Abstract
PURPOSE:
Classic Hodgkin lymphoma is highly curable with contemporary therapy. Although the limited role of surveillance imaging to detect early relapse for patients in complete remission at the end of therapy is well established, there is a paucity of data regarding role of laboratory testing in this setting.
METHODS:
Patients with newly diagnosed classic Hodgkin lymphoma uniformly treated with the Stanford V regimen from 1998-2014 and in complete remission for at least 3 months were identified in a single-center institutional database. Laboratory tests categorized by Common Terminology Criteria for Adverse Events v4.03 as grade 2 or higher were considered abnormal. Primary analysis included sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of surveillance laboratory tests for predicting relapse in the first 3 years after end of treatment.
RESULTS:
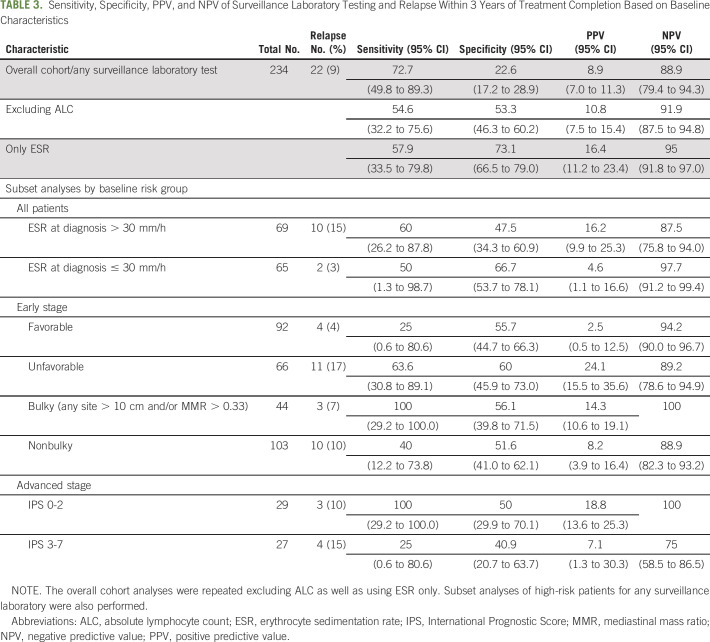
Among 235 eligible patients, 24 (10.2%) patients ultimately relapsed. In the first 3 years after end of therapy, the mean number of surveillance blood draws per patient was 7.1, (range, 1-13). These 1,661 surveillance blood draws included 4,684 individual laboratory tests, comprising 1,609 CBCs, 1,578 metabolic panels, and 1,497 erythrocyte sedimentation rates. None of the biopsies confirming relapses were prompted by any abnormal laboratory finding. The sensitivity of any surveillance laboratory test for detecting relapse within 3 years of end of treatment was 72.7% (95% CI, 49.8% to 89.3%), specificity 22.6% (95% CI, 17.2% to 28.9%), yielding a PPV of 8.9% (95% CI, 7.0% to 11.3%) and NPV of 88.9% (95% CI, 79% to 94%).
CONCLUSION:
Our study found limited clinically meaningful utility for routine surveillance laboratory testing in detecting relapse in patients with complete remission at end of treatment. Our results warrant consideration of modifications to current practice guidelines.
