Abstract
PURPOSE:
Guidelines recommend venous thromboembolism (VTE) risk assessment in outpatients with cancer and pharmacologic thromboprophylaxis in selected patients at high risk for VTE. Although validated risk stratification tools are available, < 10% of oncologists use a risk assessment tool, and rates of VTE prophylaxis in high-risk patients are low in practice. We hypothesized that implementation of a systems-based program that uses the electronic health record (EHR) and offers personalized VTE prophylaxis recommendations would increase VTE risk assessment rates in patients initiating outpatient chemotherapy.
PATIENTS AND METHODS:
Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) was a multidisciplinary program implemented by nurses, oncologists, pharmacists, hematologists, advanced practice providers, and quality partners. We prospectively identified high-risk patients using the Khorana and Protecht scores (≥ 3 points) via an EHR-based risk assessment tool. Patients with a predicted high risk of VTE during treatment were offered a hematology consultation to consider VTE prophylaxis. Results of the consultation were communicated to the treating oncologist, and clinical outcomes were tracked.
RESULTS:
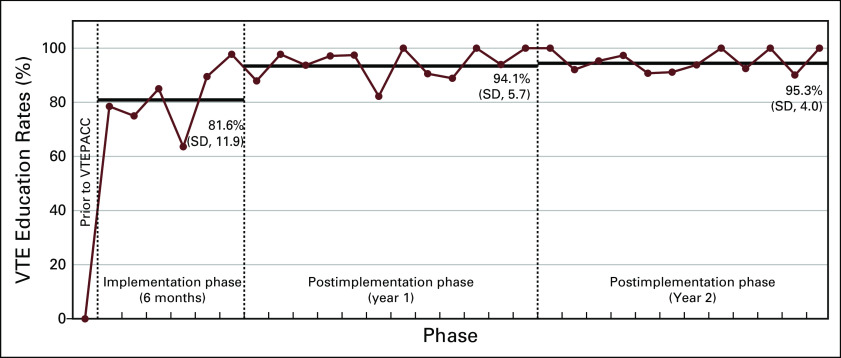
A total of 918 outpatients with cancer initiating cancer-directed therapy were evaluated. VTE monthly education rates increased from < 5% before VTEPACC to 81.6% (standard deviation [SD], 11.9; range, 63.6%-97.7%) during the implementation phase and 94.7% (SD, 4.9; range, 82.1%-100%) for the full 2-year postimplementation phase. In the postimplementation phase, 213 patients (23.2%) were identified as being at high risk for developing a VTE. Referrals to hematology were offered to 151 patients (71%), with 141 patients (93%) being assessed and 93.8% receiving VTE prophylaxis.
CONCLUSION:
VTEPACC is a successful model for guideline implementation to provide VTE risk assessment and prophylaxis to prevent cancer-associated thrombosis in outpatients. Methods applied can readily translate into practice and overcome the current implementation gaps between guidelines and clinical practice.
INTRODUCTION
Primary prevention of venous thromboembolism (VTE) has been shown to be successful in ambulatory patients with cancer receiving chemotherapy, with an average reduction in VTE rates of approximately 50%.1-6 National guidelines for VTE prophylaxis in ambulatory patients with cancer recommend an individual patient risk assessment and targeted prophylaxis for patients with cancer at the highest risk of VTE7-9 because of the variable rates of reported bleeding risk and the lack of a clear survival advantage.6,10 However, data from the Association of Community Cancer Centers reported that only 9% of oncology practitioners reported using a structured risk assessment tool in the outpatient setting, and 44% of practices reported < 10% of their outpatients with cancer had clear documentation of VTE risk.10a A successful model for guidelines implementation is needed to increase VTE risk assessment and prevention in patients with cancer.
VTE risk assessment models have been studied in outpatients with cancer in an effort to identify the 10%-20% of all patients with cancer most likely to develop a VTE and benefit most from VTE prophylaxis.11,12 The most well-studied and validated VTE risk assessment tool was developed by Khorana et al13 and focuses on outpatients with cancer initiating chemotherapy. This risk stratification tool assesses prechemotherapy WBC count, hemoglobin and platelet count, body mass index, and tumor type, and is recommended by the National Comprehensive Cancer Network to identify patients with cancer at high risk for VTE. Practical and evidence-based approaches for implementing risk assessments and prophylaxis prescribing in the oncology clinic are not available.
