conclusion, we report that meta-analysis of RCTs endorse the use of PPI with DAPT (predominantly clopidogrel based therapy) in CAD patients for prevention of GI bleeding without worsening cardiovascular outcomes. The findings of RCTs restricted analysis are in line with current professional guidelines [6] and oppose the negative observational data on the use of PPI with DAPT. Our review serves as refined summary of published literature on this issue and would allow the clinicians to compare the effects of PPI with concomitant clopidogrel based DAPT based on quality of evidence. This review also highlights the importance of conducting further well-designed RCTs comparing use of specific PPIs with different DAPT regimens (Ticagrelor and Prasugrel) to generate more durable evidence and compensate for relative scarcity of quality data on this subject.
Supplementary Material
Acknowledgments
Funding
None.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.carrev.2019.02.002.
References
- [1].American College of Emergency P, Society for Cardiovascular A, Interventions, O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61(4):e78–140. [DOI] [PubMed] [Google Scholar]
- [2].Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64(24):e139–228. [DOI] [PubMed] [Google Scholar]
- [3].Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345(7):494–502. [DOI] [PubMed] [Google Scholar]
- [4].Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352(12):1179–89. [DOI] [PubMed] [Google Scholar]
- [5].Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39(3):213–60. [DOI] [PubMed] [Google Scholar]
- [6].Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134 (10):e123–55. [DOI] [PubMed] [Google Scholar]
- [7].Bundhun PK, Teeluck AR, Bhurtu A, Huang W-Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012–2016). BMC Cardiovasc Disord 2017;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sherwood MW, Melloni C, Jones WS, Washam JB, Hasselblad V, Dolor RJ. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: a systematic review. J Am Heart Assoc 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes 2015;8(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003; 28(12):1290–9. [DOI] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ 2009;339. [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- [14].Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41(3):818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation 2012;125(8):978–86. [DOI] [PubMed] [Google Scholar]
- [17].O'Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374(9694):989–97. [DOI] [PubMed] [Google Scholar]
- [18].Gupta E, Bansal D, Sotos J, Olden K. Risk of adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention. Dig Dis Sci 2010;55(7):1964–8. [DOI] [PubMed] [Google Scholar]
- [19].Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009;301(9):937–44. [DOI] [PubMed] [Google Scholar]
- [20].Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008;51(3):256–60. [DOI] [PubMed] [Google Scholar]
- [21].Gilard M, Arnaud B, Le Gal G, Abgrall JF, Boschat J. Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J Thromb Haemost 2006;4 (11):2508–9. [DOI] [PubMed] [Google Scholar]
- [22].Guérin A, Mody R, Carter V, Ayas C, Patel H, Lasch K, et al. Changes in practice patterns of clopidogrel in combination with proton pump inhibitors after an FDA safety communication. PLoS One 2016;11(1):e0145504-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cardoso RN, Benjo AM, DiNicolantonio JJ, Garcia DC, Macedo FYB, El-Hayek G, et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart 2015;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dunn SP, Steinhubl SR, Bauer D, Charnigo RJ, Berger PB, Topol EJ. Impact of proton pump inhibitor therapy on the efficacy of clopidogrel in the CAPRIE and CREDO trials. J Am Heart Assoc 2013;2(1):e004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao QP, Sun Y, Sun YX, Wang LF, Fu L. Early use of omeprazole benefits patients with acute myocardial infarction. J Thromb Thrombolysis 2009;28(3):282–7. [DOI] [PubMed] [Google Scholar]
