Abstract
INTRODUCTION:
In 2014, a reflexive screening protocol for Lynch syndrome (LS) via an immunohistochemistry (IHC) assay was shown to be cost-effective; however, the screening rates at a predominant Hispanic-rich institution are unclear. We hypothesized that implementation of a universal tumor screening (UTS) protocol requiring screening for LS via IHC in patients with newly diagnosed colorectal cancer (CRC) at our Hispanic-rich institution would improve detection of LS by increasing screening rates.
METHODS AND MATERIALS:
This is a retrospective analysis of screening rates of 3 sequential cohorts of newly diagnosed patients with CRC between January 2012 and April 2016 at the University Health System and with follow-up at National Cancer Institute–designated Mays Cancer Center at University of Texas Health San Antonio. Cohort 1 consisted of patients screened using old screening guidelines (PRE). Cohort 2 consisted of patients screened when treating clinicians were receiving education on the new protocol (PERI). Cohort 3 consisted of patients screened after implementation of the UTS protocol (POST).
RESULTS:
The majority of 312 patients were Hispanic (62.5%), 18.1% were < 50 years, and 81.9% were ≥ 50 years of age (median age, 57 years). Of patients with CRC screened for LS via IHC, the PRE, PERI, and POST cohorts had screening rates of 31%, 64%, and 58%, respectively. We found significant differences when comparing the PRE with POST sequential cohorts (P < .01).
CONCLUSION:
The quality of Lynch syndrome–related family histories and screening rates were significantly improved after implementation in our Hispanic-rich population. Future studies are warranted to provide insight into clinical effects of increased screening, provider and patient surveillance, and screening-related systemic barriers.
INTRODUCTION
Epidemiology
Lynch syndrome (LS; ie, hereditary nonpolyposis colorectal cancer), the most common hereditary cause of colorectal cancer (CRC), accounts for 3%-5% of all patients with CRC.1,2 It has been estimated that the prevalence of LS in the United States is 1 in 370 or 0.3%, with an estimated > 98% of patients with LS going undiagnosed, which is concerning, given the abundant evidence that early detection of LS with subsequent cancer surveillance and/or risk-reducing surgeries can lower cancer-related morbidity and mortality.3 With LS, the estimated cumulative risks of CRC by 50 and 80 years of age are 13% and 42%, respectively.1,4 Furthermore, the estimated cumulative risk of endometrial cancer, the second most common LS-related cancer, by 80 years of age is 35%.1,4 Other LS-related cancers include primary cancers of the ovary, stomach, small bowel, hepatobiliary tract, CNS, genitourinary tract, and integumentary system.5,6
Diagnosis and Screening
The historical clinical criteria to screen for LS (ie, Amsterdam II and revised Bethesda guidelines) use personal and family history of malignancies to identify high-risk individuals in need of LS screening via laboratory testing, resulting in 1 in 4 patients with LS going undiagnosed.3,7,8 Additionally, approximately 90% of those meeting the historical clinical criteria did not undergo LS screening at all.9 Given the low sensitivity of these criteria, the universal tumor screening (UTS) protocol has been recommended.9
The definitive diagnosis of LS is determined by the presence of one or more germline mutations in 4 DNA mismatch repair (MMR) genes: MLH1, MSH2, MSH6, and PMS2. Mutation in one or more of these genes causes loss of MMR protein function, which subsequently leads to DNA replication errors in repetitive sequences known as microsatellite DNA.4,10 The 2 most common laboratory methods used to screen for LS are polymerase chain reaction (PCR) to detect the presence of microsatellite instability (MSI) and loss of immunohistochemistry (IHC) staining on the tumor specimen. Because the sensitivity and specificity of MSI PCR and IHC testing are virtually equivalent (85%-90% v 83%-90%, respectively), IHC has become the preferred method for LS screening because of accessibility, gene specificity, and cost effectiveness (72%-86% cheaper than MSI PCR).3,11-13 To detect sporadic CRC and prevent unnecessary germline mutation testing, data suggest inclusion of reflex testing for BRAF mutation (V600E being the most common) and/or MLH1 promoter methylation analysis in cases of MLH1 expression loss.14 Because the incidence of BRAF mutation is high (> 40% in MLH1 expression loss), implementation of a reflex testing protocol can assist in rapid and consistent differentiation between germline and somatic etiology.14