We report a multidisciplinary intervention, Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC), designed to improve guideline adherence in outpatients initiating cancer-directed therapy. Our approach focused on integration into systems of practice, incorporation of patient preferences, and inclusion of a multidisciplinary team.
OBJECTIVES
The aims of this project were to develop an effective model to improve VTE education and risk assessment rates, and increase the percentage of high-risk patients receiving VTE prophylaxis using a novel multidisciplinary and electronic health record (EHR)-based program for guideline implementation.
PATIENTS AND METHODS
VTEPACC was a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center using a preimplementation and postimplementation study design. The program was designed to provide VTE education and risk assessment to all patients with cancer initiating any cancer-directed therapy (chemotherapy, targeted therapy, or immunotherapy) in the outpatient setting at a single institution, the University of Vermont Cancer Center. Institutional review board approval was obtained (CHRMS 16-145) before initiation.
Patient eligibility was based on a histologic confirmation of cancer that required initiation of cancer-directed therapy. The therapy received by the patient was determined by the treating oncologist. Patients with all malignancy types, including lung, breast, head and neck, renal, pancreatic, upper and lower GI, gynecologic, and urologic cancer, were included. Patients with lymphoma were included; however, patients with a hematologic malignancy, such as those with leukemia, were excluded because therapy initiation was generally in the inpatient setting. Patients at any stage of disease requiring therapy were included; however, patients with early-stage disease who received radiation therapy only were not assessed as part of the VTEPACC program. Patients were excluded if they (1) received hormonal therapy only or (2) had a confirmed diagnosis of VTE at the time of VTE risk assessment. Patients with brain tumors were included at the time of study initiation and subsequently were excluded after the initiation of an investigator clinical trial enrolling this patient group at our institution. Patients who were receiving anticoagulation for another medical reason (eg, atrial fibrillation) were included in the initial risk assessment, as were patients receiving any dose of aspirin therapy.
