Abstract
For frozen embryo transplantation patients who failed to use hormone replacement cycle (HRC) transplantation for 2 consecutive times, the third time of transplantation was divided into 2 groups: HRC and natural cycle (NC), and the pregnancy rate of the 2 groups, especially the clinical pregnancy rate, was compared.
Retrospective study of 174 patients in the reproductive medicine center of an affiliated hospital of Shandong University of Traditional Chinese Medicine between January 2015 and September 2018.
The 174 patients were all infertile with regular menstruation. They had undergone 2 consecutive failed cycles of endometrial preparation with hormone replacement therapy and prepare for the third frozen embryo transplantation.
A third cycle of treatment was planned using either NC or HRC for endometrial preparation. All the embryos were obtained during the same oocyte retrieval cycle. Patients were divided into groups based on the method of endometrial preparation: 98 were classified as NC and 76 as HRC.
The pregnancy outcomes for the 2 groups were compared. Confounding factors that may affect clinical pregnancy rates were analyzed.
We found that on the day of endometrial transformation, estrogen levels and endometrial thickness in the NC group were significantly higher than those in the HRC group. There were no significant differences in the rates of biochemical pregnancy, clinical pregnancy, cumulative pregnancy, miscarriage, multiple pregnancy, ectopic pregnancy, or live birth between the 2 groups. It is concluded by binary regression analysis that the different endometrial preparation protocol have no significant effect on the CPR.
NC is as effective as HRC after 2 previous cycles of HRC. Because this was a retrospective study design, selection bias is possible, although the baseline characteristics of the 2 groups of patients were matched.
Keywords: binary regression analysis, clinical pregnancy rate, frozen embryo transfer, hormone replacement cycles, live birth rate, natural cycle, two or more failed
1. Introduction
With the development of assisted reproductive technology (ART), many patients have benefited greatly from frozen embryo transfer (FET) to achieve pregnancy.[1] In addition, FET can effectively prevent complications related to in vitro fertilization such as ovarian hyper-stimulation syndrome and multiple pregnancies.[2] FET is an important method of in vitro fertilization and several studies have shown that with the advances in refrigerant recovery technology, the clinical pregnancy rate, live birth rate, and obstetric outcomes after FET are comparable to or better than those achieved with fresh embryo transfer.[3–5]
It has been shown[6] that the synchronization of embryo and endometrium development plays an important role in the success of implantation. Clinicians have developed several strategies for preparing the endometrium during FET, including using ovulation during the natural process of follicle development and a hormone replacement regiments with exogenous estrogen and progesterone supplementation. Studies have compared the clinical pregnancy rate, sustained pregnancy rate, and live birth rate of different FET regimens, and found no intergroup differences.[7–9] However, some studies have suggested that for women with regular menstruation, it is better to use their NC for endometrial preparation, because the use of exogenous hormones in HRC may change the level of physiological hormones, leading to adverse pregnancy outcomes.[10]
Kamiya and Moriwaka[11] reported that the clinical pregnancy rate of women under 40 years old using HRC was 33.3%. This raised the question of whether it would be better for patients with regular menstruation to switch to NC after 2 consecutive failed HRCs.
2. Materials and methods
2.1. Data source
The cases included in this study were all from the Reproductive Medicine Center of the hospital affiliated with Shandong University of Traditional Chinese Medicine. The ethics committee of the Reproductive Medicine Center approved the study (Grant No. TCM20141200211). All of the cases involved transfer of autologous embryos because assisted reproduction technology treatment using donated eggs, gametes, and embryos is banned in China. All participants signed informed consent forms prior to study inclusion.
From January 2015 to September 2018, a total of 201 patients under 45 years of age in our center planned to undergo a third cycle of FET because of failure of 2 previous cycles of HRC-FET. None of the patients had severe internal or surgical complications. Eight patients with an endometrial thickness <8 mm on the day of endometrial transformation during previous transplantation cycles were excluded. Twelve patients who were prepared for the NC with human chorionic gonadotropin (HCG) and 7 patients who were treated with down-regulated HRC were also excluded. Finally, a total of 174 patients with 174 FET cycles were included in the study. None of the subjects fell off during the study. All the embryos were obtained during the same oocyte retrieval cycle. The patient did not receive any medication while awaiting the transplant (Fig. 1).
Figure 1.