INTRODUCTION
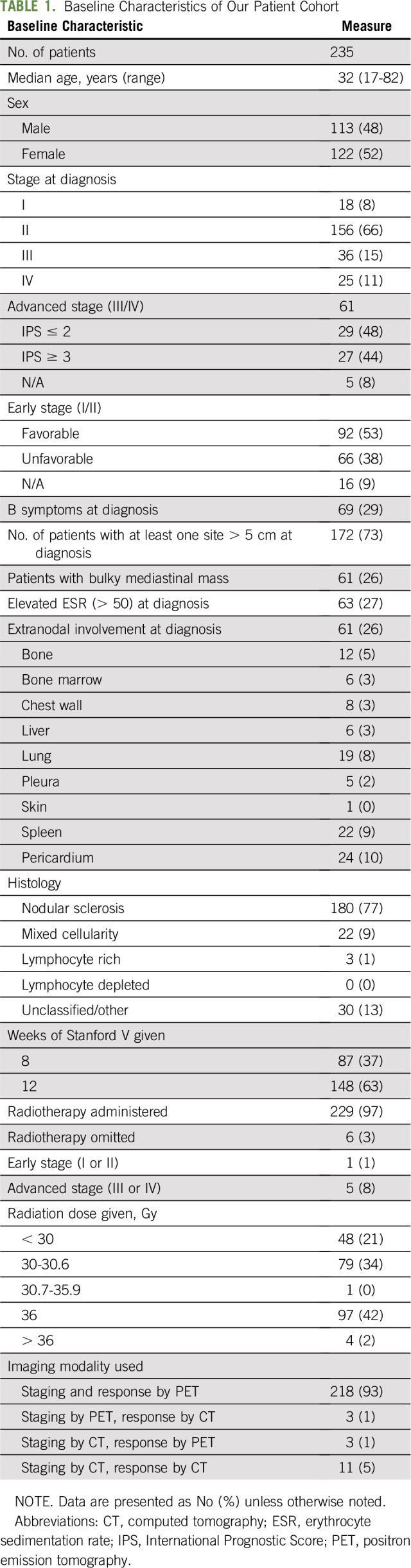
Classic Hodgkin lymphoma (CHL) is highly curable, with contemporary front-line therapies in approximately 85%-90% of patients with early-stage1-4 and 75%-80% of patients with advanced-stage disease.5-8 Historically, the rationale for surveillance for patients with CHL in first complete remission (CR) had been twofold: to detect early relapse and to evaluate for late effects of primary therapy, such as secondary cancers. Guidelines for surveillance have been carried forward from an era where extended-field and high-dose radiotherapy were the mainstay of primary therapy. Advances over the past three decades include development of safer chemotherapy regimens, reduction in the dose and field of radiation administered, and more accurate staging/response assessment with positron emission tomography/computed tomography (PET/CT). With contemporary regimens, optimal surveillance needs to be redefined to align with the improvements in safety and response assessment. An example of this is the recognition that surveillance imaging, particularly with PET/CT,9-11 was associated with high costs and few changes in management, which led to the discontinuation of routine imaging in asymptomatic patients in the current National Comprehensive Cancer (NCCN) and European Society of Medical Oncology (ESMO) CHL guidelines.12,13
In contrast to imaging guidelines, there is a paucity of data on the utility of routine laboratory testing in the detection of relapse, yet regular laboratory testing still features prominently in international surveillance guidelines. For example, NCCN guidelines recommend a CBC, erythrocyte sedimentation rate (ESR, if elevated at diagnosis), and chemistry panel “if clinically indicated.”12 ESMO guidelines recommend laboratory testing including CBC, ESR, and chemistry panel every 3 months for the first half year, then every 6 months until year 4, and yearly thereafter.13
The objective of our study was to assess if routine surveillance laboratory tests in patients treated with curative intent and in CR were useful in detecting relapse in patients with CHL in the absence of clinical signs or symptoms.
METHODS
We conducted a retrospective cohort study of patients with newly diagnosed CHL who were treated with the Stanford V1,14 regimen at our institution both on and off protocol between 1998 and 2014. Patients were identified from the Stanford lymphoma database, which includes detailed baseline and treatment information on all patients with lymphoma who have been seen in clinic at the institution. Eligibility criteria for this study included patients with a CR to primary therapy that lasted at least 3 months, with evidence of at least one surveillance visit or laboratory test while in remission. Those with primary progressive CHL or in a partial remission (PR) at the end of first-line treatment were excluded from the analysis. Patients who received primary chemotherapy and/or follow-up care outside our institution were also excluded. Data were abstracted from electronic health records for patient demographics, treatment regimen, follow-up laboratory testing data, biopsy if any and imaging dates, reasons prompting a biopsy, and relapse information. Follow-up data were collected up to 5 years after primary treatment completion. Data on death dates are imported into our database from the California Cancer Registry or from medical records.
Date of treatment completion was defined using the last date of radiation; if a patient did not receive radiation, the last date of chemotherapy was used as the date of treatment completion.
Patients were followed according to institutional guidelines, which included clinic visits and laboratory tests approximately every 3 months for years 1-2, and every 6 months for years 3-5. Laboratory data (test date, laboratory components, and results) were captured from our electronic medical records. We included laboratory components from the CBC, ESR, and chemistry panels. Specific components analyzed included WBC, hemoglobin, platelets, absolute lymphocyte count (ALC), absolute neutrophil count (ANC), albumin, AST, alanine aminotransferase, and total bilirubin. The study was approved by the Stanford Institutional Review Board.