Universal Tumor Screening
For any universal screening protocol to be effective, it must satisfy 4 prerequisites: feasibility, desirability, cost efficacy, and compliance with the principle of nonmaleficence.3 Per a previous analysis, large-scale screening for LS using IHC has been found to meet these criteria and has been considered an “attractive and even compelling option [for UTS].”3 Reflexive IHC screening for LS was recommended by several organizations, including the United States Multi-Society Task Force (USMSTF) and National Comprehensive Cancer Network (NCCN). In the largest published population-based analysis of UTS for LS, > 10,000 patients with CRC were screened for LS.4 Positive screening results were found in approximately 15% of patients, and > 3% of the patient population was diagnosed with LS by germline mutational testing.4 The application of historical clinical screening criteria (revised Bethesda guidelines) presumed that 12% of patients with LS would have remained undiagnosed.4
Purpose
Although UTS implementation has been studied at numerous institutions with large patient populations, there are no known prior studies examining implementation of UTS for LS in a minority-majority Hispanic population such as ours. Compared with non-Hispanics, it has been observed that the Hispanic population has a lower socioeconomic status leading to less access to high-quality care and uninsured status, which are potential barriers to UTS.15-17 To improve LS detection at our institution, we implemented a UTS protocol that reflexes to IHC testing to detect MMR mutations in all newly diagnosed patients with CRC. In this study, we evaluated the effectiveness of a UTS program for LS at our majority Hispanic county hospital, serving the largest minority ethnicity in the United States.18 We hypothesized that the implementation of this protocol would result in higher screening numbers compared with the historical clinical screening criteria. Additional questions included whether higher screening rates would be observed in patients < 50 years of age or in patients ≥ 50 years of age, as well as whether the UTS protocol would result in more complete taking of family histories.
METHODS AND MATERIALS
We present a single-center retrospective chart review analysis of all new diagnoses of CRC at University Health System’s Hospital (San Antonio, TX), with follow-up at National Cancer Institute–designated Mays Cancer Center at University of Texas Health San Antonio, from January 1, 2012, through April 30, 2016. Retrospective analysis was performed by manual chart review of the electronic medical record (EMR) by 2 physicians who validated the abstraction. The EMR and local tumor registry data were used to define the specified patient population. Patients with other cancers in the colorectum, including neuroendocrine tumor, GI stromal tumor, and cancers metastatic to the colon or rectum, were excluded from this analysis.
The intervention of focus was creation and implementation of a reflexive testing algorithm for MMR IHC of all new CRC diagnoses (Fig 1). Implementation of the UTS algorithm occurred by education (both verbally and via printed material) of all responsible providers, including gastroenterologists, medical oncologists, surgeons, and pathologists. The pathologists were solely responsible for verbally requesting and confirming the order for “reflexive testing” as well as MMR IHC, BRAF, and/or MLH1 hypermethylation testing, after having received appropriate education (Fig 1). The UTS algorithm was not incorporated into the EMR. IHC test results were reported to the ordering physician (either gastroenterologist who performed colonoscopy or provider who ordered the biopsy to diagnose CRC) via telephone or secure messaging. IHC results showing staining loss of MSH2, MSH6, PMS2, and nonsporadic MLH1 were referred to genetics counseling by the treating oncologist. If BRAF mutation alone or BRAF mutation and MLH1 hypermethylation together were found on reflexive testing, then referral to genetics counseling was not indicated (unless the patient met the criteria for genetic testing because of family history).
Fig 1.