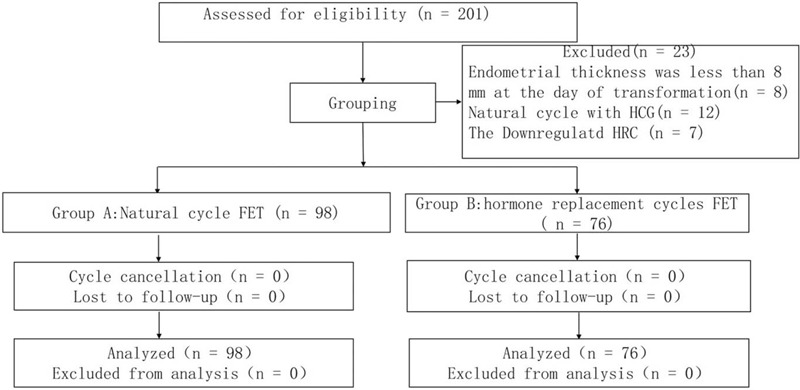
Case screening chart.
2.2. Sample size estimation
PASS2020 software (NCSS, LLC, Kaysville, UT) was used to calculate sample sizes for both groups. Test for Two Proportions was selected for calculation. Power value was set to 0.8. Alpha is set to 0.15 by default. According to the statistical data of our center, the clinical pregnancy rate of NC-FET was 0.39, and that of HRC-FET was 0.21. By calculation, each group required 65 subjects. Assuming that the follow-up loss rate of study subjects is 10%, sample size 65÷0.9 = 72 cases, Finally, 98 subjects were included in the NC group and 76 in the HRC group.
2.3. The endometrial preparation protocols
2.3.1. Natural cycle
Follicular development was monitored during the natural menstrual cycle in all patients. Starting from the tenth day of the menstrual cycle, a luteinizing hormone (LH) ovulation prediction kit was used every morning to monitor the level of urinary LH. If the contrast line of urinary LH was as strong as the control line, the patient would come to the hospital for follicular measurement until follicular discharge. On the same day, 20 mg of progesterone in oil was injected, and FET was performed on the fourth day of progesterone use.
2.3.2. Hormone replacement cycle
All of the patients received increasing doses of oral Progynova (BAYER, Germany), 4 to 6 mg per day for 12 to 20 days. Transvaginal ultrasound was used to determine that there was no follicular development and that endometrium thickness was >8 mm, and the same day, patients were injected with 40 mg of progesterone in oil combined with an oral dose of 20 mg dydrogesterone (Abbott Biologicals, the Netherlands). FET was performed on the fourth day of progesterone use.
2.4. Embryo transplantation
The method for thawing cleavage-stage embryos was as reported previously.[12] Cleavage-stage embryos were thawed on the day of transplantation and classified as grade I (good embryo) or grade II according to embryo shape, degree of fragmentation, and cell polarity. The embryos that are normally frozen in our center are cleavage-stage embryos on the third day. The maximum number of embryos transferred per cycle was 3. Priority is given to the transfer of grade 1 embryos, with no >1 grade 1 embryo per transfer.
2.5. Outcome measures
The main outcome measure was the live birth rate. Secondary outcomes included biochemical and clinical pregnancy rates, miscarriage rate, multiple pregnancy rate, and ectopic pregnancy rate. Elevated blood HCG was considered to indicate biochemical pregnancy, and clinical pregnancy was defined as the presence of a gestational sac with a fetal heart beat on ultrasound examination. Miscarriage was defined as spontaneous miscarriage between confirmation of clinical pregnancy and 24 weeks’ gestation. The cumulative pregnancy rate refers to the total chance of achieving clinical pregnancy after the transplantation of all embryos obtained from a retrieval cycle (fresh embryos and frozen embryos). Multiple pregnancy referred to the presence of ≥2 fetuses in a single uterine cavity. Ectopic pregnancy referred to the implantation of the pregnancy sac outside the uterine cavity. Live birth referred to the birth of a live fetus, regardless of the length of the pregnancy.
2.6. Statistical analysis
We used IBM SPSS Statistics version 22 (IBM Corp, Armonk NJ) for statistical analysis. Significance was defined at P < .05. Independent t test and dichotomous variable chi-squared test were used to analyze the differences between the 2 groups. The biochemical pregnancy rate and clinical pregnancy rate were calculated using the number of FET cycles as the denominator. Rates of cumulative pregnancy, live birth, miscarriage, and ectopic pregnancy were calculated using the number of clinical pregnancies as the denominator. Binary regression analysis was used to identify variables independently related to clinical pregnancy rate.