An algorithm to define laboratory tests as surveillance was developed by the study team; the algorithm was created to conform to surveillance guidelines at the time the testing was performed. Specifically, a laboratory test was classified as part of surveillance if it was done before relapse date, first biopsy date, or death date (if relapsed or died) and if it met the condition of minimum number of weeks from the previous laboratory test. Surveillance laboratory tests were defined based on the calendar year of treatment completion, the year of follow-up, and the timing of expected surveillance laboratory tests after treatment. For example, for the period 1998-2009, during the first year of surveillance, a laboratory test had to have been done at least 6 weeks after treatment completion or after the previous laboratory test to be defined as a surveillance test. During the second year of surveillance, this timing changed to at least 8 weeks from the previous laboratory tests for a test to be classified as a surveillance test; tests conducted before 8 weeks were not considered surveillance.
For each surveillance laboratory component, we assessed if the test result was normal or abnormal on the basis of test result values. For laboratory tests that could be categorized based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, we defined an abnormal laboratory component as one that was at least grade 2. For tests that do not have a CTCAE grading scale (elevated WBC, elevated ANC, elevated platelets, and elevated ESR), any value outside the normal range was defined as abnormal for the purposes of this analysis. If a patient had at least one abnormal surveillance laboratory component before a biopsy, the patient was classified as having an abnormal surveillance laboratory test.
The primary goals for this study were to estimate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of any surveillance laboratory tests for relapse detection within the first 3 years of follow-up. If any surveillance laboratory test in the 3-year period after treatment completion was abnormal, the patient was classified as having an abnormal surveillance laboratory test. The gold standard for this analysis was the presence of a biopsy-proven relapse or initiation of subsequent therapy (for patients without a biopsy-proven relapse). The 95% CIs for sensitivity and specificity were calculated using the Clopper-Pearson confidence intervals15 and for the PPV and NPV using standard logit confidence intervals.16 The secondary goals were to estimate the sensitivity, specificity, PPV, and NPV of surveillance laboratory tests for relapse detection within the first 3 years of follow-up in specific subsets of patients on the basis of the following baseline risk factors: (1) elevated ESR (> 30 mm/h) at diagnosis/nonelevated ESR at diagnosis; (2) early stage (stage I-II) favorable/unfavorable (per European Organisation for Research and Treatment of Cancer criteria); (3) early stage bulky (> 10 cm or mediastinal mass ratio > 0.33)/nonbulky; (4) advanced stage (stage III-IV) International Prognostic Score (IPS) 0-2/IPS 3-7. After observing the high frequency of abnormal ALC and ESR, post hoc analyses were performed to estimate sensitivity, specificity, PPV, and NPV of (1) only ESR surveillance laboratory tests, and (2) surveillance laboratory tests after excluding ALC. Analyses were conducted using SAS v 9.4 (SAS Institute, Cary, NC) and R package.17
RESULTS
Between January 1998 and December 2014, 235 patients with newly diagnosed CHL met the eligibility criteria for our study. The median age was 32 years (range, 18-82 years), and 174 (74.1%) had early-stage disease (Table 1). Two hundred twenty-one patients (94%) had their end-of-treatment response assessment by PET/CT. Twenty-four patients (10%) relapsed at a median time from end of treatment of 8 months (range, 3.4-80.5 months). One hundred ninety-four patients (83%) had at least 3 years of follow-up at our institution (Table 1).
TABLE 1.
Baseline Characteristics of Our Patient Cohort
The median number of blood draws per patient over the 3 years examined was 8 (interquartile range, 5-13). Overall there were a total of 1,661 surveillance blood draws, which included 1,609 CBCs, 1,578 basic metabolic panels, and 1,497 ESRs. One hundred eighty (77%) patients had at least one abnormal laboratory component by our definition over the first 3 years of follow-up. Figure 1 shows the distribution of laboratory testing by patient. There were several more abnormal tests in the first few weeks after treatment completion, which became less frequent over time, and very few patients had relapse.
Fig 1.