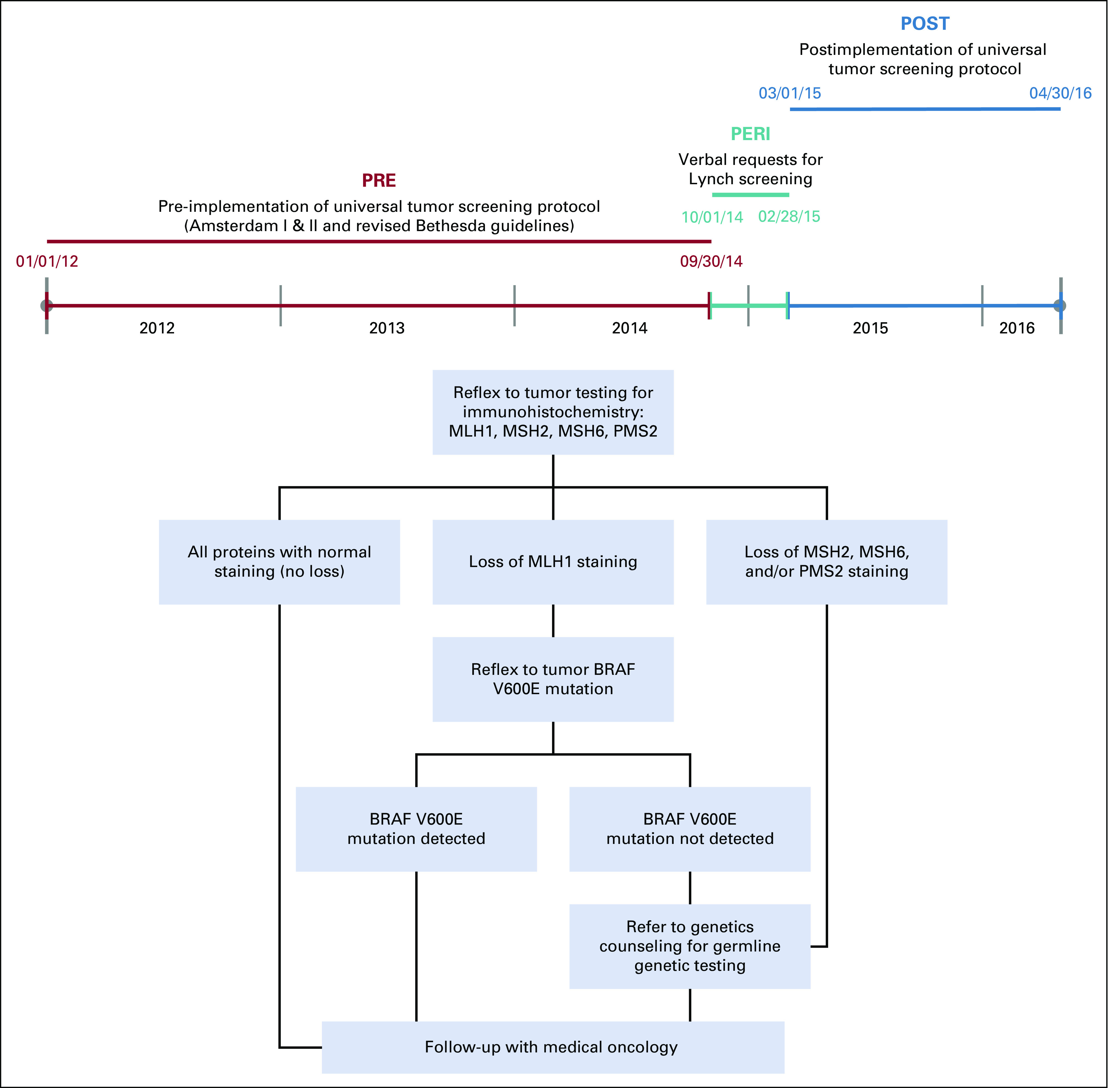
Timeline and defined cohort interventions representing the different patient populations who underwent the program’s algorithm for universal tumor screening via immunohistochemistry testing. PRE, intervention cohort comprising patients screened for Lynch syndrome (LS) per old guidelines (Amsterdam I, Amsterdam II, and revised Bethesda) from January 1, 2012, to September 9, 2014. PERI, intervention cohort comprising patients screened for LS after clinician education on new guidelines from October 1, 2014, to February 28, 2015. POST, intervention cohort comprising patients screened for LS after implementation of US from March 1, 2015, to April 30, 2016. MLH1, MSH2, MSH6, PMS2, DNA mismatch repair (MMR) genes. BRAF V600E mutation, somatic mutation not consistent with germline mutation of LS.