3. Results
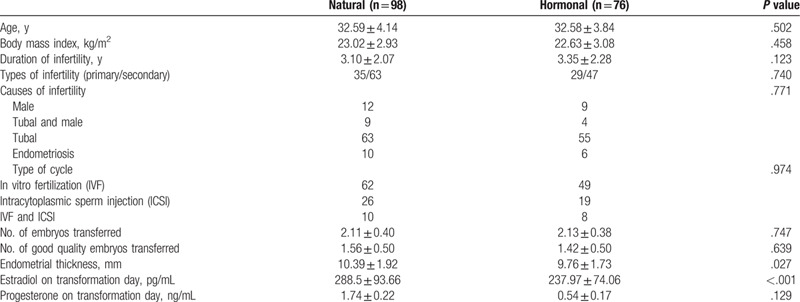
During the 3-year and 8-month observational study, data were collected from 174 subjects for a total of 174 transplant cycles, and all data were retained. The baseline characteristics of patients in the 2 groups were consistent, as was the number of transferred embryos (P = .747). The number of high-quality embryos was also similar (P = .639). On the day of endometrial transformation, endometrial thickness was significantly greater in the NC group than in the HRC group (P = .027). The estradiol level in the NC group on the day of endometrial transformation was significantly higher than that in the HRC group (P < .001). The progesterone level in the NC group on the day of endometrial transformation was also higher than that in the HRC group, but the difference was not significant (Table 1).
Table 1.
Characteristics of patients in the 2 groups.
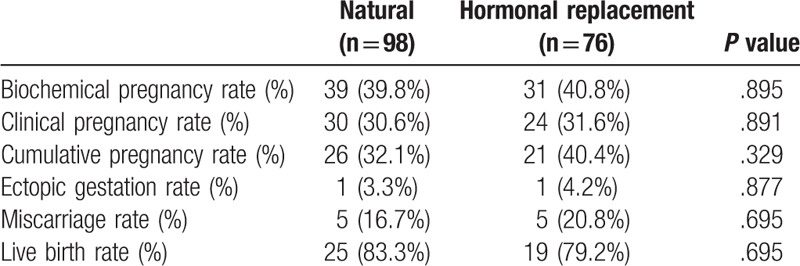
A comparison of the pregnancy outcomes of the 2 groups demonstrated that there were no significant differences between the groups in biochemical pregnancy rate (P = .895), clinical pregnancy rate (P = .891), cumulative pregnancy rate (P = .329), miscarriage rate (P = .695), ectopic pregnancy rate (P = .877), or live birth rate (P = .695). No multiple pregnancies occurred in either group (Table 2).
Table 2.
Comparison of pregnancy outcomes in the 2 groups.
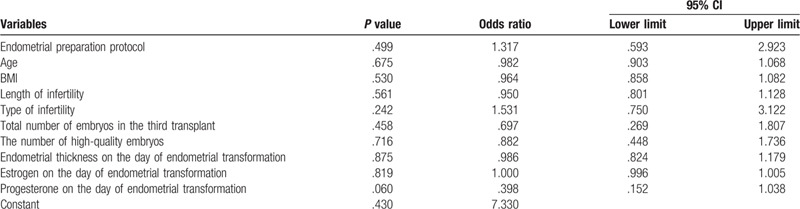
The multivariate model included endometrial preparation protocol, women's age, BMI, length of infertility, type of infertility, total number of embryos in the third transplant, the number of high-quality embryos, endometrial thickness on the day of endometrial transformation, estrogen and progesterone on the day of endometrial transformation. Binary regression analysis was then performed to adjust for potential confounding factors. Through multiple analysis, no variables with significant influence on CPR were found. Of course, it also indicates that after 2 consecutive replacement cycle transplants, the use of replacement cycle or natural cycle endometrial preparation in the third transplant cycle has no significant effect on CPR (Table 3).
Table 3.
Analysis of binary regression results affecting clinical pregnancy rate.