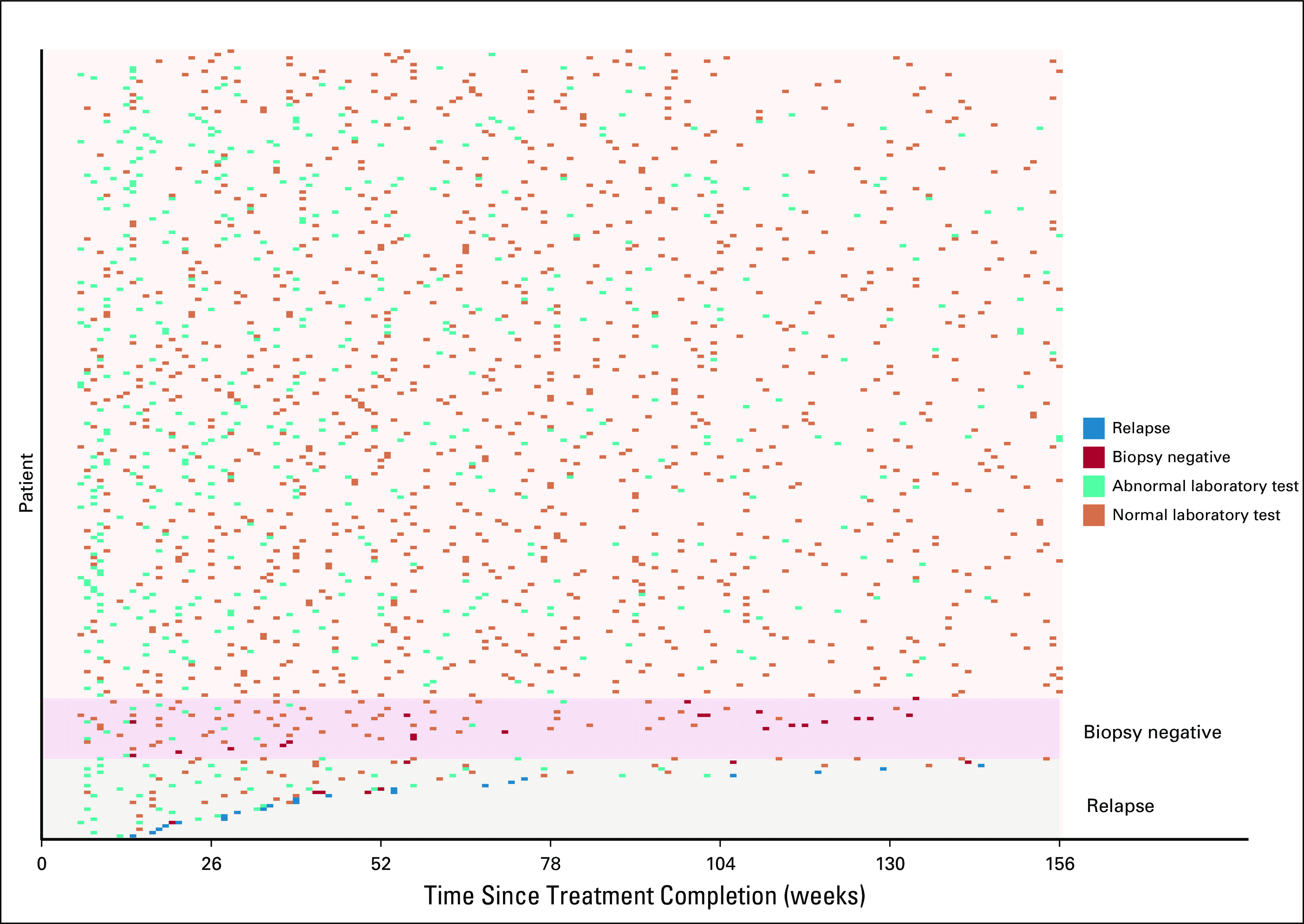
Surveillance laboratory testing over 3 years.
The frequency of specific abnormal laboratory components is listed in Table 2. Notably, the ALC was abnormal 25% of the time and ESR was abnormal 9.6% of the time. All other components analyzed were infrequently abnormal, ranging from 0.1%-3.6%. A swimmer’s plot in Figure 2 illustrates the patterns of abnormal testing performed on all patients who subsequently relapsed.
TABLE 2.