Patients were distributed into 3 sequential cohorts: (1) the pre-intervention (PRE) cohort consisted of newly diagnosed patients with CRC screened for LS using the historical clinical screening criteria (between January 1, 2012, and September 30, 2014); (2) the peri-intervention (PERI) cohort consisted of newly diagnosed patients with CRC screened for LS after education of providers on UTS (between October 1, 2014, and February 28, 2015). During this PERI period, IHC testing was ordered by individual treating physicians; (3) the postintervention (POST) cohort consisted of patients screened for LS after implementation of a reflexive UTS protocol, which included MMR IHC, BRAF, and/or MLH1 hypermethylation ordered by the pathologist (between March 1, 2015, and April 30, 2016; Fig 1). As part of the UTS algorithm, the treating physicians were responsible for referral to genetics counseling, when appropriate.
The primary endpoint of this project was to detect the increased proportion of patients screened for LS via IHC testing. The secondary endpoints included the quality of reported family history obtained by the clinician, incidence of positive family history for LS-related cancers, and proportion of patients who received genetics counseling. Quality or adequacy of taking the family history was assessed based on the presence of 2 criteria: cancer type and age at diagnosis. If both criteria were not documented, family history was considered inadequate. As a post hoc analysis, we evaluated for statistical differences in baseline patient characteristics that may have affected the successful implementation of UTS. The baseline characteristics included ethnicity, sex, mean age at diagnosis, presence or absence of CRC risk factors (smoking, alcohol abuse, obesity with a body mass index > 30 kg/m2, diabetes, and history of cholecystectomy), presence or absence of cancer family history, stage at diagnosis, and insurance status at diagnosis.
Statistical analysis was performed using R version 3.6.1 (2019-07-05). Patients were separated into 3 cohorts: PRE, PERI, and POST. Patient records missing relevant data points for analysis were excluded from the study population. Patients in each cohort were identified as < 50 years of age or ≥ 50 years of age for the analysis of LS screening rates via IHC. Pearson’s χ2 test was performed comparing PRE, PERI, and POST cohorts for race/ethnicity, sex, insured status, presence of risk factors, family history, IHC testing, KRAS testing, germline testing, and genetics counseling. P values < .05 were found for insurance status, presence of risk factors, family history, IHC testing KRAS testing, germline testing, and genetic counseling. Post hoc tests comparing PRE versus POST cohorts were performed for each of these variables by Welch two-sample t test with a 95% confidence level. P values for comparisons of cohorts, which were screened for LS via IHC and subsequently received genetic counseling, were unable to be performed due to low sample size and zero variance in the POST population.
RESULTS
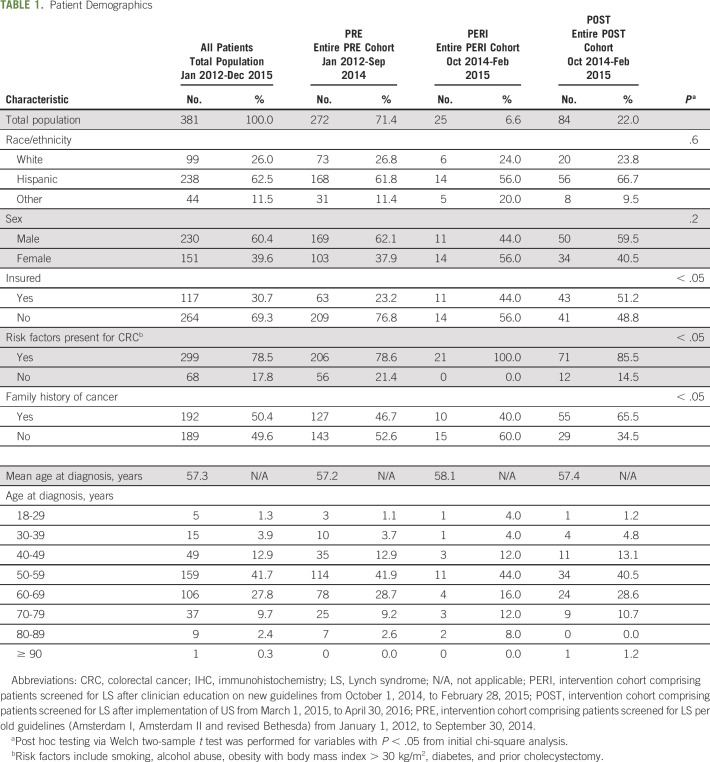
Baseline patient characteristics were similar across all sequential cohorts (Table 1). Of the 381 patients included in this analysis, there were 230 male patients (60.4%) and 151 female patients (39.6%). There were 69 patients (18.1%) < 50 years of age and 312 patients (81.9%) ≥ 50 years of age. The median age was 57 years (interquartile range, 51-63 years). Hispanics accounted for 62.5% of patients (n = 238), and non-Hispanics accounted for 37.5% of patients (n = 143). There were also 117 insured patients (30.7%) and 264 uninsured patients (69.3%). Of the patients with evaluable stage at diagnosis, 10.8% were stage I, 31.4% were stage II, 36.1% were stage III, and 21.7% were stage IV. For a subset of patients, baseline patient characteristic analyses were not performed because of the absence of sufficient EMR documentation.