4. Discussion
The implantation window refers to the period during which the endometrium allows blastocyst implantation, which is regulated by estrogen and progesterone.[13] The NC completely relies on endogenous steroids to complete the preparation of the endometrium before embryo transfer, without the intervention of exogenous hormones. However, using the NC for FET requires frequent follicular monitoring to determine the timing of ovulation and prevent cycle cancellation. HRC refers to the use of exogenous estrogen and progesterone for endometrial preparation, which is conducive to the accurate determination of ovulation time and the formulation of an embryo transfer plan, whereby the rate of cancellation of the cycle is significantly reduced, especially for women with irregular menstruation. HRC can effectively reduce the frequency of patient monitoring and is convenient for doctors and patients.[14] In this study, the benefits of HRT cycles were well documented, despite the fact that both hormone levels and endometrial thickness met the transplant criteria during the first 2 HRT cycles, the pregnancy was not successful. We found no clear cause.
Some studies have shown no difference in live birth rates between NC-FET and HRC-FET in women with normal menstrual cycles.[15,16] In contrast, other study has reported that, in women with a normal ovulation cycle, the NC is the best choice for endometrial preparation before FET, and has a better pregnancy outcome.[17]
In a previous study, maternal age, maternal body mass index, and the number and quality of embryos transferred were reported to be the main factors influencing the results of FET.[18] In the present study there was no significant difference in the baseline characteristics of the 2 groups, so selection bias between the 2 groups was largely avoided.
In the present study, endometrial thickness in the HRC group was lower than that in the NC group (P = .027). However, the clinical pregnancy rates and live births were similar in the 2 groups. The reason for this may be that endometrial thickness is regulated by ovarian hormone levels and does not fully reflect the microenvironment and histology of the endometrium.[19]
Higher estradiol levels may interfere with implantation, leading to decreased endometrial receptivity and premature closure of the implantation window.[20] A previous study[21] showed that excessive estradiol exposure had a negative effect on the endometrium. In the present study, the estrogen level on the day of endometrial transformation was significantly higher in the NC group than in the HRC group, but there was no significant difference in the pregnancy rates of the 2 groups, possibly because the estrogen level in the NC group was similar to that in a normal ovulation period. However, the mechanism underlying this finding needs further study.
It has been suggested[16] that miscarriage rates after HRC and NC are comparable. However, in a retrospective analysis[22] of 666 natural and 466 hormonal FET cycles, the miscarriage rate after HRC (23%) was significantly higher than that after NC (11.4%). Our data also suggested that the miscarriage rate after HRC was higher than that after NC, but the difference was not significant.
5. Conclusion
Our results confirm that there is no significant difference in the clinical pregnancy rate and live birth rate after NC or HRC for patients who have undergone multiple failed cycles of FET and who have regular menstruation. It is concluded by binary regression analysis that the different endometrial preparation protocols have no significant effect on the CPR. There is no need to distinguish between the use of NC or HRC. Because of the small sample size in the present study, we did not compare NC with HCG or down-regulated HRC. It has been shown that the use of HCG can produce better endometrial thickness and achieve higher clinical and sustained pregnancy rates.[23] It has also been reported that the use of down-regulated HRC can significantly improve pregnancy and live birth rates.[24] Clinical data are needed to demonstrate the advantages and disadvantages of the last 2 FET treatment options.
Acknowledgments
The authors thank all the colleagues engaged in medical statistics and patients involved in this study.
Author contributions
Conceptualization: Zhengao Sun.
Data curation: Conghui Pang.
Formal analysis: Lin Guo.
Modification: Yanyan Bi.
Software: Conghui Pang, Yanyan Bi.
Validation: Zhengao Sun.
Visualization: Yanyan Bi, Zhijuan Wu.
Writing – original draft: Conghui Pang, Xiaoyan Xu.
Writing – review & editing: Kehua Wang, Fang Lian.
Footnotes
Abbreviations: ART = assisted reproductive technology, FET = frozen embryo transfer, hCG = human chorionic gonadotrophin, HRC = hormone replacement cycle, ICSI = intracytoplasmic sperm injection, IVF = in vitro fertilization, NC = natural cycle.
How to cite this article: Pang C, Guo L, Bi Y, Wang K, Lian F, Wu Z, Xu X, Sun Z. A comparison of pregnancy rate between natural cycle and hormone replacement cycle in patients who underwent frozen embryo transfer using 2 consecutive hormone replacement regiments: A STROBE-compliant retrospective study. Medicine. 2020;99:37(e22163).
This work was supported by the National Natural Science Foundation of China under Grant [No: 81874484].