Frequency of Abnormal Surveillance Laboratory Components
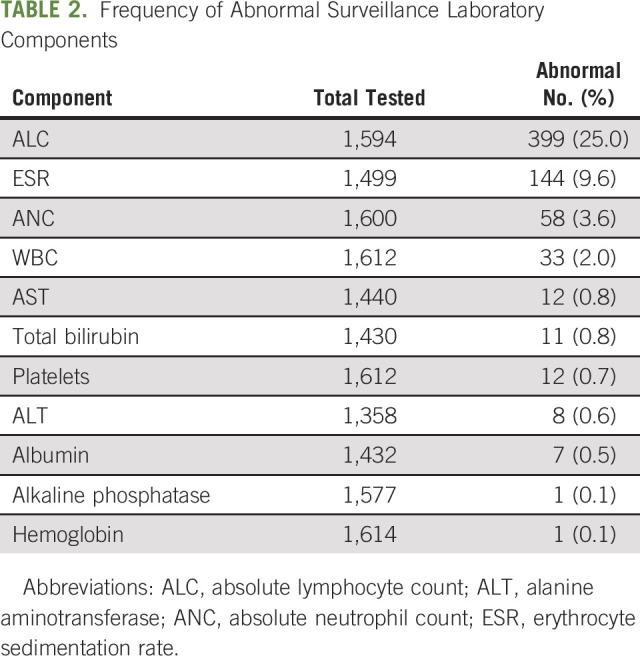
Fig 2.
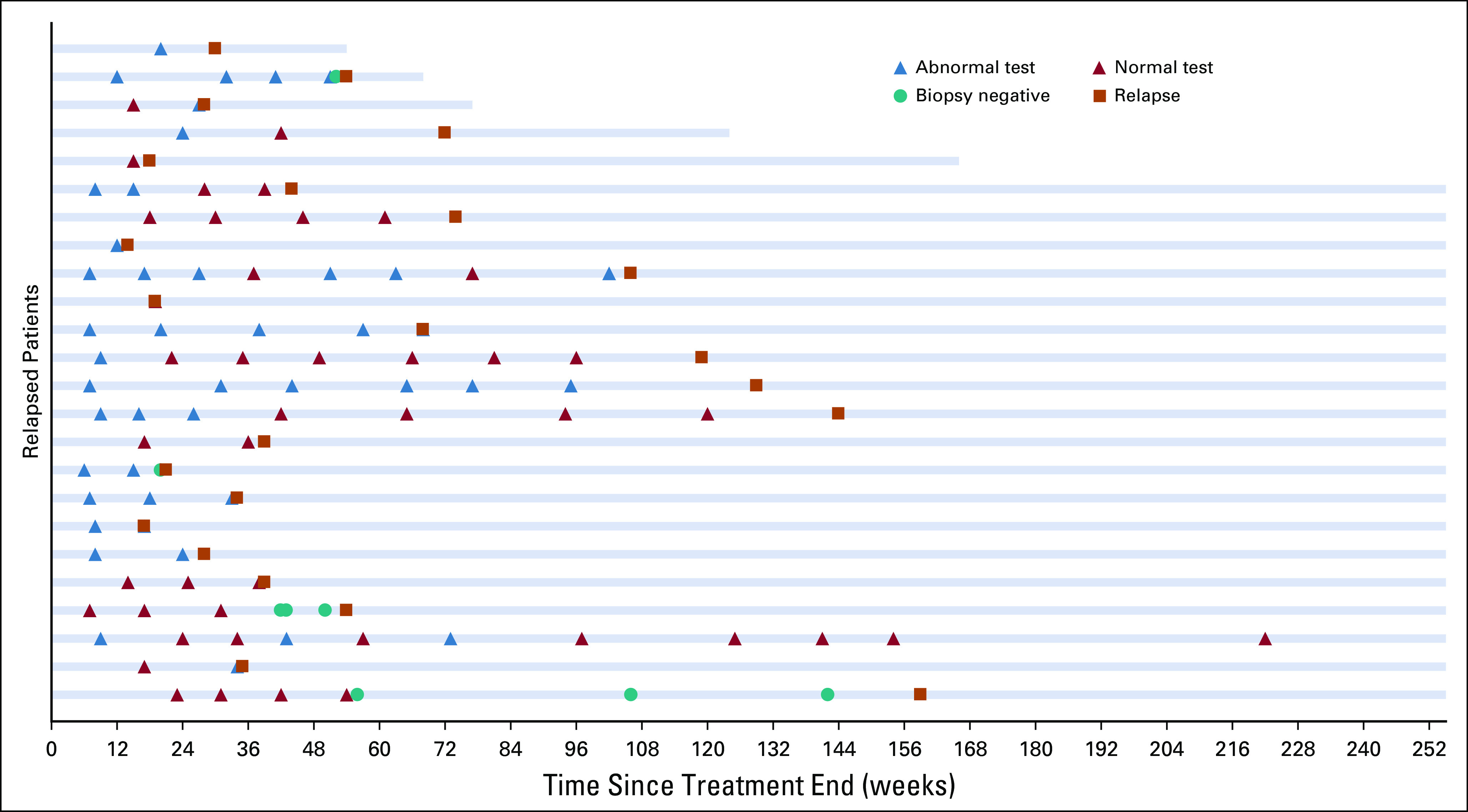
Swimmer’s plot of the data from Figure 1 for relapsed patients only. One patient relapsed at week 350.
