Background:
Dissecting cellulitis of the scalp (DCS) is a part of the follicular occlusion tetrad (hidradenitis, acne conglobata, and pilonidal disease). It is a spectrum disorder that can be severe and refractory to medical management. The authors describe 3 such cases successfully treated with surgical resection and reconstruction and present a scoring system for timely referral of such patients to a reconstructive surgical team.
Methods:
A literature review of all available cases of DCS was undertaken, and the treatments and outcomes were reviewed. Our institution has had 3 recent cases that demonstrated delayed presentation common in the severe spectrum of this condition. All underwent radical surgical resection and reconstruction with skin grafting that was very positively received by all the patients.
Results:
Three cases of DCS were treated with radical scalpectomy, and split-thickness skin grafting was done with a good cosmetic outcome and a high degree of subjective patient satisfaction. All would have received timely referral if the presented scoring system had been applied earlier.
Conclusions:
DCS is a rare but debilitating condition that may progress to a medically refractory condition requiring surgical intervention. Surgical resection and skin grafting offer a durable cure, but delayed presentations are common. Use of a scoring system may reduce the time to surgical referral for refractory cases.
INTRODUCTION
Dissecting cellulitis of the scalp (DCS), also known as perifolliculitis capitis abscedens et suffodiens or Hoffman’s disease, is a rare cicatricial alopecia. It has also been described as a neutrophilic cicatricial alopecia of unknown origin.1 Initially, described by Spitzer2 in 1903, the condition was given its eponymous name in 1908.3 It is identified as one of the follicular occlusion (FO) triad or tetrad, including hidradenitis suppurativa (HS), acne conglobata, and pilonidal disease.4–9 The primary pathologic event is obstruction of the pilar infundibulum through hyperkeratosis, accumulation of obstructed follicular products with rupture, and an intense inflammatory reaction at the bulb of the hair follicle.10 This leads to the formation of pustules that develop into tracts ultimately coalescing to chronically inflamed tissue.4 Pathologic examination can show reduced follicle number with evidence of follicular rupture and presence of neutrophils. It has been associated with sternoclavicular hyperostosis, polyarticular arthritis, and human leukocyte antigen B27 (HLA-B27) seronegative spondyloarthropathy.11
DCS ranges from involving an isolated area of the scalp to involving the entire calvaria representing a complex management challenge. Frequently, a variety of treatment options indicates a lack of clear superiority of one approach over another. In the case of DCS, it may also represent the spectrum of the disease which at one end can be severe and socially isolating for which surgery is the only treatment that offers long-term disease control without any medical side effects.12
The global impact of DCS is unknown. This is unsurprising as DCS is recognized as a rare disease by the National Institutes of Health. On the other hand, HS, also defined as a rare disease by National Institutes of Health, has been more extensively studied and demonstrated to be associated with lower socioeconomic status.13 HS demonstrates a prevalence between 0.31% and 4% but with rising health care costs in outpatient setting following index diagnosis.14,15 Health care costs increased following the initial diagnosis from $1349 to $4428 for Medicare patients and from $859 to $2662 for Medicaid patients with claims also doubling from 6.6 to 12.6 and 9.7 to 18.1 for Medicare and Medicaid patients, respectively.14 This implies that, in the case of HS, continued varied treatments are used with patients returned to outpatient clinics frequently without a durable cure.
Here, we present a review of DCS and the current state of knowledge. We recommend our grading system to aid in the identification of patients who may benefit from a more aggressive surgical approach after medical management. We present 3 cases of patients with severe DCS who, after years of medical management, were elected to undergo a scalpectomy with skin grafting, with a durable and most gratifying outcome.
METHODS
Our institution has had 3 recent cases of DCS, and all had significant delays in their surgical referral. All had diffuse severe disease and underwent a scalpectomy with split-thickness skin graft reconstruction done by the senior author (C.E.S.). The surgery consists of marking the disease area and extent of the dissection followed by infiltration with lidocaine–epinephrine and resection by scalpel. Areas of purulence are cultured at the time of resection. Raney clips are applied liberally upon dissection of scalp tissue to aid in hemostasis. The depth of the resection is down to the galeal plane with careful attention to avoid an improper plane and injury to a branch of the facial nerve. The diseased scalp is removed as one piece, and the Raney clips are then removed one by one followed by hemostasis. Bacitracin irrigation is used, and a simple moist dressing is applied. Patients are admitted until pain control is established with local wound care. No antibiotic therapy is given beyond 24 hours of the index operation or in the outpatient setting. Once a healthy bed of granulation tissue is established, a second operation is performed for coverage. A dermatome is used to harvest a 0.012-inch split-thickness skin graft, which is then meshed 3:1 and secured with a suture. All patients experienced a degree of skin graft loss presumably due to sloughing. All had a durable pain-free cure of their disease with a good cosmetic outcome. No formal validated satisfaction score is available for DCS; however, all patients were subjectively very pleased with their outcome without a formal outcome score. Literature does support satisfaction with surgical resection for hidradenitis, and subjective satisfaction of our patients suggests this is similar for DCS.16 Further reconstruction with follicular units was not favored due to the central pathologic entity obstructing the follicular infundibulum. Additionally, no patients requested follicular reconstruction when it was discussed following reconstructive surgery.
For our review of DCS, a literature search using PubMed was performed using the terms DCS, perifolliculitis capitis abscedens et suffodiens, and Hoffman’s disease scalp. A total of 253 articles were excluded from this study. After removing duplicates, a literature review was performed on the remaining 68 articles.