- [26].Ng FH, Tunggal P, Chu WM, Lam KF, Li A, Chan K, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol 2012;107 (3) :389–96. [DOI] [PubMed] [Google Scholar]
- [27].Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363(20):1909–17. [DOI] [PubMed] [Google Scholar]
- [28].Cardoso RN, Benjo AM, DiNicolantonio JJ, Garcia DC, Macedo FY, El-Hayek G, et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart 2015;2(1):e000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21(19):2917–30. [DOI] [PubMed] [Google Scholar]
- [30].Barada K, Karrowni W, Abdallah M, Shamseddeen W, Sharara AI, Dakik HA. Upper gastrointestinal bleeding in patients with acute coronary syndromes: clinical predictors and prophylactic role of proton pump inhibitors. J Clin Gastroenterol 2008;42 (4) :368–72. [DOI] [PubMed] [Google Scholar]
- [31].Charlot M, Ahlehoff O, Norgaard ML, Jorgensen CH, Sorensen R, Abildstrom SZ, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent ofclopidogrel use: a nationwide cohort study. Ann Intern Med 2010;153(6):378–86. [DOI] [PubMed] [Google Scholar]
- [32].Evanchan J, Donnally MR, Binkley P, Mazzaferri E. Recurrence of acute myocardial infarction in patients discharged on clopidogrel and a proton pump inhibitor after stent placement for acute myocardial infarction. Clin Cardiol 2010;33(3):168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gaglia MA Jr, Torguson R, Hanna N, Gonzalez MA, Collins SD, Syed AI, et al. Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am J Cardiol 2010;105(6):833–8. [DOI] [PubMed] [Google Scholar]
- [34].Gaspar A, Ribeiro S, Nabais S, Rocha S, Azevedo P, Pereira MA, et al. Proton pump inhibitors in patients treated with aspirin and clopidogrel after acute coronary syndrome. Revista portuguesa de cardiologia: orgao oficial da Sociedade Portuguesa de Cardiologia. Port J Cardiol 2010;29(10):1511–20. [PubMed] [Google Scholar]
- [35].Hudzik B, Szkodzinski J, Danikiewicz A, Wilczek K, Romanowski W, Lekston A, et al. Effect of omeprazole on the concentration of interleukin-6 and transforming growth factor-beta1 in patients receiving dual antiplatelet therapy after percutaneous coronary intervention. Eur Cytokine Netw 2010;21(4):257–63. [DOI] [PubMed] [Google Scholar]
- [36].Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy 2010;30(8):787–96. [DOI] [PubMed] [Google Scholar]
- [37].Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010;152(6):337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sarafoff N, Sibbing D, Sonntag U, Ellert J, Schulz S, Byrne RA, et al. Risk of drug-eluting stent thrombosis in patients receiving proton pump inhibitors. Thromb Haemost 2010;104(3):626–32. [DOI] [PubMed] [Google Scholar]
- [39].Tentzeris I, Jarai R, Farhan S, Brozovic I, Smetana P, Geppert A, et al. Impact of concomitant treatment with proton pump inhibitors and clopidogrel on clinical outcome in patients after coronary stent implantation. Thromb Haemost 2010;104(6):1211–8. [DOI] [PubMed] [Google Scholar]
- [40].Yasu T, Ikee R, Miyasaka Y, Chubachi H, Saito S. Efficacy and safety of concomitant use of rabeprazole during dual-antiplatelet therapy with clopidogrel and aspirin after drug-eluting stent implantation: a retrospective cohort study. Yakugaku Zasshi 2010;130(12):1743–50. [DOI] [PubMed] [Google Scholar]
- [41].Zairis MN, Tsiaousis GZ, Patsourakos NG, Georgilas AT, Kontos CF, Adamopoulou EN, et al. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol 2010;26(2):e54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Banerjee S, Weideman RA, Weideman MW, Little BB, Kelly KC, Gunter JT, et al. Effect of concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention. Am J Cardiol 2011;107(6):871–8. [DOI] [PubMed] [Google Scholar]
- [43].Ren YH, Zhao M, Chen YD, Chen L, Liu HB, Wang Y, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J 2011; 124(6):856–61. [PubMed] [Google Scholar]
- [44].Harjai KJ, Shenoy C, Orshaw P, Usmani S, Boura J, Mehta RH. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: an analysis from the Guthrie Health Off-Label Stent (GHOST) investigators. Circ Cardiovasc Interv 2011;4(2):162–70. [DOI] [PubMed] [Google Scholar]