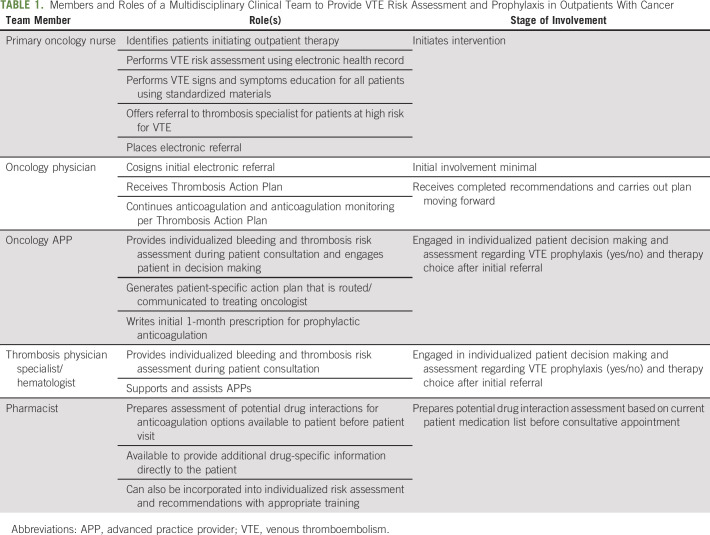
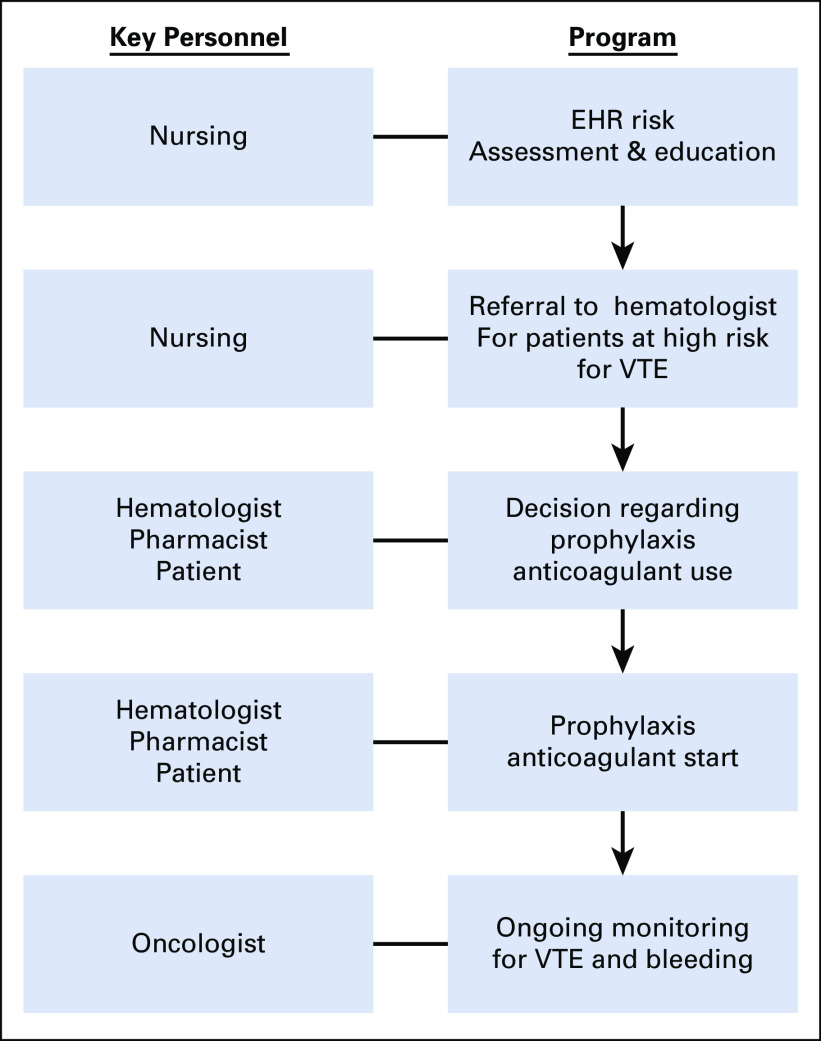
The VTEPACC program comprised 4 key structural components (Fig 1), including (1) an EHR-based assessment tool for bleeding and thrombosis risk to identify high-risk patients with cancer for targeted VTE prophylaxis; (2) a data capture and electronic reporting methodology; (3) an electronic alert for referral to the thrombosis program for high-risk patients; and (4) an EHR-based communication tool to enhance physician and nursing communication, that is, the Thrombosis Action Plan using EPIC EHR software. The model is based on a multidisciplinary care team including oncologists, hematologists, advanced practice providers (APPs), nurses, and pharmacists (Table 1).
Fig 1.
Elements of the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic Program. EHR, electronic health record; VTE, venous thromboembolism.
TABLE 1.
Members and Roles of a Multidisciplinary Clinical Team to Provide VTE Risk Assessment and Prophylaxis in Outpatients With Cancer
An overview of the VTEPACC care pathway is shown in Figure 2. Outpatients with cancer coming to the ambulatory cancer clinic for their initial treatment received both verbal education and written material regarding VTE risk and signs and symptoms of thrombosis by a nurse. An assessment of VTE risk was performed by an oncology nurse using the Khorana and Protecht scores built into the EHR. Patients with a predicted high risk of VTE during treatment (defined as ≥ 3 points on the Khorana or Protecht score) were offered a hematology consultation, where patient-specific VTE prophylaxis recommendations were made. Results of the consultation, including individualized drug and dosing recommendations, were electronically communicated to the treating oncologist with the initial recommended prescription written by the hematologist in a majority of patients. The Data Supplement contains examples of the communication tool, Thrombosis Action Plan, and the electronic risk assessment tool. Data quality monitoring, education rates, risk assessment rates, and referral rates for high-risk patients were reviewed monthly using electronic data dashboards.
Fig 2.
Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic Care Pathway. EHR, electronic health record; VTE, venous thromboembolism.