DCS Reconstructive Cases
Case 1
A 32-year-old obese man with diabetes and HS in the axillae and groins presented for surgical evaluation of DCS (Fig. 1). He had DCS for the past 7 years and was referred by his dermatologist. His multiple failed medical therapies included chronic antibiotic therapy, isotretinoin, prednisone, steroid injections, etanercept, and radiation therapy. He suffered from recurrent drainage and pain requiring a pain clinic referral and long-term opiate use. At the time of evaluation, he was on prednisone and cephalexin. On physical examination, his scalp showed numerous pustular lesions and sinus tracts, which involved the parietal, occipital, and vertex regions. He was taken to the operating room for near-total scalp excision. The plane of excision was at the subgaleal plane. Approximately 3 weeks after the excision, he underwent debridement and wound closure with 2:1 meshed split-thickness skin grafts measuring approximately 500 cm2. A nonadhesive dressing was applied to the skin grafts, and the patient was admitted for 6 days for pain control and graft monitoring. On the day of discharge, the grafts had excellent take, and he was discharged with daily dressing changes. The patient was extremely satisfied with the result of his surgery. At 1 year, the patient had no pain and no evidence of any recurrent disease. He visited the clinic after 2 years with one small area of superficial breakdown from local minor trauma and still did not have any evidence of recurrence.
Fig. 1.
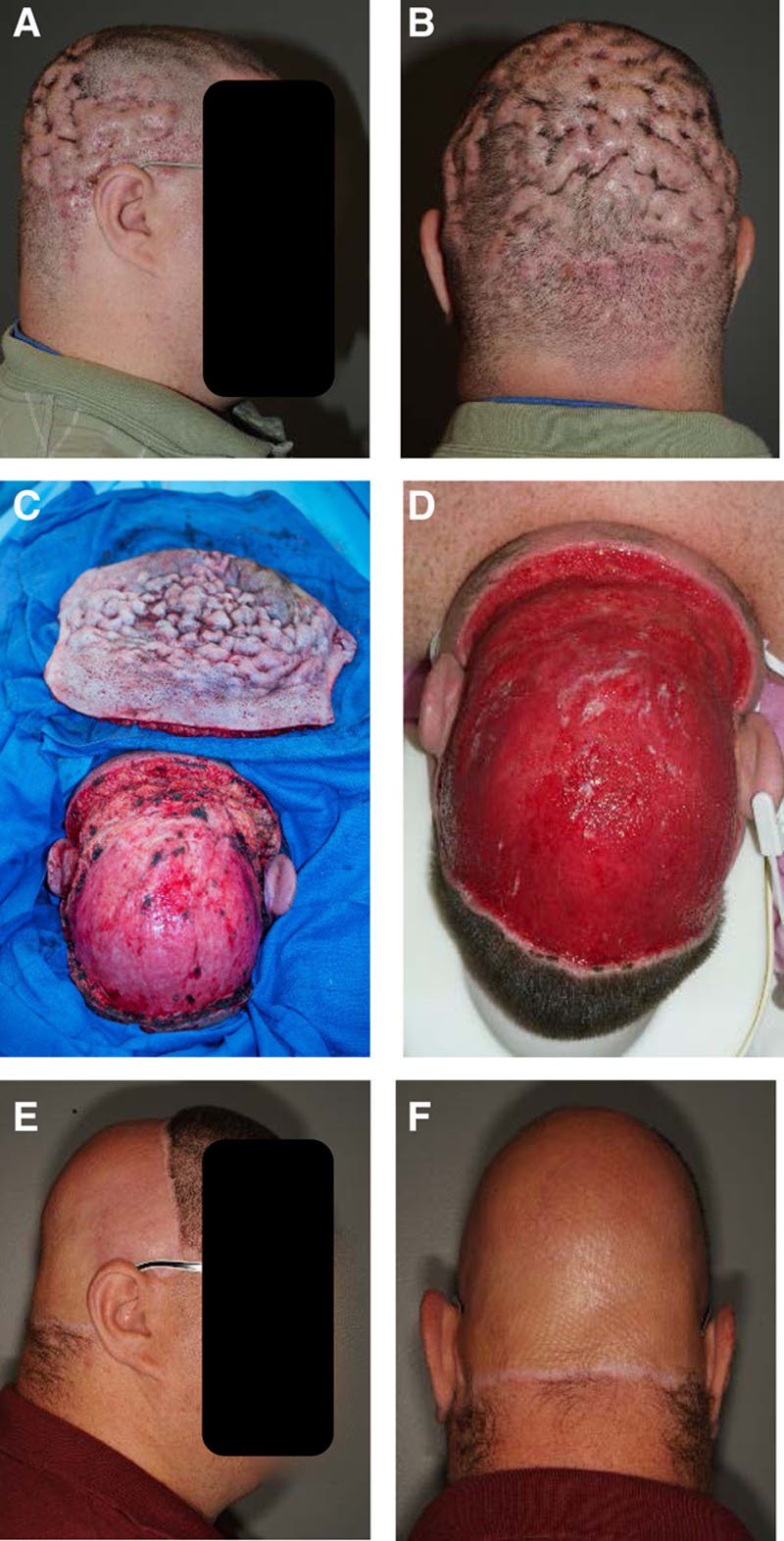
A 32-year-old obese man with DCS (A and B) requiring near-total scalp excision (C) followed by 3 weeks of dressing changes (D). At 1-year follow-up, there was no recurrence of disease (E and F).
Case 2
A 52-year-old obese male truck driver presented for DCS evaluation (Fig. 2). He had tried multiple medical treatments, but his DCS had steadily worsened. He had been in this condition for the past 5 years, and he was on minocycline, as prescribed by his dermatologist.
Fig. 2.