TABLE 1.
Patient Demographics
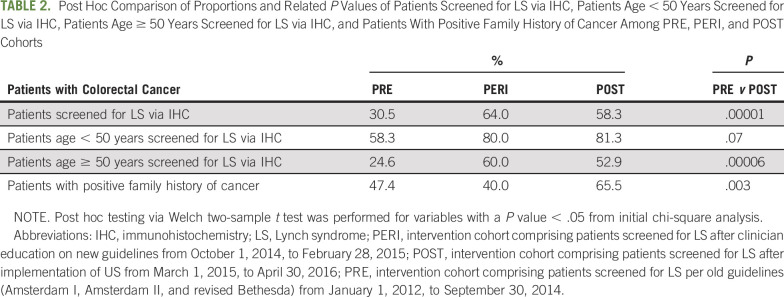
The proportions of patients screened for LS via IHC testing out of all patients diagnosed with CRC were 31% (n = 83) in the PRE cohort, 64% (n = 16) in the PERI cohort, and 58% (n = 49) in the POST cohort (Table 2). When comparing the PRE and POST cohorts, we found statistically significant differences between the proportions of patients with CRC screened for LS via IHC (P < .01). Among patients < 50 years of age at diagnosis, we found no significant difference in the proportion of patients screened for LS via IHC between the PRE (58%) and POST (81%) groups (Table 2). There was a significant difference in incidence of UTS in patients diagnosed at ≥ 50 years of age when we compared PRE versus POST cohorts (24.6% v 52.9%; P < .001, respectively; Table 2).
TABLE 2.
Post Hoc Comparison of Proportions and Related P Values of Patients Screened for LS via IHC, Patients Age < 50 Years Screened for LS via IHC, Patients Age ≥ 50 Years Screened for LS via IHC, and Patients With Positive Family History of Cancer Among PRE, PERI, and POST Cohorts
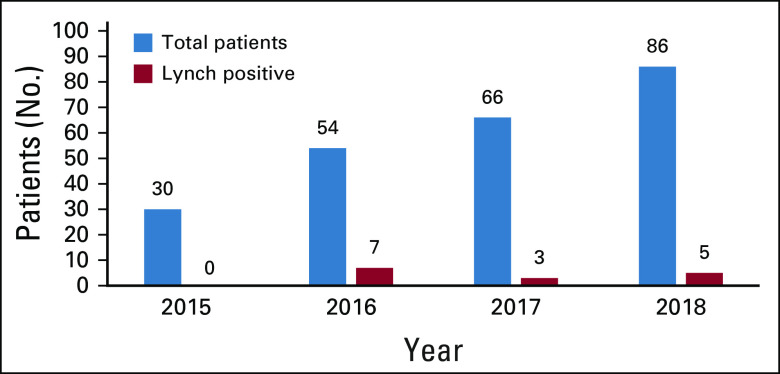
Of all patients screened for LS, 12.1%, 18.8%, and 4.1% screened positive with IHC in the PRE, PERI, and POST cohorts, respectively, with 58.8% and 41.2% being MLH1/PMS2 and MSH2/MSH6 deficient, respectively. Any MLH1 staining loss detected by IHC was observed in 2.6%, 8.0%, and 1.2% for the PRE, PERI, and POST cohorts, respectively. Of this specified subgroup, BRAF mutation and/or MLH1 hypermethylation testing was appropriately performed in 14.3%, 12.5%, and 100.0% for the PRE, PERI, and POST cohorts, respectively. Zero patients with a BRAF mutation in any cohort were subsequently referred to genetics counseling. Among those patients with IHC staining loss for MSH2, MSH6, or PMS2 or nonsporadic loss of MLH1, 30.0%, 66.7%, and 100.0% were referred to genetic counseling in the PRE, PERI, and POST cohorts, respectively. Of those patients referred to genetics counseling, 46.7% in the PRE cohort, 75.0% in the PERI cohort, and 80.0% in the POST cohort completed the process, including counseling and testing. Of those patients who completed germline testing, 13.3% were diagnosed with LS (50% MLH1, 50% PMS2, 0% MSH2, and 0% MSH6). Of those patients who did not complete the process, 66.7% were medically uninsured. Additional post hoc analysis of patient data between 2015 and 2018 showed that of 236 patients referred to the GI genetics clinic, 6.4% (n = 15) were diagnosed with LS based on germline testing results (Appendix Fig A1, online only).