For our primary analysis, 234 patients had at least one surveillance laboratory test during the first 3 years of follow-up, of whom 22 (9.4%) relapsed during the first 3 years of follow-up (Table 3). Any surveillance laboratory test had a sensitivity of 72.7% (95% CI, 49.8% to 89.3%), specificity of 22.6% (95% CI, 17.2% to 28.9%), PPV of 8.9% (95% CI, 7.0% to 11.3%), and NPV of 88.9% (95% CI, 79.4% to 94.3%). Excluding ALC from the analysis yielded a PPV of 10.8% (95% CI, 7.5% to 15.4%), and only using ESR yielded a PPV of 16.4% (95% CI, 11.2% to 23.4%; Table 3).
TABLE 3.
Sensitivity, Specificity, PPV, and NPV of Surveillance Laboratory Testing and Relapse Within 3 Years of Treatment Completion Based on Baseline Characteristics
The results of our secondary analyses on patient subsets are shown in Table 3. The PPV remained low in patients with adverse risk characteristics at diagnosis, including patients with elevated ESR (16.2%; 95% CI, 9.9% to 25.3%), early-stage unfavorable (24.1%; 95% CI, 15.5% to 35.6%), early-stage bulky (14.3%; 95% CI, 10.6% to 19.1%), and advanced-stage disease with IPS > 2 (7.1%; 95% CI, 1.3% to 30.3%).
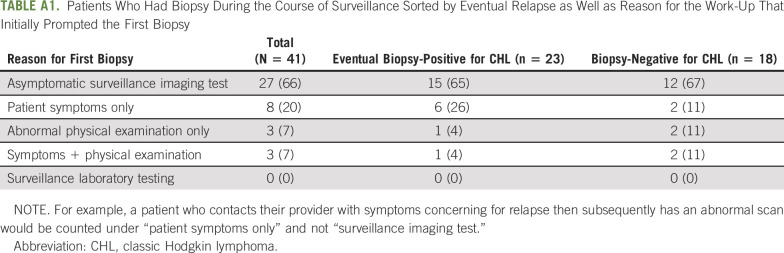
Forty-one patients had a biopsy for suspected relapse, of which 23 confirmed relapsed disease. One patient proceeded with subsequent therapy on the basis of highly suspicious imaging findings in an area that could not easily undergo biopsy. However, none of the patients who had a biopsy or relapsed had additional work-up prompted by an abnormal laboratory value. Surveillance imaging prompted the biopsy for most patients (27/41; 66%), regardless of whether the patient eventually relapsed or not (Appendix Table A1, online only).
DISCUSSION
In this single-center, retrospective review of patients with uniformly treated CHL who achieved a CR after primary therapy, we found that surveillance laboratory tests had minimal impact in detecting relapse or altering patient management. Surveillance laboratory testing was not associated with clinically meaningful sensitivity, specificity, PPV, and NPV.18 Despite the time and resources that went into this testing, none of the patients in our cohort had a biopsy prompted by an abnormal laboratory test result; most biopsies were prompted as a result of surveillance imaging.
We designed our study to use clinically meaningful definitions for abnormal laboratory tests to avoid minimally abnormal results skewing our analysis. The CTCAE grading system allowed us to systematically evaluate each laboratory component and only identify grade 2 or higher laboratory values as abnormal. Despite the stringent criteria, 180 (77%) patients had at least one abnormal laboratory test by our criteria during the first 3 years of follow-up.