The primary quality outcomes were the number of patients receiving VTE risk assessment and education, and number of high-risk patients receiving VTE prophylaxis. Secondary endpoints included number of patients accepting a referral to the thrombosis program. Ascertainment of VTE education rates and risk assessment were captured via a searchable function embedded within the risk assessment flow sheet. This same tool also captured patient preference with regard to thrombosis referral (yes/no) and prior VTE history. The tool contains drop-down elements as well as an automatic Khorana and Protecht score calculator.
VTE education and risk assessment rates before VTEPACC were based on chart review, and oncologists were also queried directly regarding general practice patterns that may not be captured in the chart to further verify the preintervention rates. The primary clinical outcome of VTE (pulmonary embolism of deep vein thrombosis) was based on International Classification of Disease (10th revision) codes (with validation of 20% of patients by chart review).
After a 1-year planning and preparation phase, there was a 6-month implementation phase, where study metrics were monitored, followed by a 2-year postimplementation phase. Before the start of the implementation, all members of the clinical and study team met monthly to prepare for the project initiation. Monthly meetings of team members were conducted as part of an iterative Plan-Do-Study-Act process, continued throughout the project, and were informed by updated dashboard metrics.
RESULTS
VTE Risk Assessment for Outpatients With Cancer Initiating Therapy
A total of 918 sequential patients were evaluated during the 2-year postimplementation phase. VTE risk assessment and education rates were < 5% before VTEPACC. During the implementation phase, education and risk assessment rates increased to 81.6% (range, 63.6%-97.7%) per month and further to 94.7% (range, 82.1%-100%) per month for the full 2-year postimplementation phase when all systems were in place (Fig 3). Based on monthly team meetings that reviewed study metrics, program improvements were made in the EHR documentation, new nurse onboarding processes were implemented, and streamlining of the thrombosis consult initiation was performed, triggered by primary nursing. It was through this iterative process that VTE education and risk assessment rates were able to increase.
Fig 3.
Venous thromboembolism (VTE) education and risk assessment rates after implementation of the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic Program (VTEPACC) Program. EHR, electronic health record, SD, standard deviation.
High-Risk Patients Receiving Anticoagulation Therapy
During the 2-year postimplementation period, a total of 213 outpatients with cancer (23.2%) initiating therapy were found to be at high risk for developing a VTE. This proportion remained stable over the duration of the reporting period (± 2%). Of the high-risk patients, 70.9% were referred to a hematologist, pharmacist, or APP who was part of the Thrombosis and Hemostasis Program, and 93.4% of referred patients were subsequently assessed (eg, arrived for the visit). Reasons for nonreferral included (1) the patient was already on full-dose anticoagulation for another reason, and (2) the patient refused referral. A common barrier encountered that precluded the Thrombosis and Hemostasis Program visit was the need for an additional clinic visit; this barrier was successfully addressed later in the implementation phase by offering visits that coincided with chemotherapy infusion dates and times. For the high-risk patients referred, 93.8% received personalized prophylactic anticoagulation therapy (approximately half received a prophylactic dose of a direct oral anticoagulant [DOAC]), one third received a prophylactic dose of low molecular weight heparin (LMWH), and the remainder received unfractionated heparin and warfarin (< 3%).
VTE Rates in Outpatients With Cancer Initiating Therapy on VTEPACC
The major clinical outcome tracked after VTEPACC initiation was VTE in the first 6 months of therapy. Patients identified as being at high risk for VTE during initial risk assessment had an 8.0% risk of VTE at 6 months during the 2-year study phase. This compares with a VTE rate of 12.8% in the implementation phase. The VTE rate at 6 months in the study phase was 6.1% in the medium-risk group and 2.1% in the low-risk group.
DISCUSSION
VTEPACC represents a successful guideline implementation initiative that includes a multidisciplinary team of oncologists, hematologists, APPs, pharmacists, and nurses, and an EHR-based risk assessment that was nursing driven. We substantially increased VTE education and risk assessment rates and achieved a high thromboprophylaxis rate in patients who were found to be at high risk for VTE. Outpatients with cancer initiating therapy were accepting of a thrombosis referral that involved an individual risk assessment and discussion of both thrombosis and bleeding risk, and the majority of these patients received VTE prophylaxis.