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Givens CR, Markun LC, Ryan IP, et al. Outcomes of natural cycles versus programmed cycles for 1677 frozen-thawed embryo transfers. Reprod Biomed Online 2009;19:380–4.. [DOI] [PubMed] [Google Scholar]
- [2].Zheng Y, Li Z, Xiong M, et al. Hormonal replacement treatment improves clinical pregnancy in frozen-thawed embryos transfer cycles: a retrospective cohort study. Am J Transl Res 2013;6:85–90.. [PMC free article] [PubMed] [Google Scholar]
- [3].Shapiro BS, Daneshmand ST, Garner FC, et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011;96:344–8.. [DOI] [PubMed] [Google Scholar]
- [4].Kansal Kalra S, Ratcliffe SJ, Milman L, et al. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril 2011;95:548–53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maheshwari A, Pandey S, Shetty A, et al. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 2012;98:368.e1–77.e9.. [DOI] [PubMed] [Google Scholar]
- [6].Fazleabas AT, Strakova Z. Endometrial function: cell specific changes in the uterine environment. Mol Cell Endocrinol 2002;186:143–7.. [DOI] [PubMed] [Google Scholar]
- [7].al-Shawaf T, Yang D, al-Magid Y, et al. Ultrasonic monitoring during replacement of frozen/thawed embryos in natural and hormone replacement cycles. Hum Reprod 1993;8:2068–74.. [DOI] [PubMed] [Google Scholar]
- [8].Sathanandan M, Macnamee MC, Rainsbury P, et al. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study. Hum Reprod 1991;6:685–7.. [DOI] [PubMed] [Google Scholar]
- [9].Groenewoud ER, Cantineau AE, Kollen BJ, et al. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2013;19:458–70.. [DOI] [PubMed] [Google Scholar]
- [10].Morozov V, Ruman J, Kenigsberg D, et al. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet 2007;24:119–23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kamiya H, Moriwaka O. [Human embryo cryopreservation]. Hum Cell 1997;10:39–44.. [PubMed] [Google Scholar]
- [12].Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 2007;67:73–80.. [DOI] [PubMed] [Google Scholar]
- [13].Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol 1992;6:351–71.. [DOI] [PubMed] [Google Scholar]
- [14].Dal Prato L, Borini A, Cattoli M, et al. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril 2002;77:956–60.. [DOI] [PubMed] [Google Scholar]
- [15].Mounce G, McVeigh E, Turner K, et al. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril 2015;104:915.e1–20.e1.. [DOI] [PubMed] [Google Scholar]
- [16].Kawamura T, Motoyama H, Yanaihara A, et al. Clinical outcomes of two different endometrial preparation methods for cryopreserved-thawed embryo transfer in patients with a normal menstrual cycle. Reprod Med Biol 2007;6:53–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Konc J, Kanyo K, Varga E, et al. The effect of cycle regimen used for endometrium preparation on the outcome of day 3 frozen embryo transfer cycle. Fertil Steril 2010;94:767–8.. [DOI] [PubMed] [Google Scholar]
- [18].Lahav-Baratz S, Koifman M, Shiloh H, et al. Analyzing factors affecting the success rate of frozen-thawed embryos. J Assist Reprod Genet 2003;20:444–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang EM, Han JE, Kim YS, et al. Use of the natural cycle and vitrification thawed blastocyst transfer results in better in-vitro fertilization outcomes: cycle regimens of vitrification thawed blastocyst transfer. J Assist Reprod Genet 2011;28:369–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ma WG, Song H, Das SK, et al. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA 2003;100:2963–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fritz R, Jindal S, Feil H, et al. Elevated serum estradiol levels in artificial autologous frozen embryo transfer cycles negatively impact ongoing pregnancy and live birth rates. J Assist Reprod Genet 2017;34:1633–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Veleva Z, Tiitinen A, Vilska S, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod 2008;23:878–84.. [DOI] [PubMed] [Google Scholar]
- [23].Casper RF, Yanushpolsky EH. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril 2016;105:867–72.. [DOI] [PubMed] [Google Scholar]
- [24].El-Toukhy T, Taylor A, Khalaf Y, et al. Pituitary suppression in ultrasound-monitored frozen embryo replacement cycles. A randomised study. Hum Reprod 2004;19:874–9.. [DOI] [PubMed] [Google Scholar]


