Abstract
The geriatric nutritional risk index (GNRI) is associated with the prognosis of many diseases. However, the association between the GNRI and the prognosis of patients aged ≥65 years with severe community-acquired pneumonia (SCAP) has not been studied. We aimed to evaluate the prognostic value of GNRI in elderly SCAP patients.
This study retrospectively analyzed the clinical data of 346 patients aged ≥65 years with SCAP from December 2013 to September 2019. Patients were divided into 4 groups by the GNRI. The chi-square test or student's t test was used to compare the differences between the groups. Logistic regression analysis was used to evaluate the factors that affect prognosis. The receiver operating characteristic curve was used to compare the prognostic performance of the GNRI with other indicators. A GNRI-based nomogram was established based on the result of the multivariate analysis.
Two hundred nine (60.4%) patients had a poor prognosis. GNRI scores were significantly lower in the poor prognosis group than in the group with a good prognosis. In the multivariate analysis, gender, mean arterial pressure, neutrophil counts, and the GNRI were independently correlated with the prognosis of elderly patients. The GNRI was a significantly better predictor for poor prognosis than other indicators. The GNRI-based nomogram had excellent prediction capabilities.
GNRI is a simple and effective prognostic indicator for elderly patients with SCAP, and a GNRI-based nomogram can aid in developing individualized treatment plans for elderly patients with SCAP.
Keywords: elderly, geriatric nutritional risk index, prognosis, severe community-acquired pneumonia
1. Introduction
Community-acquired pneumonia (CAP) is an infectious inflammation of the lung parenchyma that occurs outside the hospital, including pathogen infection with a specific incubation period and onset within the average incubation period after admission. Severe pneumonia is identified in pneumonia patients with continuous hypoxemia or acute respiratory failure requiring ventilation support, circulatory failure such as hypotensive shock, or other organ dysfunction. Severe pneumonia can occur in both community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP). In this study, severe community-acquired pneumonia (SCAP) was studied. Due to weakened immunity, malnutrition, coexistence of multiple diseases, and increased bacterial resistance in the elderly, the likelihood of pneumonia developing into severe pneumonia is significantly increased in this group of patients. Out of all hospitalized CAP patients, 10% to 22% experience severe pneumonia and require critical care.[1] SCAP progresses rapidly with a fatality rate as high as 50%.[2]
The elderly are at a higher risk of nutritional problems due to their poor nutritional status, many basis diseases, weakened gastrointestinal function resulting in insufficient food intake and poor digestion and absorption, and other physiological characteristics. In addition, energy and protein requirement increases as a result of disease catabolism, disorders of intestinal flora, gastrointestinal dysfunction caused by hypoxia and antibiotics use, and the abnormal psychological state in hospital aggravating disease stress. Affected by their physiological and pathological characteristics, elderly patients with severe pneumonia tend to develop rapidly into malnutrition, which leads to a series of serious consequences: delayed repair of lung tissue damage can undermine the immune function, respiratory muscle and lung function leads to respiratory failure and shock, aggravating lung infection results in secondary infection and complications, ultimately aggravating baseline diseases and could even lead to death. The incidence of protein-energy malnutrition in acutely hospitalized elderly patients is 20% to 50%.[3] Malnutrition is a major independent predictor of adverse outcomes in elderly hospitalized patients.[4] Therefore, early recognition and timely correction of high-risk nutritional status are critical for improving the prognosis of elderly patients with SCAP.
The geriatric nutritional risk index (GNRI), proposed by Bouillanne et al[5] in 2005, evolved from the nutritional risk index and is a nutrition-related indicator that can be used to predict the prognosis of diseases in the elderly. The GNRI score is easy, fast, and affordable, and it can be estimated by measuring height, body mass, and serum albumin levels. The GNRI has been correlated with the outcome of various diseases in the elderly. However, the association between GNRI and the prognosis of elderly SCAP patients has not been reported. In this study, we are the first to investigate the value of the GNRI in assessing the prognosis of patients with SCAP.