The difficulty in meeting guidelines for outpatients with cancer nationwide to improve VTE prophylaxis underscores the need to develop successful models of care delivery. Guidelines recommend the use of a validated risk assessment tool to distinguish high- versus low-risk patients.7,8,14 Both the Khorana risk score and Protecht score are validated tools to assess this risk, and a prior study found that use of a computerized system that assessed elements of the Khorana score found in the EHR was effective in identifying high-risk patients.15 However, additional guidance regarding implementation, guided individualized decision making, and choice of anticoagulant is missing. We hypothesized that at least 1 component that contributed to a lack of guideline implementation was the lack of expertise in anticoagulation drugs as well as bleeding and thrombosis risk assessment. We addressed this through a multidisciplinary approach using thrombosis experts to guide oncologists and patients regarding anticoagulant options as well as pharmacists to assess drug-drug interactions. In addition, VTEPACC emphasized a consistent and central role for nursing to effectively deploy the program. We analogized VTE risk assessment and education to neutropenic fever risk assessment and education at our oncology clinic. This approach accurately reflects the real risks of subsequent hospitalization, morbidity, and death, and resulted in > 95% of patients receiving guideline-recommended care.
Recent guidelines have expanded the number of drug options (apixaban, rivaroxaban, or LMWH) for primary prophylaxis in high-risk patients with cancer.14 Primary prophylaxis with LMWH reduces the risk of symptomatic VTE in outpatients with cancer treated with chemotherapy.6 Direct oral anticoagulants are also effective. In the AVERT trial (Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients) among intermediate- to high-risk patients (Khorana score ≥ 2), a significant reduction in VTE was seen in the apixaban group (4.2%) compared with the group receiving placebo (10.2%; hazard ratio, 0.41)16. Rivoraxaban efficacy in the primary prevention of a combined endpoint of symptomatic and asymptomatic VTE was also suggested in the CASSINI trial (Rivoraxaban for Preventing Venous Thromboembolism in High-Risk Ambulatory Patients With Cancer) in patients with a Khorana score ≥ 2 initiating cancer-directed therapy.17 In both trials, an increase in bleeding was seen mitigating some of the benefits of treatment. Given these findings, systems of care that incorporate both thrombosis and bleeding risk as well as individualized patient preference are a necessity. In the VTEPACC model, 50% of patients received DOAC prophylaxis, and pharmacy evaluation for drug interactions with both DOAC and LMWH were performed. We also observed that DOACs had the lowest number of drug interactions18.
The principal goal of thromboprophylaxis is VTE prevention in subgroups of patients who will most benefit. In the clinical trial setting, VTE occurred in 11% of high-risk patients receiving placebo in a study of nadroparin prophylaxis and 10.2% of placebo patients in the AVERT trial (Khorana score ≥ 2).16,19 A recent systematic review found the 6-month incidence of VTE was 11% in patients with a Khorana score ≥ 3.20 In the VTEPACC population, the rate of VTE at 6 months from study initiation was 12.8% and was approximately 40% lower, at 8.2% after full implementation of VTEPACC. These results support VTEPACC as effective in reducing VTE in a real-world setting.
The prospective evaluation and treatment of a large number of consecutive patients in this outpatient cohort allows for applicability of our study results to patients with cancer initiating therapy in the outpatient setting. The major limitation of this study is the single-center design. The applicability to smaller practices is not known, and a perceived barrier could be the lack of hematologists, although a large number of patients in VTEPACC were seen by APPs trained in thrombosis and bleeding related to patients with cancer, and in subsequent work, we are assessing the impact of pharmacist-driven patient consultations. In addition, education and risk assessment rates may not account for all patients because the ability to capture appropriate patients via the EHR across all clinics and treatment regimens was challenging.
The effectiveness of a multidisciplinary approach to address VTE risk assessment and prophylaxis in oncology patients underscores the need for leveraging multiple areas of expertise to optimize clinical outcomes. Implementation of VTEPACC using a multidisciplinary team and EHR-based approach improved VTE risk assessment and education. VTEPACC was successfully able to identify patients at high risk for developing a VTE and offer personalized VTE prophylaxis. Successful implementation of guidelines to prevent cancer-associated thrombosis could have a significant impact on patient morbidity and mortality.