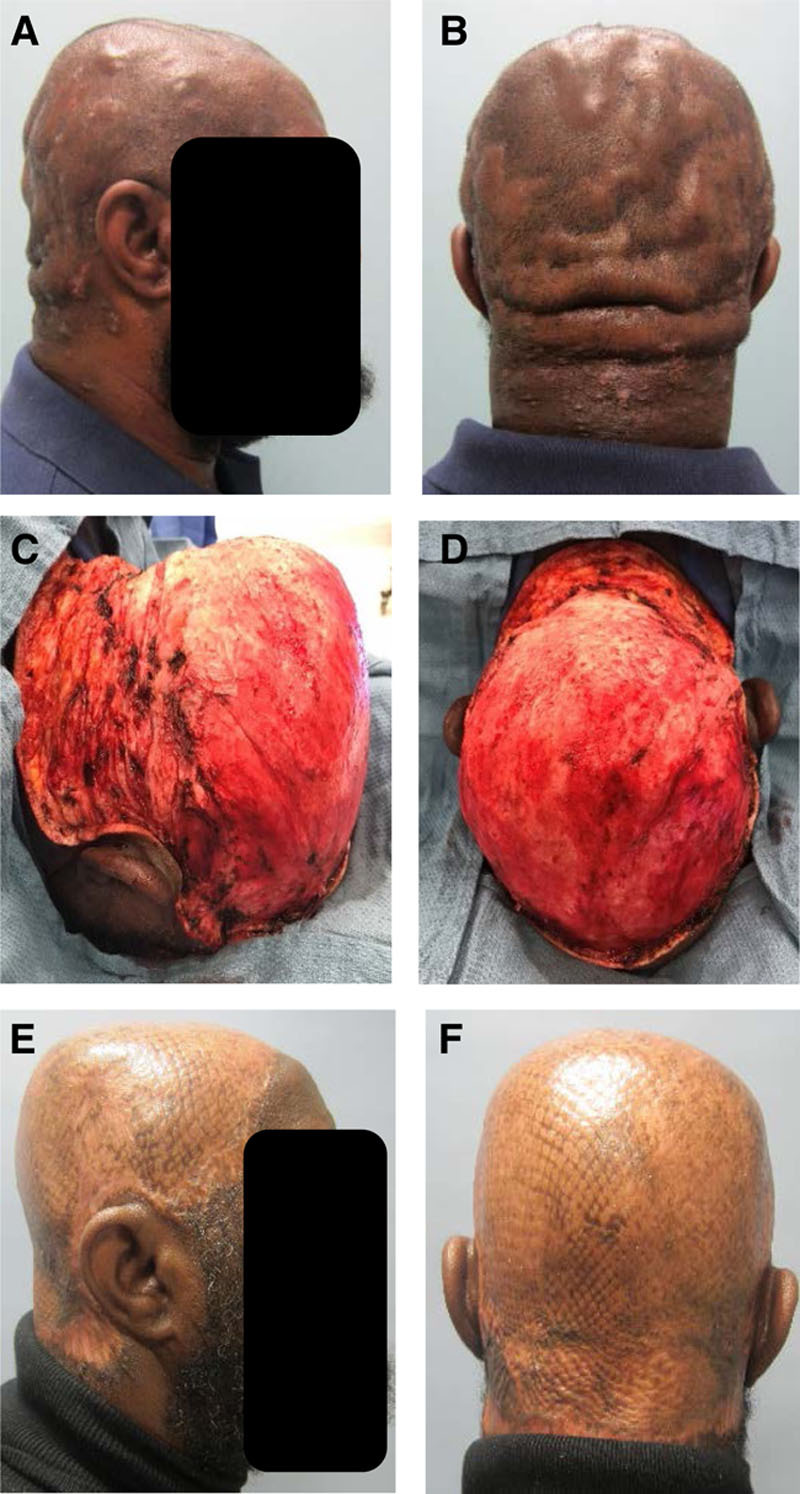
A 52-year-old obese man with DCS failing medical treatments (A and B) requiring near-total scalp excision (C and D). At 4 months after surgery, he had no pain and no evidence of recurrence (E and F).
On examination, his scalp and posterior neck showed the stigmata of dissecting cellulitis of the hair-bearing skin. There was significant pain and drainage from his entire hair-bearing scalp. He underwent excision of the entire scalp, which was extensively involved. This was in the subgaleal plane and required cauterization of the superficial temporal vessels to be able to excise the anterior portions. Approximately 3 weeks later, he underwent debridement and closure with 3:1 meshed split-thickness skin graft (STSG) measuring approximately 900 cm2. A nonadhesive dressing was applied to the skin grafts with a bolster head wrap, and the patient was admitted postoperatively for 2 days. On postoperative day 9, the bolster head wrap was removed with excellent graft take with a small frontal area of right-temporal area needing additional dressing changes. At his most recent follow-up appointment, 4 months after the surgery, he had no pain and no evidence of recurrence.
Case 3
A healthy 27-year-old obese male truck driver presented for evaluation of DCS (Fig. 3). He was self-referred and has been suffering from DCS for the past 4 years. The patient reported that he had been on multiple medications (including isotretinoin) and had recently seen a dermatologist, who prescribed him additional medications that he did not want to start. He had a diffuse pain in the hair-bearing portion of his scalp and was on chronic opiates. On physical examination, his scalp showed classic male pattern baldness, but the hair-bearing portions in the temporoparietal and occipital regions had diffuse DCS with multiple abscesses. He underwent debridement of all hair-bearing portions in the subgaleal plane. Approximately 3 weeks later, he underwent debridement and closure with 2:1 meshed STSG of approximately 660 cm2. A nonadhesive dressing was applied to skin grafts with a bolster head wrap, and the patient was admitted postoperatively for 4 days for pain management. On postoperative day 8, the head wrap was removed with 75% graft take, with some graft loss over the occipital region due to pressure. This area slowly epithelialized and contracted and did not require any additional grafting. The patient had no pain or evidence of recurrence and was able to return to work without any issues.