2. Methods
2.1. Research objects
This study retrospectively analyzed the medical records of 346 patients aged ≥65 years with SCAP hospitalized in the first affiliated hospital of Guangxi medical university from December 2013 to September 2019. The inclusion criteria were: patients aged ≥65 years, patients were diagnosed with SCAP based on diagnostic criteria, the patients’ medical records and blood tests were complete. The exclusion criteria were: the admission time in the hospital was <24 hours, patients with incomplete hospital information and blood draw results, patients with hematological and immune system diseases. We used the IDSA/ATS (Infectious Diseases Society of America/American Thoracic Society) CAP severity criteria that include 2 major criteria and 9 minor criteria as diagnostic criteria for SCAP. The validated definition of SCAP included either 1 major or ≥3 minor criteria.[6] A total of 18,880 CAP patients aged ≥65 years were hospitalized between December 2013 and September 2019, of which 471 (2.49%) met the SCAP criteria. We excluded 125 patients, including 94 patients without height or weight measurements during hospitalization, 21 patients whose length of admission was <24 hours, and 10 patients with hematological or immune system diseases. Finally, 346 elderly patients with SCAP were enrolled in the study (Fig. 1). This study was approved by the hospital ethics committee of the first affiliated hospital of Guangxi medical university. The Ethical approval number was 2020 (KY-E-018). The study is in strict compliance with the Helsinki declaration's provisions, and all data were kept confidential.
Figure 1.
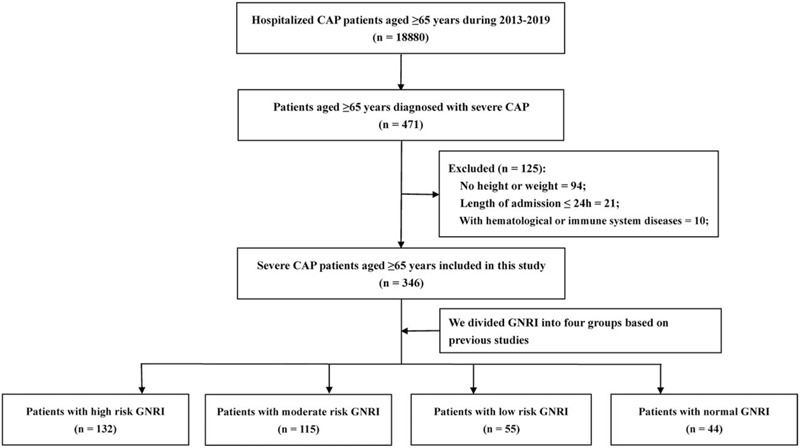
The process of case inclusion and exclusion in this study. CAP = community-acquired pneumonia; SCAP = severe community-acquired pneumonia.
2.2. Data collection
We collected clinical data of patients in the first week after admission to the ICU. The collected clinical and pathological features included gender, age, hospitalization days, the hospital situation, height, weight, comorbidities (diabetes, hypertension, myocardial infarction, congestive heart failure, cerebrovascular disease, chronic kidney disease, cancer, and respiratory failure), and vital signs (temperature, heart rate, breathing rate, and blood pressure). Venous blood was collected for routine blood analyses (hemoglobin, and leucocyte, platelet, neutrophil, lymphocyte, and monocytes counts), total bilirubin, serum albumin, serum creatinine, and blood urea nitrogen (BUN).
2.3. Definition of GNRI and other parameters
The GNRI was calculated as follows: GNRI = 1.489 × serum albumin concentration (g/L) + 41.7 × current body weight/ideal weight.[7] The ideal weight was defined as height (m) × height (m) × 22. When the current body weight was greater than the ideal weight, the ratio was set to 1. As in previous studies,[5] we divided the GNRI into 4 groups: high risk (GNRI < 82), moderate risk (82 ≤ GNRI < 92), low risk (92 ≤ GNRI < 98), and normal (GNRI ≥ 98). The mean arterial pressure (MAP) was calculated as diastolic pressure + 1/3 pulse pressure difference, the body mass index (BMI) as weight (kg)/height2 (m), the neutrophil-to-lymphocyte ratio (NLR) as neutrophil count (109/L)/lymphocyte count (109/L), the platelet-to-lymphocyte ratio (PLR) as platelet count (109/L)/lymphocyte count (109/L), the lymphocyte-to-monocyte ratio (LMR) as lymphocyte count (109/L)/monocyte count (109/L).