Among patients with a positive family history of cancer, we observed that < 25% in each sequential cohort had an adequate family history recorded in the EMR (PRE, 8.8%; POST, 20.2%). Adequacy of family history–taking of patients with a positive family history of cancer improved significantly (P < .05) after clinicians were educated on new laboratory testing guidelines and implementation of UTS. Adequate family history–taking after intervention also improved because we observed an increase in the detection of positive family history (PRE, 47.4% v POST, 65.5%; P < .01).
DISCUSSION
In this retrospective analysis, we examined the efficacy of UTS implementation in detecting LS in all patients with new diagnoses of CRC within a predominantly Hispanic and uninsured patient population at a county metropolitan hospital in Texas. Three sequential cohorts consisted of a predominance of male uninsured Hispanic patients > 50 years of age and were not significantly different in regard to baseline patient characteristics. This is not surprising, given that the risk of CRC in patients with LS is greatest among men > 50 years of age.1,4
Despite patient population size limitations, the project’s primary endpoint, a significant improvement in incidence of LS screening, was achieved. The observed statistically significant improvements in LS screening occurred after clinician education on the new screening guidelines and after implementation of the UTS protocol.
We implemented UTS in the postintervention cohort but unfortunately did not meet the goal of 100% compliance. We can attribute this to human error, because the pathologists were unable to follow the implemented algorithm with complete compliance. There was initial resistance to habit formation of behavioral change as expected by the ordering pathologists. We believe the interventional time period (PERI cohort) was not long enough to allow for adaptation of behavioral change to occur. We did not observe a significant improvement in LS screening incidence in patients < 50 years of age. We attribute this to a substantially smaller patient population in this age group (n = 69; 18.1%)1,4. More importantly, because the highest risk of CRC and LS is seen in patients ≥ 50 years of age, we focused more on the significant improvement in LS screening in patients ≥ 50 years of age after clinician education and implementation of UTS.