Fig. 3.
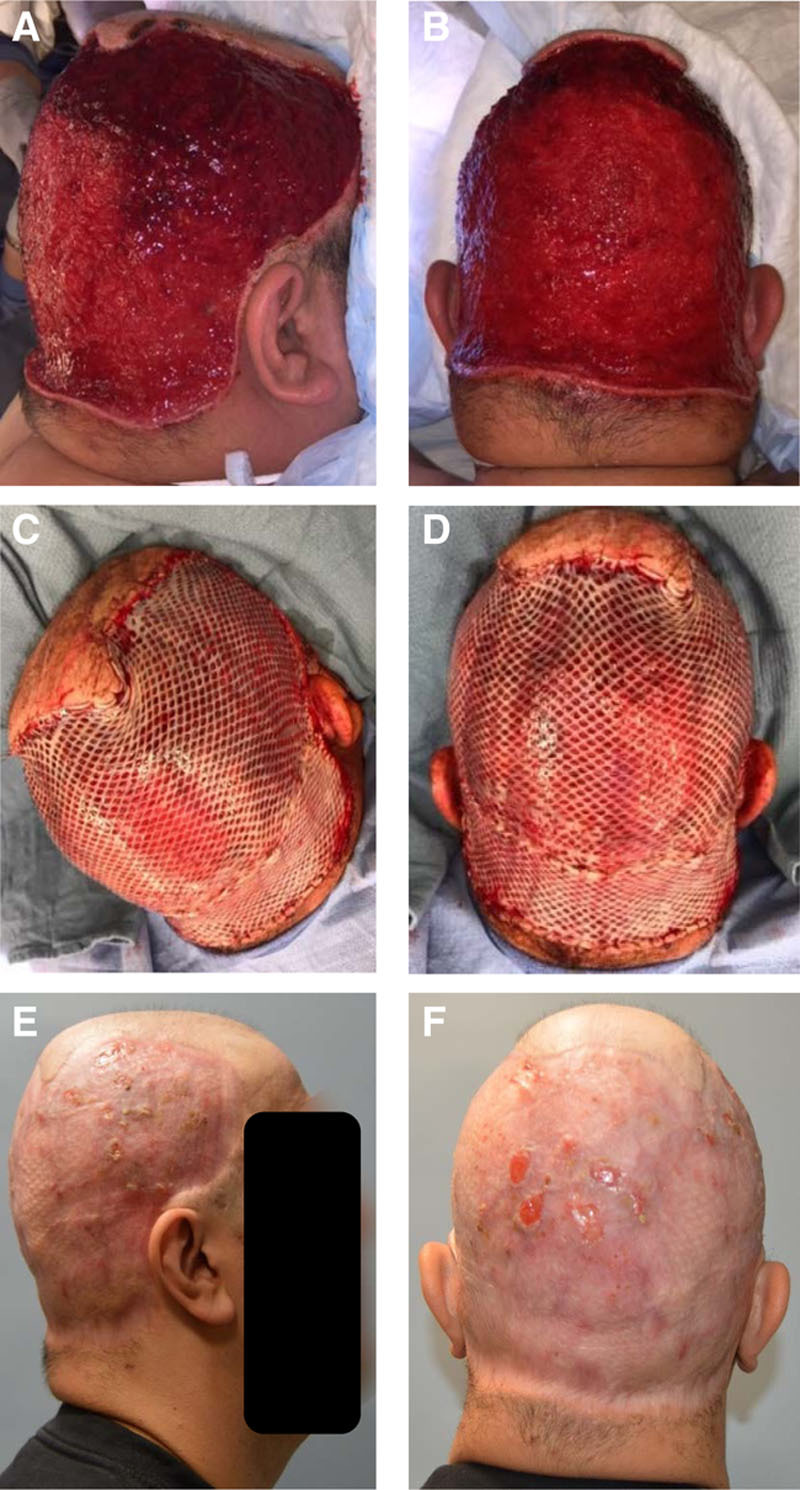
A 27-year-old obese man with DCS underwent debridement of all hair-bearing scalp (A and B). STSG closure after 3 weeks (C and D). At 9-month follow-up, there was no recurrence of disease (E and F).
DISCUSSION
Review of DCS
DCS is in essence a cicatricial alopecia characterized by multiple inflammatory pustules with abscess formation and development of chronic discharging tracts and sinuses. It has been described to be a part of the FO triad, which includes acne conglobata, HS or acne inversa, and perifolliculitis capitis abscedens et suffodiens.5,17 The FO tetrad adds pilonidal disease.4,6–9 Members of this tetrad are normally diagnosed without mention of the others; however, there are reports of these diseases affecting individuals concurrently.6,7,18 Although debate remains regarding the actual cause of these diseases, a common pathogenesis has been suggested. This includes abnormal keratinization with obstruction of the follicular infundibulum, leading to rupture in a manner similar to that of hidradenitis.9,19 This rupture leads to an intense inflammatory reaction. Over time the patients develop deep sinus tracts heralding diagnosis nominally 34 months following the onset of the symptoms.7 The sinuses become more intense and eventually coalesce to develop as a chronic suprainfection that can only be suppressed with antibiotic therapy.4,19,20 Scientific work into the pathogenesis of DCS reflects its rarity as a disease entity. Histology, as described by Scheinfeld,8 reveals lesions with dense neutrophilic, lymphocytic, histiocytic, and plasma cellular infiltrate early on. A more chronic lesion will exhibit granuloma consisting of lymphocytes, plasma cells, and foreign-body giant cells.