2.4. Outcomes
We reviewed each patient's medical records and defined patients who died during hospitalization or were discharged automatically due to adverse prognosis as patients with a poor prognosis, and defined patients who were discharged with improvement as having a good prognosis. Dying patients and patients discharged within 1 day were not included in the final analysis.
2.5. Statistical analysis
The student's t test was used to compare continuous variables, while the Chi-square test or Fisher exact test were used to compare classified variables. The standard deviation (SD) was used to describe continuous parametric data, while the median and inter quartile range (IQR) were used to describe continuous non-parametric data. The distribution of classification variables was expressed as percentages. A logistic regression analysis was used to assess independent predictors of patient outcomes, and all parameters with P < .05 in univariate analyses were included in the multivariate analysis. The multivariate logistic regression analysis was performed by using the stepwise variable selection method and we calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each parameter. Receiver operating characteristic (ROC) curves were used to compare the predictive prognostic efficacy of the GNRI, NLR, PLR, and LMR and to calculate their area under the curves (AUC). A P-value < .05 was considered statistically significant, and SPSS version 24.0 (IBM SPSS, IBM Corporation, Armonk, NY) and R software (version 3.5.3; http://www.r-project.org) were used for statistical analysis of all data.
3. Results
3.1. Clinicopathologic factors
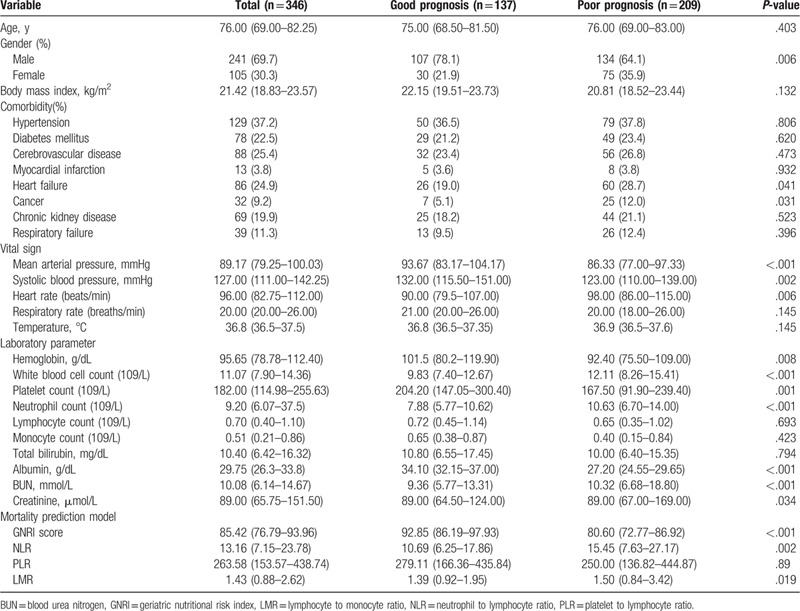
This study included 241 male and 105 female, with an average age of 76 years (range from 65 to 107 years). The numbers of patients with high risk GNRI, moderate risk GNRI, low risk GNRI, and normal GNRI were 132 (38.2%), 115 (33.2%), 55 (15.9%), and 44(12.7%), respectively. Of these, 209 (60.4%) patients had a poor prognosis, and 137 (39.6%) had a good prognosis, the characteristics of these groups are presented in Table 1. We explored the correlation between clinical-pathological factors and outcomes, including age, gender, BMI, comorbidity, vital signs, and the laboratory parameters GNRI, NLR, PLR, and LMR. We found that gender, heart failure, cancer, MAP, systolic blood pressure, heart rate, white blood cells, hemoglobin, platelets, neutrophils, albumin, BUN, blood creatinine, GNRI, NLR, and LMR were significantly different between the good and poor prognosis group.