Few studies have focused on the role of laboratory testing for survivors of CHL. Isolated detection of relapse by ESR has been described in few studies.19,20 A Stanford study of 709 patients with early-stage CHL treated with primary radiotherapy between 1969 and 1994 found that 157 (22%) patients relapsed, of whom only one had a relapse detected based on an abnormal ESR.19 A separate older single-center series of 107 patients and 22 relapses found 2 occurrences of an abnormal laboratory test prompting the detection of relapse.20
Our results are also comparable to a report of a combined analysis of patients with CHL or non-Hodgkin lymphoma by Hawkes et a,l21 in which there were few changes in management on the basis of laboratory abnormalities and no difference in survival between patients with or without laboratory abnormalities in the 3 months before relapse. Although the analysis by Hawkes et al21 did not use the same stringent criteria for defining an abnormal blood test in their analysis, the NPV seen in our primary and secondary analyses were comparable, suggesting that excluding mildly abnormal laboratory tests did not affect our analysis.
We found a high rate of lymphopenia in our cohort, with 25% of laboratory values grade 2 or higher. Lymphopenia after a chemotherapy regimen like Stanford V is well described and related to the prednisone used as part of the regimen.6 Because of this finding, we performed a post hoc analysis that excluded this laboratory component and found similar results to our primary analysis.
We also did not see a substantial difference in the PPV in higher-risk cohorts where the likelihood of treatment failure is greater. Although only 9% of the overall cohort relapsed within 3 years, the rates of relapse in higher-risk patients were similar. For this reason, we found no impact of an elevated ESR at diagnosis (15%), early-stage unfavorable (17%), early-stage bulky (7%), and advanced-stage IPS > 2 (15%). One caveat is that we included only patients who achieved a CR for at least 3 months. Therefore, many of the treatment failures in the high-risk subgroup may have occurred before this time and therefore would not have been included in our analysis.
Although one of the strengths of our study is that patients were treated uniformly, there are limitations related to the retrospective nature of the analysis and laboratory data decoupled from the clinic visit, which required an algorithm to identify laboratory testing performed for surveillance. We also could not identify if any additional laboratory testing might have been performed outside our institution. In addition, patients with advanced-stage disease were under-represented in our cohort compared with early stage. However, the main driver of the poor utility of surveillance laboratory testing is the low relapse rate, and in Table 3 the rates of relapse were similar between patients with early- and advanced-stage disease. Although Stanford V is not widely used outside of our institution, in a randomized phase III study there was not significant difference in outcomes when compared with the more commonly used doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) regimen.6 Therefore, we believe the results of this study can be extrapolated to ABVD and ABVD-like regimens.
To our knowledge, this is the largest study examining the utility of surveillance laboratory testing on patients with CHL treated with contemporary therapy. Despite restricting our cohort to patients in whom surveillance testing would likely be beneficial, we were unable to find a clear benefit in asymptomatic patients. Although the high NPV of surveillance testing may provide reassurance to patients, 77% of patients had at least one abnormal blood test, whereas only 9% ultimately relapsed. This high rate of unexplained abnormality seen in our study and others leads to a poor PPV in asymptomatic patients and unnecessary patient anxiety22 and may lead to additional imaging and biopsy procedures.
We believe that our results are provocative, and we hope they will be discussed by guideline committees across the globe (NCCN and ESMO) when recommendations for surveillance are being considered. Although surveillance laboratories may be useful in select settings (eg, monitoring for hypothyroidism in patients treated with neck radiation), these results do not justify their routine use in the detection of relapse. Preliminary results of novel methods of relapse detection such as circulating tumor DNA may prove to be more sensitive/specific, and larger studies are required to validate these results before they can be incorporated clinically.23
Appendix
TABLE A1.
Patients Who Had Biopsy During the Course of Surveillance Sorted by Eventual Relapse as Well as Reason for the Work-Up That Initially Prompted the First Biopsy
PRIOR PRESENTATION
Presented at the 11th International Symposium on Hodgkin Lymphoma, Cologne, Germany, October 28, 2018; and the 61st American Society of Hematology Annual Meeting, San Diego, CA, December 3, 2018.
SUPPORT
Supported in part by Cancer Center Support Grant No. 5P30CA124435 and Stanford National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award No. UL1 RR025744; in particular, the research was facilitated by the Biostatistics Shared Resource.