DCS is predominantly found in men of Afro-Caribbean descent in early adulthood or middle age, although occasionally females and Caucasian individuals have been reported to be affected.21,22 The exact cause of DCS remains elusive and is likely multifactorial. Associations with sternoclavicular hyperostosis as well as polyarticular arthritis and HLA-B27 seronegative spondyloarthropathy have been described.11,20 Anecdotal evidence of an association with Crohn’s disease is also reported in the literature.23 Bacterial culture of purulence, that is sterile along with the effectiveness of anti-inflammatory immunomodulators, highlights an abnormal inflammatory process.24 Other reports of positive cultures combined with the fact that DCS can improve with antibiotic therapy imply an element of disease potentiation with suprainfection.24 Fluoroquinolone effectiveness has been attributed to their inherit anti-inflammatory activity.8 The ability of laser destruction of the hair follicle in disease control indicates that the follicle is a necessary portion of the pathogenesis. Modern literature has highlighted additional descriptive terms (including dissecting terminal hair folliculitis) because this appears to be the initiating active region in the disease.25 Familial examples are limited to one case of 2 brothers and may represent an environmental stimulus to genetically predisposed individuals rather than a true genetic dissonance.26 Rarely, pediatric cases have been reported with the earliest being 7 years of age.10,19 An androgenic role has been implied by its predilection for men in 20–50s age range and an isolated report of development of the condition in a user of recreational anabolic steroids.27
There are a wide variety of treatments that have been reported, including suppressive antibiotic therapy,4,8,19,20,28 zinc sulfate,29,30 isotretinoin,31–34 corticosteroids,35 antiandrogens,36 biologic agents,4,37–39 laser therapy,40–43 aminolevulinic acid-photodynamic therapy,44–46 radiation therapy,47 limited surgical therapy,48 and radical surgical resection (scalpectomy).11,49–51 Each of these therapies has its own mechanism of action. Antibiotic therapy is used to treat infection or suprainfection that is thought to be a primary driving force behind continued inflammation.4,8,19,20,27 Additionally, antibiotics have been used for their direct anti-inflammatory activity.8 Zinc sulfate’s mechanism of action in DCS is frequently described as poorly defined due to its immunostimulant effect.29 Zinc has been widely appreciated as essential for a myriad of pathways within the skin.52 Some basic science research points out a clear effect of zinc supplementation on the inflammatory response.53 Isotretinoin’s mechanism is thought to involve the induction of apoptosis of the sebocytes, and its popularity as a first-line medical therapy is clear.31–34,54 Both the topical and intralesional functions of steroids were based on their broad anti-inflammatory activity.35 Biologic agents initially developed for rheumatoid arthritis have found a role in the treatment of a plethora of conditions in which inflammation is a central actor. The central mechanism is blockade of tumor necrosis factor and its downstream cytokine signaling.55 Laser treatment with a targeted chromophore of melanin is based on epilation because the hair follicle is central to this pathologic entity.40–42 Additionally, a carbon dioxide laser has been used as a high-precision dissection tool.43 Photodynamic therapy is thought to be effective due to the formation of reactive oxygen species, as studied in acne vulgaris.56 Radiation therapy works through destruction of hair follicle with persistent results in a small case series with a reasonable cosmetic outcome.47 Surgical resection with grafting also functions by removing full-thickness skin, including the hair follicle. All damaged and scarred tissues are removed at the loose areolar tissue plane of the scalp. The wound is allowed to granulate, and a skin graft is applied for definitive coverage and results in a long-term durable treatment for this disease.
A key area for a clinician to focus on is to identify those patients who will progress to late-stage disease and need surgical referral. Imaging (including ultrasound and magnetic resonance imaging) has been performed as an adjunct to diagnosis and to determine the extent of the disease.4,57 A formal grading system for DCS has recently been suggested.58 By this classification, all our cases would represent stage IIIc disease. The 2 grading systems for HS are Hurley and Sartorius. Modifications of these and development of newer grading systems are ongoing endeavors.59,60 Despite this definite approach to nomenclature, considerable debate remains in which some have even advocated for removal of the older more common designations such as HS.25 A caveat can be found in the recent Cochrane reviews, and updated reviews have lamented the poor quality of data on treatment options.9,61 Of the medical therapies, dramatic photographic improvement of severe disease has been documented with isotretinoin.31 Additional short case series (3 cases) have shown remission following a 9- to 11-month course of isotretinoin with remission sustained at 10 months to 2.5 years later following this.62 Cases of successful treatment with isotretinoin in white patients with sustained remission have also been reported albeit with a much shorter follow-up (3 months).22 Some authors have recommended this as a first-line treatment.31
There appears to be a defined subset of patients that, despite the wide array of treatment options, fail medical management or experience significant side effects necessitating cessation of medical therapy. Traditionally, surgical intervention using a targeted surgical approach or a broad approach (including scalpectomy with split-thickness skin grafting) has been done to these cases with good results.1,11,48,51,63–65 Application of negative pressure wound dressing has also been described, which may lessen the risk of slough following skin graft application.11 An approach of limited serial excision has been described.1 Additionally, serial excision while maintaining a patient on suppressive antibiotic regiment has also been performed successfully.48 Scalpectomy with split-thickness skin grafting in a radiated patient was reported by the US Navy surgeons with no recurrence or breakdown in 8 months.51 A 2 case series was reported highlighting the comparison of refractory clinical scenario of patients with DCS and the postoperative outcome, which allows rapid permanent disease control.63 A series of 3 patients all with >120 cm2 of diseased scalp were treated in a similar fashion with rapid social reintegration and no recurrences at 1 year.64 Occasionally, the recurrent cycles of infection can lead to sepsis and a deteriorating clinical scenario in which case surgical intervention must be promptly instituted.49
Delays in surgical treatment are not benign. Failure of nonoperative therapy results in a delayed surgical treatment and may have consequences beyond that of patient suffering. These include a report of a patient developing osteomyelitis of the cranium requiring 3 months of antibiotics therapy and multiple skin grafting attempts.65 Development of diffuse, classically aggressive squamous cell carcinoma (Marjolin ulcer) has been reported with a fatal case being documented in 1981.66
It must be emphasized that those suffering from the more severe manifestations of this disease frequently undergo multiple trial treatments as the disease progresses. Due to unsightly alopecia, painful lesions, and malodorous discharge, these patients experience a significant impact upon their economic and social lives. Frequently they are unable to maintain gainful employment and become social isolates. For these refractory patients, the option for scalpectomy with split-thickness skin grafting gives a new beginning and, in our experience, is universally welcomed.