Table 1.
General characteristics of the patients.
3.2. Correlation between the GNRI score and survival outcomes
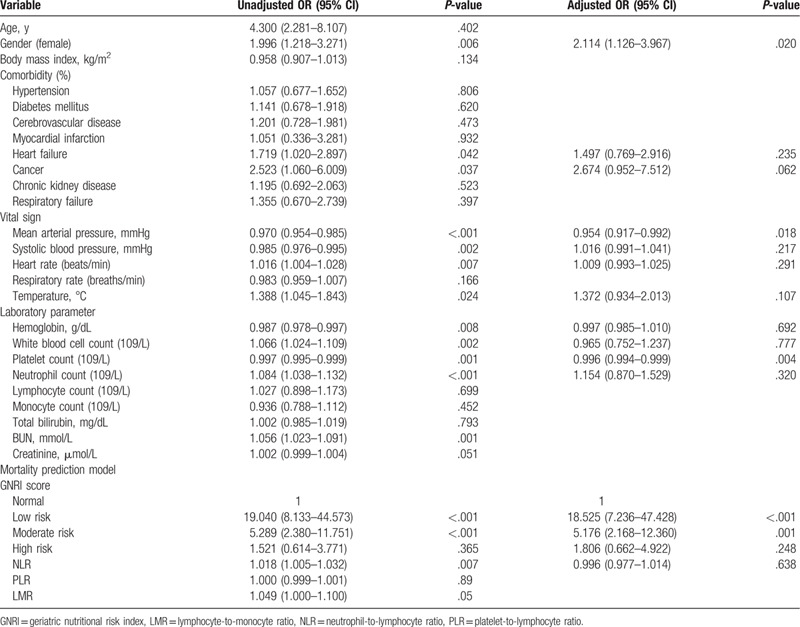
The mortality rates of the high risk, moderate risk, low risk, and normal group of GNRI were 22.7%, 30.9%, 60.9%, and 84.8%, respectively. To further evaluate the prognostic predictors of SCAP, we incorporated each factor into a logistic regression analysis. In the univariate analysis, we found that gender, heart failure, cancer, MAP, systolic blood pressure, heart rate, temperature, hemoglobin, white blood cell count, platelet count, neutrophil count, GNRI, and NLR were associated with SCAP prognosis. All variables with a P-value <.05 in the univariate analyses were incorporated into a multivariate analysis. Only gender, MAP, neutrophil counts, and GNRI were independent factors influencing the prognosis of SCAP in the elderly (Table 2).
Table 2.
Independent predictors of hospital mortality in older patients.
3.3. Comparison of the ROC curves of GNRI, NLR, PLR, and LMR to predict survival of patients with SCAP
To compare the ability of GNRI, NLR, PLR, and LMR to predict the survival of patients with SCAP, we plotted the ROC curve and calculated the AUC (Fig. 2). Our data showed that GNRI (0.800, 95% CI: 0.753–0.847, P < .001) had a significant predictive prognostic efficacy than the NLR 0.612 (95% CI: 0.552–0.672, P < .001), PLR 0.542 (95% CI: 0.481–0.603, P = .187), and LMR 0.557 (95% CI: 0.496–0.618, P = .076).
Figure 2.