ACKNOWLEDGMENT
The authors thank additional and past members of the project team, including Jacob Barker, Jordan Tolstoi, Katie Michaud, Kate Devine, Yongli Ji, and Mike Gianni.
AUTHOR CONTRIBUTIONS
Conception and design: Chris E. Holmes, Steven Ades, Susan Gilchrist, Daniel Douce, Britny Rogala, Mary Cushman, Allison Kaigle Holm
Administrative support: Allison Kaigle Holm
Provision of study materials or patients: Emily Parenteau, Karen Libby, Chris Holmes, Steven Ades, Mary Cushman
Collection and assembly of data: Chris E. Holmes, Steven Ades, Karen Libby, Mary Cushman, Allison Kaigle Holm
Data analysis and interpretation: Chris E. Holmes, Steven Ades, Susan Gilchrist, Daniel Douce, Britny Rogala, Mary Cushman, Allison Kaigle Holm
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Successful Model for Guideline Implementation to Prevent Cancer-Associated Thrombosis: Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/site/ifc/journal-policies.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Susan Gilchrist
Consulting or Advisory Role: Outcomes4Me
Britny Rogala
Speakers' Bureau: Genentech
Mary Cushman
Research Funding: Sphingotech (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 2. Riess H, Pelzer U, Deutschinoff G, et al: A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): Results of the CONKO 004 trial. J Clin Oncol 27, 2009 (abstr LBA4506) [Google Scholar]
- 3.Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283–1292. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Akl EA, Gunukula S, Barba M, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011;(4):CD006652. doi: 10.1002/14651858.CD006652.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Akl EA, Schünemann HJ. Routine heparin for patients with cancer? One answer, more questions. N Engl J Med. 2012;366:661–662. doi: 10.1056/NEJMe1113672. [DOI] [PubMed] [Google Scholar]
- 6.Di Nisio M, Porreca E, Candeloro M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12:CD008500. doi: 10.1002/14651858.CD008500.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Bohlke K, Falanga A: Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2015;11:e442–e444. doi: 10.1200/JOP.2015.004473. [DOI] [PubMed] [Google Scholar]
- 9.Key NS, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update summary. J Oncol Pract. 2019;15:661–664. doi: 10.1200/JOP.2015.004473. [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1111/ajco.12770. Thein KZ, Yeung S-CJ, Oo TH, et al: Risk of bleeding from primary thromboprophylaxis (PTP) in patients with solid cancer receiving chemotherapy: A systematic review and meta-analysis of randomized controlled trials (RCT). Blood 128:3820, 2016. [DOI] [PubMed] [Google Scholar]
- 10a. Association of Community Cancer Centers: Venous Thromboembolism. Identifying Cancer Patients at Risk, 2016. https://www.accc-cancer.org/docs/projects/resources/pdf/vte-identifying-cancer-patients-at-risk-2016.
- 11.Streiff MB. Association between cancer types, cancer treatments, and venous thromboembolism in medical oncology patients. Clin Adv Hematol Oncol. 2013;11:349–357. [PubMed] [Google Scholar]
- 12.Connolly GC, Francis CW. Cancer-associated thrombosis. Hematology (Am Soc Hematol Educ Program) 2013;2013:684–691. doi: 10.1182/asheducation-2013.1.684. [DOI] [PubMed] [Google Scholar]
- 13.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1200/JCO.19.01461. Key NS, Khorana AA, Kuderer NM, et al: Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 38:496-520, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Lustig DB, Rodriguez R, Wells PS. Implementation and validation of a risk stratification method at The Ottawa Hospital to guide thromboprophylaxis in ambulatory cancer patients at intermediate-high risk for venous thrombosis. Thromb Res. 2015;136:1099–1102. doi: 10.1016/j.thromres.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 18.Ng H, Rogala B, Ades S, et al. : Prospective evaluation of drug-drug interactions in ambulatory cancer patients initiated on prophylactic anticoagulation. J Oncol Pharm Practice 2020. DOI: 10.1177/1078155220901569 [DOI] [PubMed] [Google Scholar]
- 19.Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern Emerg Med. 2012;7:291–292. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]
- 20.Mulder FI, Candeloro M, Kamphuisen PW, et al. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica. 2019;104:1277–1287. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]