For male patients, a reconstruction that approximates a bald scalp is an acceptable cosmetic outcome. The largest series has shown a strong male preponderance of disease.58 All our 3 cases are men, and the outcome was subjectively favorable. Reconstruction of scalp hair was not performed nor was it requested following surgery. For female patients, this approach may be less appreciated. Oncology literature demonstrates hair loss as of great concern to females undergoing treatment for breast cancer.67 A clear discussion with photographs would be strongly encouraged before undertaking this procedure for a female patient.
It should be noted that surgery also provides a durable cure for this disease if the resection is adequately carried out. One-year follow-up after excision with wide (3 cm) margins has shown the lack of recurrence of the disease.68 This also allows a clinician to offer this approach to a patient who would prefer this to the cost and compliance requirements of medical therapy. The over-the-counter cost of isotretinoin without insurance coverage can be as high as $579.57 per month.
Proposed DCS Grading System for Medical/Surgical Management
For DCS, we suggest a grading scheme based on the following 5 principles:
-
Percentage of scalp involvement
a. <50% 1 point
b. >50% 2 points
-
Number of flare ups
a. Each flare 1 point
-
Duration of symptoms
a. Each 6 months of active disease 1 point
-
Number of medical therapies tried
a. Each treatment modality 1 point
-
Social isolation score
a. Limitations in job/hobbies 1 point
b. No family/friend visitors 2 points
For those with scores of 6 or less, we recommend continuing medical therapy; however, those with scores of 7 or greater should receive at minimum a discussion of surgical options or referral to a plastic surgeon for a similar discussion. With rare diseases, it takes time to generate a complete data set to validate expert recommendations; despite the time constraint, it is important to start appreciating the occasionally chronic and refractory nature of this disease and offer surgical resection with grafting and allow the patient to decide between the options presented. In our cases, we frequently found all patients relieved with the possibility of being disease-free and thankful following intervention.
CONCLUSIONS
In conclusion, we present 3 cases of DCS that were successfully and durably treated by scalpectomy and skin grafting. All patients were rendered durably pain free following scalpectomy in the subgaleal plane and had good cosmetic results. We recommend trial of medical management for early-stage disease and application of our grading system to enable timely referral of those cases that declare themselves refractory to medical management.
Footnotes
Published online 18 August 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Garcia C. Commentary on cutting out the tracts. Dermatol Surg. 2017;43:740. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer LV. Dermatitis follicularis et perifollicularis conglobata. Dermatology. 1903;10:109–120 [Google Scholar]
- 3.Hoffmann E. Folliculitis et perifolliculitis capitis abscedens et suffodiens: case presentation. Dermatol Z 1908;15:122–123 [Google Scholar]
- 4.Takahashi T, Yamasaki K, Terui H, et al. Perifolliculitis capitis abscedens et suffodiens treatment with tumor necrosis factor inhibitors: a case report and review of published cases. J Dermatol. 2019;46:802–807 [DOI] [PubMed] [Google Scholar]
- 5.Sun KL, Chang JM. Special types of folliculitis which should be differentiated from acne. Dermatoendocrinol. 2017;9:e1356519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasanth V, Chandrashekar BS. Follicular occlusion tetrad. Indian Dermatol Online J. 2014;5:491–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421–423 [DOI] [PubMed] [Google Scholar]
- 8.Scheinfeld N. Dissecting cellulitis (perifolliculitis capitis abscedens et suffodiens): a comprehensive review focusing on new treatments and findings of the last decade with commentary comparing the therapies and causes of dissecting cellulitis to hidradenitis suppurativa. Dermatol Online J. 2014;20:22692. [PubMed] [Google Scholar]
- 9.Ingram JR, Woo P-N, Chua SL, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev. 2015;2015:CD010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaopande VL, Kulkarni MM, Joshi AR, et al. Perifolliculitis capitis abscedens et suffodiens in a 7 years male: a case report with review of literature. Int J Trichology. 2015;7:173–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerome MA, Laub DR. Dissecting cellulitis of the scalp: case discussion, unique considerations, and treatment options. Eplasty. 2014;14:ic17. [PMC free article] [PubMed] [Google Scholar]
- 12.Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274–294 [DOI] [PubMed] [Google Scholar]
- 13.Deckers IE, Janse IC, van der Zee HH, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol. 2016;75:755–759.e1 [DOI] [PubMed] [Google Scholar]