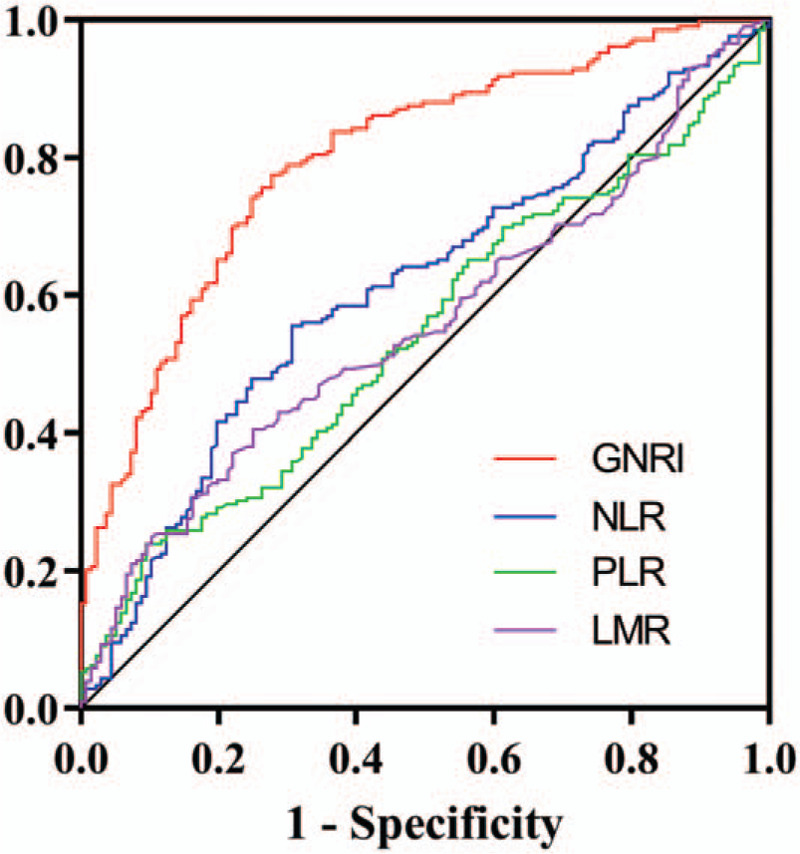
Area under the receiver operating characteristic curves of GNRI and other indicators for the prediction of survival. GNRI = geriatric nutritional risk index; LMR = lymphocyte to monocyte ratio; NLR = neutrophil to lymphocyte ratio; PLR = platelet to lymphocyte ratio.
3.4. Establishing GNRI-based nomograms to predict outcomes
Based on the results of the multivariate logistic regression analysis, we developed a GNRI-based nomogram to predict the prognosis of elderly patients with SCAP (Fig. 3). GNRI, gender, MAP, and platelet count were included in the risk model, and MAP and platelet counts were used as continuous variables to improve the prediction accuracy. In this analysis, the points of each factor could be counted, and the death risk can also be predicted. The C-index for the nomogram was 0.808 (95% CI: 0.760–0.856), and the calibration curves for the probability of death showed good consistency between the prediction of the nomogram and actual observations (Fig. 4).
Figure 3.
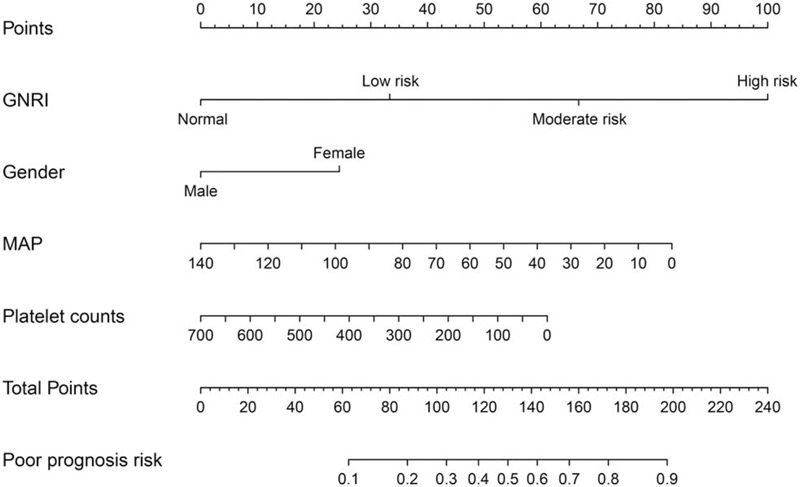
Construction of the GNRI-based nomogram. GNRI = geriatric nutritional risk index, MAP = mean arterial pressure.