In the future, to attain 100% compliance, we plan to incorporate this algorithm into the electronic pathologic reporting system. In a prior study conducted by pathologists and gynecologic oncologists at the University of Washington, a reflexive LS screening program was implemented. An algorithm to conduct MMR IHC testing for all new diagnoses of endometrial cancer in patients ≤ 60 years of age was incorporated into the electronic pathology reporting system. The pathologists were required to follow the specified algorithm to sign out each endometrial cancer pathology report. Prior to implementation, approximately 15% of patients did not receive appropriate MMR IHC testing. After implementation, > 3% of patients still did not receive appropriate testing. This improvement was statistically significant (P < .05) but does emphasize that even with a stop-gap reflexive testing algorithm, human error persists. It was hypothesized that human error in this study was most likely due to miscommunication, failure to follow through, and busy workload at an academic medical center. It was suggested that the addition of an automated ordering system, when indicated by the reflex testing algorithm, would hopefully lead to 100% compliance.19
As a secondary endpoint, we evaluated the incidence of positive screening results and found a similar proportion based on prior studies without significant differences between cohorts.4 More importantly, the majority of these patients were appropriately referred to genetics counseling after clinician education and UTS implementation, and zero patients were inappropriately referred to genetics counseling based on IHC results (ie, MLH1 expression loss with BRAF mutation). This is noteworthy because before UTS implementation, appropriate referral rates were less than one third. We also found that a previously defined significant barrier to LS diagnosis, that is, patient noncompliance with genetics counseling and testing, improved significantly during and after UTS implementation.20,21 Of those patients who were nonadherent, the majority were medically uninsured, which is important, given that uninsured status is a previously defined poor risk factor for CRC outcomes.15-17
Although UTS has been shown to identify more patients with LS compared with historical screening protocols, implementation of UTS still faces many barriers and is vastly underused despite being recommended by the USMSTF and NCCN.3,9 In a prior survey of more than 100 cancer programs, fewer than 75% of National Cancer Institute–designated comprehensive cancer centers and only 15% of community hospitals surveyed were performing UTS for LS among patients with CRC.22 The barriers to UTS identified in this study were primarily attributed to lack of necessary resources and education on criteria and implementation of UTS.22
Other studies have also evaluated barriers to UTS for LS. The most significant barrier was identified as patient noncompliance in the genetics counseling and testing process due to perceived lack of benefit by the patient.20,21 In an effort to address this barrier, a model that involves streamlined UTS testing (automatic reflex to BRAF testing and genetics counseling referral), increased collaboration with genetic counselors (including UTS result tracking, genetics counseling referral facilitation, and disclosure of UTS results to patients), and assistance in overcoming barriers to follow-up, such as introducing genetic counseling at postoperative appointments, has been proposed.20
The importance of family history in assessing LS risk is evident in previous clinical guidelines for screening.9 In our study, the incidence of positive family history for malignancy (approximately 50%) was similar across all sequential cohorts. We also demonstrated that adequacy of family history–taking improved after implementation of UTS, which correlated with increased detection of positive family history, which may allow for screening for other hereditary cancer syndromes.
Because LS only accounts for approximately 3%-5% of all CRC, the incidence of positive LS screening in our population was low, as expected. However, we believe successful implementation of UTS for LS improved clinician awareness of the importance for LS screening in CRC and resulted in an increase in referrals to genetics counseling for germline mutation testing to diagnose LS. Between 2015 and 2018, we were able to definitively diagnose 15 patients (6.4% of all patients referred to the GI genetics clinic) with LS after the institution of UTS for LS, which is above the expected rate of 3%-5%.1,2 The primary reason for collecting data on genetics counseling and testing after completion of cohort intervention periods was to allow sufficient time for completion of the complex process, which has known barriers to completion.20,21
Limitations and Future Studies
Limitations of this study include the absence of multivariate analysis resulting in multiple independent t tests performed when analyzing the outcome variables, the small patient population of the study cohorts, and unequal distribution of time periods among the 3 sequential cohorts, which limited the power to determine statistically significant differences in LS screening incidence and diagnosis between the cohorts. This can be improved with future expansion of this study to include more patients to adequately power the study. Additionally, our pathologists manually ordered the reflex testing once a CRC diagnosis was made and resulted in human error. This contributed substantially to lower UTS rates after implementation. This can be improved in future studies by instituting an automated EMR-based reflex laboratory test screening and ordering algorithm that would prevent human error.19
The high rate of uninsured Hispanic patients proved to be a major barrier to completion of genetics counseling and subsequent detection of LS in our predominantly Hispanic uninsured patient population. In 2 landmark population-based analyses (one at a large single-center academic institution and one being a multicenter international analysis), the impact of ethnicity or race on UTS implementation and results was not analyzed.4,21 Prior studies have noted disparities in the incidence, stage at diagnosis, and cancer-related mortality of CRC diagnosis in the Hispanic population that could be attributed to lower socioeconomic status and higher uninsured rate.15-17,23,24 To our knowledge, this is the first known analysis of UTS implementation for LS in a predominantly Hispanic uninsured patient population. Since this analysis, our genetics program has been working on grants and other programs to help with the cost of germline testing. The clinical effects of Hispanic ethnicity and uninsured status separately and together on LS detection and outcomes should be explored in future analysis.