- 14.Marvel J, Vlahiotis A, Sainski-Nguyen A, et al. Disease burden and cost of hidradenitis suppurativa: a retrospective examination of US administrative claims data. BMJ Open. 2019;9:e030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:(2 Pt 1)191–194 [DOI] [PubMed] [Google Scholar]
- 16.Prens LM, Huizinga J, Janse IC, et al. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chicarilli ZN. Follicular occlusion triad: hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp. Ann Plast Surg. 1987;18:230–237 [DOI] [PubMed] [Google Scholar]
- 18.Koshelev MV, Garrison PA, Wright TS. Concurrent hidradenitis suppurativa, inflammatory acne, dissecting cellulitis of the scalp, and pyoderma gangrenosum in a 16-year-old boy. Pediatr Dermatol. 2014;31:e20–e21 [DOI] [PubMed] [Google Scholar]
- 19.Arneja JS, Vashi CN, Gursel E, et al. Management of fulminant dissecting cellulitis of the scalp in the pediatric population: Case report and literature review. Can J Plast Surg. 2007;15:211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hintze JM, Howard BE, Donald CB, et al. Surgical management and reconstruction of Hoffman’s disease (dissecting cellulitis of the scalp). Case Rep Surg. 2016;2016:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stites PC, Boyd AS. Dissecting cellulitis in a white male: a case report and review of the literature. Cutis. 2001;67:37–40 [PubMed] [Google Scholar]
- 22.Koca R, Altinyazar HC, Ozen OI, et al. Dissecting cellulitis in a white male: response to isotretinoin. Int J Dermatol. 2002;41:509–513 [DOI] [PubMed] [Google Scholar]
- 23.Syed TA, Ul Abideen Asad Z, Salem G, et al. Dissecting cellulitis of the scalp: a rare dermatological manifestation of Crohn’s disease. ACG Case Rep J. 2018;5:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh V. Dissecting cellulitis of the scalp in 2 girls. Dermatologica. 1990;180:48–50 [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Plewig G. Should hidradenitis suppurativa/acne inversa best be renamed as “dissecting terminal hair folliculitis”? Exp Dermatol. 2017;26:544–547 [DOI] [PubMed] [Google Scholar]
- 26.Bjellerup M, Wallengren J. Familial perifolliculitis capitis abscedens et suffodiens in two brothers successfully treated with isotretinoin. J Am Acad Dermatol. 1990;23:(4 Pt 1)752–753 [DOI] [PubMed] [Google Scholar]
- 27.Kurtzman DJB, Alexander CE. Image gallery: dissecting cellulitis of the scalp following anabolic steroid use. Br J Dermatol. 2017;177:e160. [DOI] [PubMed] [Google Scholar]
- 28.Greenblatt DT, Sheth N, Teixeira F. Dissecting cellulitis of the scalp responding to oral quinolones. Clin Exp Dermatol. 2008;33:99–100 [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Aiba S, Tagami H. Successful treatment of dissecting cellulitis and acne conglobata with oral zinc. Br J Dermatol. 1999;141:1137–1138 [DOI] [PubMed] [Google Scholar]
- 30.Jacobs F, Metzler G, Kubiak J, et al. New approach in combined therapy of perifolliculitis capitis abscedens et suffodiens. Acta Derm Venereol. 2011;91:726–727 [DOI] [PubMed] [Google Scholar]
- 31.Marquis K, Christensen LC, Rajpara A. Dissecting cellulitis of the scalp with excellent response to isotretinoin. Pediatr Dermatol. 2017;34:e210–e211 [DOI] [PubMed] [Google Scholar]
- 32.Koudoukpo C, Abdennader S, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 7 cases confirming the efficacy of oral isotretinoin. Ann Dermatol Venereol. 2014;141:500–506 [DOI] [PubMed] [Google Scholar]
- 33.Karpouzis A, Giatromanolaki A, Sivridis E, et al. Perifolliculitis capitis abscedens et suffodiens successfully controlled with topical isotretinoin. Eur J Dermatol. 2003;13:192–195 [PubMed] [Google Scholar]
- 34.Taylor AE. Dissecting cellulitis of the scalp: response to isotretinoin. Lancet. 1987;2:225. [DOI] [PubMed] [Google Scholar]
- 35.Adrian RM, Arndt KA. Perifolliculitis capitis: successful control with alternate-day corticosteroids. Ann Plast Surg. 1980;4:166–169 [PubMed] [Google Scholar]
- 36.Goldsmith PC, Dowd PM. Successful therapy of the follicular occlusion triad in a young woman with high dose oral antiandrogens and minocycline. J R Soc Med. 1993;86:729–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarini AA, Trüeb RM. 3 cases of dissecting cellulitis of the scalp treated with adalimumab: control of inflammation within residual structural disease. Arch Dermatol. 2010;146:517–520 [DOI] [PubMed] [Google Scholar]
- 38.Brandt HR, Malheiros AP, Teixeira MG, et al. Perifolliculitis capitis abscedens et suffodiens successfully controlled with infliximab. Br J Dermatol. 2008;159:506–507 [DOI] [PubMed] [Google Scholar]
- 39.Mansouri Y, Martin-Clavijo A, Newsome P, et al. Dissecting cellulitis of the scalp treated with tumour necrosis factor-α inhibitors: experience with two agents. Br J Dermatol. 2016;174:916–918 [DOI] [PubMed] [Google Scholar]
- 40.Krasner BD, Hamzavi FH, Murakawa GJ, et al. Dissecting cellulitis treated with the long-pulsed Nd:YAG laser. Dermatol Surg. 2006;32:1039–1044 [DOI] [PubMed] [Google Scholar]