Figure 4.
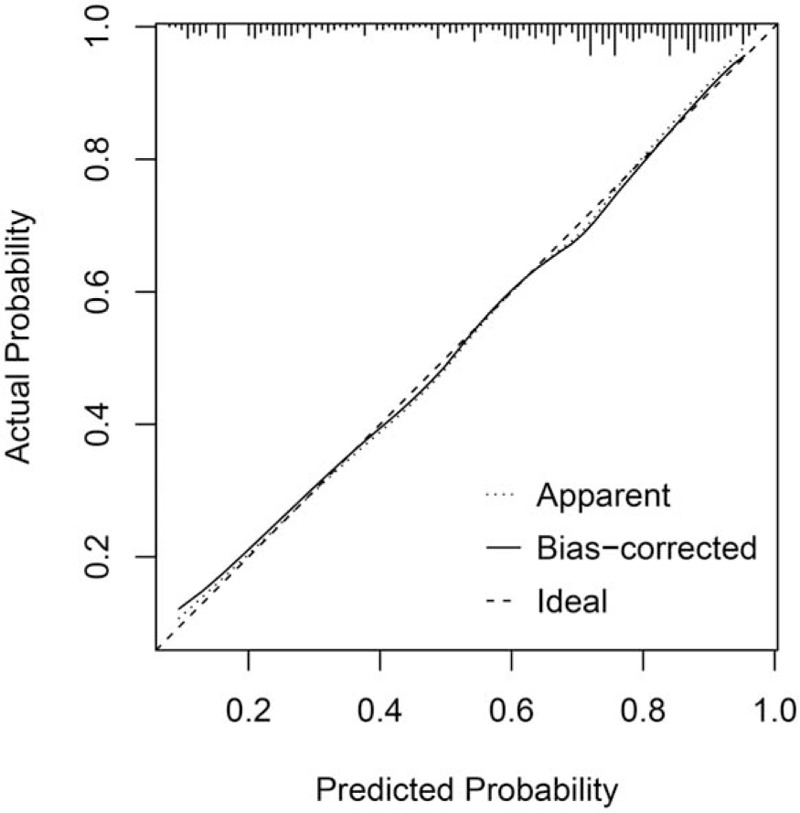
The calibration curves for the GNRI-based nomogram. Notes: The x axis presents the predicted probability and the y axis shows the actual probability. The calibration lines fit along with the 45 reference. GNRI = geriatric nutritional risk index.
4. Discussion
SCAP, especially in the elderly, is often accompanied by malnutrition, and the elderly are susceptible to infections due to age-related decline in their immune function, age-related structural and functional changes in organs, and increased underlying disease risk. Malnutrition is one of the most common causes of immune decline.[8] Malnutrition is susceptible to infection, and infection can lead to increased malnutrition, thereby entering a vicious cycle resulting in a more severe infection that is difficult to control.[9] Therefore, it is crucial to recognize high-risk nutritional status early to improve the prognosis of elderly patients with SCAP.
GNRI is a recently proposed indicator to evaluate the inflammatory nutritional status of patients. Owing to its simple calculation, it has been gradually applied in the prognosis evaluation of various chronic diseases. GNRI has prognostic value in patients with esophageal cancer,[10] hepatocellular carcinoma,[11] colorectal carcinoma,[12] and non-small-cell lung carcinoma.[13] Lee et al,[7] found that GNRI is associated with the prognosis of patients with sepsis, and that patients with a lower GNRI had higher mortality rates. GNRI is also increasingly considered as a predictor of nutritional risk and prolonged hospital stay. As reported by Gärtner et al,[14] GNRI is a valuable tool for assessing in-hospital mortality and predicting length of stay, and Cereda et al,[15] reported that elderly patients with a low GNRI had increases in the length of hospitalization. Abd-El-Gawad et al,[3] compared the ability of various nutrition-related parameters to predict the prognosis of patients and found that GNRI had a high predictive value in assessing nutritional status and forecasting nutrition-related complications in elderly patients. However, as far as we know, there have been no clinical studies confirming the application of GNRI in elderly patients with SCAP. In this study, we discussed the prognostic value of GNRI in elderly patients with SCAP for the first time.