AUTHOR CONTRIBUTIONS
Conception and design: Ryan C. Lynch, Richard T. Hoppe, Ranjana Advani
Provision of study material or patients: Richard T. Hoppe, Ranjana Advani
Collection and assembly of data: Ryan C. Lynch, Solomon Henry, Douglas Wood, Sarah Daadi, Ranjana Advani
Data analysis and interpretation: Ryan C. Lynch, Vandana Sundaram, Manisha Desai, Richard T. Hoppe, Ranjana Advani
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Utility of Routine Surveillance Laboratory Testing in Detecting Relapse in Patients With Classic Hodgkin Lymphoma in First Remission: Results From a Large Single-Institution Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ryan C. Lynch
Research Funding: Cyteir, Juno Therapeutics, Bayer, Incyte, Rhizen Pharmaceuticals, TG Therapeutics, Takeda
Vandana Sundaram
Employment: Varian Medical Systems (I)
Stock and Other Ownership Interests: Varian Medical Systems (I)
Travel, Accommodations, Expenses: Varian Medical Systems (I)
Manisha Desai
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Janssen Research & Development (Inst)
Richard T. Hoppe
Stock and Other Ownership Interests: Johnson & Johnson, Pfizer
Ranjana Advani
Honoraria: Takeda
Consulting or Advisory Role: Genentech/Roche, Gilead Sciences, Bayer, Cell Medica, Seattle Genetics, AstraZeneca, Takeda, Kyowa, Kite Pharma, Portola Pharmaceuticals, Celgene, Sanofi
Research Funding: Millennium (Inst), Seattle Genetics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Janssen (Inst), Celgene (Inst), Agensys (Inst), Merck (Inst), Kura (Inst), Regeneron (Inst), Infinity Pharmaceuticals (Inst), Forty Seven (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Advani RH, Hoppe RT, Baer D, et al. Efficacy of abbreviated Stanford V chemotherapy and involved-field radiotherapy in early-stage Hodgkin lymphoma: Mature results of the G4 trial. Ann Oncol. 2013;24:1044–1048. doi: 10.1093/annonc/mds542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 3.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 4.André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 5.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Press OW, Li H, Schöder H, et al. US Intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34:2020–2027. doi: 10.1200/JCO.2015.63.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AI, Zuckerman DS, Van den Abbeele AD, et al. Surveillance imaging of Hodgkin lymphoma patients in first remission: A clinical and economic analysis. Cancer. 2010;116:3835–3842. doi: 10.1002/cncr.25240. [DOI] [PubMed] [Google Scholar]
- 10.Patel V, Buckstein M, Perini R, et al. Computed tomography and positron emission tomography/computed tomography surveillance after combined modality treatment of supradiaphragmatic Hodgkin lymphoma: A clinical and economic perspective. Leuk Lymphoma. 2013;54:2168–2176. doi: 10.3109/10428194.2013.767902. [DOI] [PubMed] [Google Scholar]
- 11.Jerusalem G, Beguin Y, Fassotte MF, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol. 2003;14:123–130. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe RT, Advani RH, Ai WZ, et al. NCCN guidelines insights: Hodgkin lymphoma, version 1.2018. J Natl Compr Canc Netw. 2018;16:245–254. doi: 10.6004/jnccn.2018.0013. [DOI] [PubMed] [Google Scholar]
- 13.Eichenauer DA, Aleman BMP, Andre M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19–iv29. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 14.Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: A subset analysis of the North American Intergroup E2496 Trial. J Clin Oncol. 2015;33:1936–1942. doi: 10.1200/JCO.2014.57.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 16.Mercaldo ND, Lau KF, Zhou XH. Confidence intervals for predictive values with an emphasis to case-control studies. Stat Med. 2007;26:2170–2183. doi: 10.1002/sim.2677. [DOI] [PubMed] [Google Scholar]
- 17. Wickham H: ggplot2: Elegant Graphics for Data Analysis (ed 2). New York, NY, Springer, 2016, pp 276. [Google Scholar]
- 18.Trevethan R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front Public Health. 2017;5:307. doi: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin’s disease: Role of routine follow-up studies. J Clin Oncol. 1997;15:1123–1130. doi: 10.1200/JCO.1997.15.3.1123. [DOI] [PubMed] [Google Scholar]
- 20.Dryver ET, Jernström H, Tompkins K, et al. Follow-up of patients with Hodgkin’s disease following curative treatment: The routine CT scan is of little value. Br J Cancer. 2003;89:482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkes EA, Loh Z, Estacio O, et al. Routine blood investigations have limited utility in surveillance of aggressive lymphoma in asymptomatic patients in complete remission. Br J Cancer. 2018;119:546–550. doi: 10.1038/s41416-018-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21:2262–2266. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spina V, Bruscaggin A, Cuccaro A, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131:2413–2425. doi: 10.1182/blood-2017-11-812073. [DOI] [PubMed] [Google Scholar]