- 41.Boyd AS, Binhlam JQ. Use of an 800-nm pulsed-diode laser in the treatment of recalcitrant dissecting cellulitis of the scalp. Arch Dermatol. 2002;138:1291–1293 [DOI] [PubMed] [Google Scholar]
- 42.Chui CT, Berger TG, Price VH, et al. Recalcitrant scarring follicular disorders treated by laser-assisted hair removal: a preliminary report. Dermatol Surg. 1999;25:34–37 [DOI] [PubMed] [Google Scholar]
- 43.Glass LF, Berman B, Laub D. Treatment of perifolliculitis capitis abscedens et suffodiens with the carbon dioxide laser. J Dermatol Surg Oncol. 1989;15:673–676 [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Ma Y, Xiang LH. Successful treatment of recalcitrant dissecting cellulitis of the scalp with ALA-PDT: case report and literature review. Photodiagnosis Photodyn Ther. 2013;10:410–413 [DOI] [PubMed] [Google Scholar]
- 45.Feng Y, Zhang Y, Guo H, et al. Treatment of dissecting cellulitis of the scalp with 10% ALA-PDT. Lasers Surg Med. 2019;51:332–338 [DOI] [PubMed] [Google Scholar]
- 46.Zhan Y, Chen X, Zhou Y, et al. Dissecting cellulitis of the scalp successfully treated with ALA-PDT: case report. Photodiagnosis Photodyn Ther. 2018;24:182–184 [DOI] [PubMed] [Google Scholar]
- 47.Chinnaiyan P, Tena LB, Brenner MJ, et al. Modern external beam radiation therapy for refractory dissecting cellulitis of the scalp. Br J Dermatol. 2005;152:777–779 [DOI] [PubMed] [Google Scholar]
- 48.Powers MC, Mehta D, Ozog D. Cutting out the tracts: staged excisions for dissecting cellulitis of the scalp. Dermatol Surg. 2017;43:738–740 [DOI] [PubMed] [Google Scholar]
- 49.Bellew SG, Nemerofsky R, Schwartz RA, et al. Successful treatment of recalcitrant dissecting cellulitis of the scalp with complete scalp excision and split-thickness skin graft. Dermatol Surg. 2003;29:1068–1070 [DOI] [PubMed] [Google Scholar]
- 50.Housewright CD, Rensvold E, Tidwell J, et al. Excisional surgery (scalpectomy) for dissecting cellulitis of the scalp. Dermatol Surg. 2011;37:1189–1191 [DOI] [PubMed] [Google Scholar]
- 51.Moschella SL, Klein MH, Miller RJ. Perifolliculitis capitis abscedens et suffodiens. Report of a successful therapeutic scalping. Arch Dermatol. 1967;96:195–197 [PubMed] [Google Scholar]
- 52.Norris D. Zinc and cutaneous inflammation. Arch Dermatol. 1985;121:985–989 [PubMed] [Google Scholar]
- 53.Guéniche A, Viac J, Lizard G, et al. Protective effect of zinc on keratinocyte activation markers induced by interferon or nickel. Acta Derm Venereol. 1995;75:19–23 [DOI] [PubMed] [Google Scholar]
- 54.Nelson AM, Gilliland KL, Cong Z, et al. 13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126:2178–2189 [DOI] [PubMed] [Google Scholar]
- 55.Monaco C, Nanchahal J, Taylor P, et al. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. J Am Acad Dermatol. 2010;63:195–211; quiz 211 [DOI] [PubMed] [Google Scholar]
- 57.Cataldo-Cerda K, Wortsman X. Dissecting cellulitis of the scalp early diagnosed by color Doppler ultrasound. Int J Trichology. 2017;9:147–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C-N, Chen W, Hsu CK, et al. Dissecting folliculitis (dissecting cellulitis) of the scalp: a 66-patient case series and proposal of classification: dissecting folliculitis of the scalp in Taiwan. JDDG J Dtsch Dermatol Ges. 2018;16:1219–1226 [DOI] [PubMed] [Google Scholar]
- 59.Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch hidradenitis suppurativa expert group. Acta Derm Venereol. 2017;97:412–413 [DOI] [PubMed] [Google Scholar]
- 60.Chiricozzi A, Faleri S, Franceschini C, et al. AISI: a new disease severity assessment tool for hidradenitis suppurativa. Wounds. 2015;27:258–264 [PubMed] [Google Scholar]
- 61.Ingram JR. Interventions for hidradenitis suppurativa: updated summary of an original Cochrane review. JAMA Dermatol. 2017;153:458–459 [DOI] [PubMed] [Google Scholar]
- 62.Scerri L, Williams HC, Allen BR. Dissecting cellulitis of the scalp: response to isotretinoin. Br J Dermatol. 1996;134:1105–1108 [PubMed] [Google Scholar]
- 63.Dellon AL, Orlando JC. Perifolliculitis capitis: surgical treatment for the severe case. Ann Plast Surg. 1982;9:254–259 [DOI] [PubMed] [Google Scholar]
- 64.Williams CN, Cohen M, Ronan SG, et al. Dissecting cellulitis of the scalp. Plast Reconstr Surg. 1986;77:378–382 [DOI] [PubMed] [Google Scholar]
- 65.Ramasastry SS, Granick MS, Boyd JB, et al. Severe perifolliculitis capitis with osteomyelitis. Ann Plast Surg. 1987;18:241–244 [DOI] [PubMed] [Google Scholar]
- 66.Curry SS, Gaither DH, King LE., Jr Squamous cell carcinoma arising in dissecting perifolliculitis of the scalp. A case report and review of secondary squamous cell carcinomas. J Am Acad Dermatol. 1981;4:673–678 [DOI] [PubMed] [Google Scholar]
- 67.Coates A, Abraham S, Kaye SB, et al. On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–208 [DOI] [PubMed] [Google Scholar]
- 68.Bachynsky T, Antonyshyn OM, Ross JB. Dissecting folliculitis of the scalp. A case report of combined treatment using tissue expansion, radical excision, and isotretinoin. J Dermatol Surg Oncol. 1992;18:877–880 [DOI] [PubMed] [Google Scholar]