Future prospective studies are warranted to provide insight into clinical effects of increased screening, including surveillance by provider and patient, and systemic barriers to universal IHC. In the future, we aim to increase collaboration with genetics counseling via automated referrals with positive screening results and increase accessibility and convenience of patient care. Currently, our institution is addressing the barriers to improve long-term follow-up, which is crucial in preventing and detecting early-stage LS-related malignancies.
The primary endpoint was reached in terms of significant improvement in incidence of LS screening after implementation of UTS. Adequacy of family history–taking (in patients with a positive family history of cancer) also significantly improved postintervention. With adequate family history–taking, there was an increase in detection of LS-related cancers as well as screening for other hereditary cancer syndromes. We observed a higher than expected rate of LS diagnosis in our unique Hispanic uninsured patient population, which deserves special consideration in future studies.
Appendix
Fig A1.
Number of patients seen in the GI genetics clinic and diagnosed with Lynch syndrome by germline testing, by year.
PRIOR PRESENTATION
Presented at the Texas Society of Clinical Oncology Annual Conference, San Antonio, TX, September 14, 2019, and ASCO Quality Care Symposium, San Diego, CA, March 3-4, 2017.
SUPPORT
Supported by the National Institutes of Health (CA054174) and the National Institute on Aging (AG044271).
AUTHOR CONTRIBUTIONS
Conception and design: Tyler W. Snedden, Anna Taranova, Samina Qamar, Sukeshi Patel Arora
Administrative support: Anna Taranova, Roberto Villarreal
Provision of study materials or patients: Roberto Villarreal
Collection and assembly of data: Tyler W. Snedden, Anna Taranova, Roberto Villarreal, Sukeshi Patel Arora
Data analysis and interpretation: Tyler W. Snedden, Andrew McCracken, Anusha Vaidyanathan, Anna Taranova, Roberto Villarreal, Sukeshi Patel Arora
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Implementation of Universal Tumor Screening of Colorectal Cancer for Detection of Lynch Syndrome at a Hispanic-Rich County Hospital
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tyler W. Snedden
Honoraria: M3 Global Research, Medscape Surveys, All Global Circle
Sukeshi Patel Arora
Speakers' Bureau: Bayer, Exelixis
Travel, Accommodations, Expenses: Lexicon, Ipsen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 2.Burt RW, DiSario JA, Cannon-Albright L. Genetics of colon cancer: Impact of inheritance on colon cancer risk. Annu Rev Med. 1995;46:371–379. doi: 10.1146/annurev.med.46.1.371. [DOI] [PubMed] [Google Scholar]
- 3.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993;71:677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 9.Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933–940. doi: 10.1038/gim.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligtenberg MJ, Kuiper RP, Geurts van Kessel A, et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer. 2013;12:169–174. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 11.Palomaki GE, McClain MR, Melillo S, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: A cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 14.Loughrey MB, Waring PM, Tan A, et al. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer. 2007;6:301–310. doi: 10.1007/s10689-007-9124-1. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65:457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 16.Le H, Ziogas A, Lipkin SM, et al. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17:1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 17.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: A retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Census Bureau: The United States Census Bureau Population Estimate. https://www.census.gov/. 2018.
- 19.Kilgore MR, McIlwain CA, Schmidt RA, et al. Reflex test reminders in required cancer synoptic templates decrease order entry error: An analysis of mismatch repair immunohistochemical orders to screen for Lynch syndrome. J Pathol Inform. 2016;7:48. doi: 10.4103/2153-3539.194840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cragun D, DeBate RD, Vadaparampil ST, et al. Comparing universal Lynch syndrome tumor-screening programs to evaluate associations between implementation strategies and patient follow-through. Genet Med. 2014;16:773–782. doi: 10.1038/gim.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heald B, Plesec T, Liu X, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013;31:1336–1340. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant SR, Walker GV, Guadagnolo BA, et al. Variation in insurance status by patient demographics and tumor site among nonelderly adult patients with cancer. Cancer. 2015;121:2020–2028. doi: 10.1002/cncr.29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafri NS, Gould M, El-Serag HB, et al. Incidence and survival of colorectal cancer among Hispanics in the United States: A population-based study. Dig Dis Sci. 2013;58:2052–2060. doi: 10.1007/s10620-012-2454-3. [DOI] [PubMed] [Google Scholar]