- [45].Nakayama A, Morita H, Ando J, Fujita H, Ohtsu H, Nagai R. Adverse cardiovascular outcomes associated with concurrent use of clopidogrel or ticlopidine and proton-pump inhibitors in patients undergoing percutaneous coronary intervention. Heart Vessel 2013;28(3):292–300. [DOI] [PubMed] [Google Scholar]
- [46].Rossini R, Capodanno D, Musumeci G, Lettieri C, Lortkipanidze N, Romano M, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis 2011;22(3):199–205. [DOI] [PubMed] [Google Scholar]
- [47].Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation 2011;123(5):474–82. [DOI] [PubMed] [Google Scholar]
- [48].Aihara H, Sato A, Takeyasu N, Nishina H, Hoshi T, Akiyama D, et al. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry. Catheter Cardiovasc Interv 2012;80(4):556–63. [DOI] [PubMed] [Google Scholar]
- [49].Bhurke SM, Martin BC, Li C, Franks AM, Bursac Z, Said Q. Effect of the clopidogrel-proton pump inhibitor drug interaction on adverse cardiovascular events in patients with acute coronary syndrome. Pharmacotherapy 2012;32(9):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Burkard T, Kaiser CA, Brunner-La Rocca H, Osswald S, Pfisterer ME, Jeger RV. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med 2012;271(3):257–63. [DOI] [PubMed] [Google Scholar]
- [51].Chitose T, Hokimoto S, Oshima S, Nakao K, Fujimoto K, Miyao Y, et al. Clinical outcomes following coronary stenting in Japanese patients treated with and without proton pump inhibitor. Circ J 2012;76(1):71–8. [DOI] [PubMed] [Google Scholar]
- [52].Douglas IJ, Evans SJW, Hingorani AD, Grosso AM, Timmis A, Hemingway H, et al. Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs. BMJ [Br Med J] 2012;345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hauptle R, Weilenmann D, Schneider T, Haile SR, Ammann P, Knellwolf C, et al. Individualised PPI prescription in patients on combination antiplatelet therapy and upper gastrointestinal events after percutaneous coronary intervention: a cohort study. Wien Med Wochenschr 2012;162(3–4):67–73. [DOI] [PubMed] [Google Scholar]
- [54].Lin CF, Shen LJ, Wu FL, Bai CH, Gau CS. Cardiovascular outcomes associated with concomitant use of clopidogrel and proton pump inhibitors in patients with acute coronary syndrome in Taiwan. Br J Clin Pharmacol 2012;74(5):824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Macaione F, Montaina C, Evola S, Novo G, Novo S. Impact of dual antiplatelet therapy with proton pump inhibitors on the outcome of patients with acute coronary syndrome undergoing drug-eluting stent implantation. ISRN Cardiol 2012;2012:692761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ortolani P, Marino M, Marzocchi A, De Palma R, Branzi A. One-year clinical outcome in patients with acute coronary syndrome treated with concomitant use of clopidogrel and proton pump inhibitors: results from a regional cohort study. J Cardiovasc Med (Hagerstown) 2012;13(12):783–9. [DOI] [PubMed] [Google Scholar]
- [57].Jiang Z, Wu H, Duan Z, Wang Z, Hu K, Ye F, et al. Proton-pump inhibitors can decrease gastrointestinal bleeding after percutaneous coronary intervention. Clin Res Hepatol Gastroenterol 2013;37(6):636–41. [DOI] [PubMed] [Google Scholar]
- [58].Zou JJ, Chen SL, Tan J, Lin L, Zhao YY, Xu HM, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One 2014;9(1):e84985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hsieh CF, Huang WF, Chiang YT, Chen CY. Effects of clopidogrel and proton pump inhibitors on cardiovascular events in patients with type 2 diabetes mellitus after drug-eluting stent implantation: a nationwide cohort study. PLoS One 2015;10(8):e0135915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Weisz G, Smilowitz NR, Kirtane AJ, Rinaldi MJ, Parvataneni R, Xu K, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: the ADAPT-DES study. Circ Cardiovasc Interv 2015;8(10). [DOI] [PubMed] [Google Scholar]
- [61].Gargiulo G, Costa F, Ariotti S, Biscaglia S, Campo G, Esposito G, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J 2016;174:95–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