GNRI is calculated based on serum albumin, body weight, and height, among which serum albumin and body weight are strong independent outcome predictors in elderly patients in previous studies.[16–19] Serum albumin is not only used to evaluate malnutrition but also as an indirect indicator of inflammation.[20,21] Serum albumin is affected by extracellular fluid volume,[22] and body weight is also impacted by hydration status, but changes in hydration status are in sharp contrast to changes in albumin concentration. Therefore, the 2 indicators in GNRI minimize the effect of confounding variables such as hydration status, resulting in a better prediction of nutrition-related death.
In this study, we found that female patients, and patients with comorbidities heart failure, and cancer, are more likely to have a poor prognosis. Similarly, patients with a low MAP and systolic blood pressure and a high heart rate tend to have a poor prognosis. Furthermore, we found that patients with a poor prognosis had lower hemoglobin, platelets, and albumin levels, while having higher white blood cell and neutrophil counts, BUM, and creatinine than those with a good prognosis. It is worth noting that there were significant differences between the GNRI and LMR in the mortality prediction model between the 2 groups, and patients with a high GNRI and LMR tended to have a better prognosis. By comparing the mortality rates of patients in the GNRI group, we found that with the gradual decrease of GNRI, the mortality rate of patients gradually increased. Through multivariate logistic regression analysis, we further found that GNRI was an independent prognostic factor in elderly patients with SCAP. In addition, female gender, a low MAP, and a low platelet count were also independent risk factors for patient prognosis. Studies have shown that women are generally less healthy in old age than men, and are more likely to suffer from malnutrition, resulting in worse outcomes. These factors may be related to differences in genes between men and women.[23] Low MAP indicates that the body is in a state of insufficient tissue and organ perfusion and hemodynamic instability, which is more likely to lead to a poor prognosis. MAP has previously been associated with organ perfusion and mortality.[24,25] In addition, MAP was also identified as an independent risk factor for death in patients admitted to the ICU.[26] Many recent studies have demonstrated that platelets are involved in the body's immune regulation and inflammatory response, and that the degree of thrombocytopenia is related to the severity of the disease, potentially leading to higher mortality.[27,28]
Peripheral blood leukocytes and their subsets are effective and easily obtained indicators for the evaluation of inflammation. However, the sensitivity and specificity of traditional infection indicators such as WBC and neutrophils alone are low. Recently, the NLR was reported to be closely related to the systemic inflammatory response and is widely used to evaluate the prognosis of various chronic diseases.[29,30] de Jager et al,[31] suggested that it can be used to predict the prognosis of patients with pneumonia. They showed it was superior to the traditional indicators, such as NEU and WBC, in predicting mortality. This may be due to the excessive activation of neutrophils in severe infections, which can cause the destruction of organ parenchymal cells and lead to multiple organ dysfunction.[32] Lymphocytopenia indicates that the body is in an immunosuppressive state and cannot effectively regulate the specific immune response to resist pathogen infection, leading to a poor prognosis.[33] In addition, PLR has been identified as a potential marker of systemic infections,[34] and MLR is thought to be associated with the prognosis of Klebsiella pneumonia infection.[35] In this study, we further compared the AUC of GNRI, NLR, PLR, and LMR, and concluded that the GNRI was the most suitable mortality prediction model for evaluating the prognosis of elderly patients with SCAP. In addition, in a multivariate analysis, we showed that only GNRI was an independent factor affecting the prognosis of patients with SCAP, in contrast to NLR, PLR, and LMR.
To facilitate the individual evaluation of the prognosis of patients with SCAP, we established a GNRI-based nomogram, which showed an increased risk of poor prognosis in female patients and a further increased risk of death with lower GNRI, MAP, and platelet count levels (Fig. 2). When comparing these risk factors, GNRI and MAP had a more significant effect on mortality than platelet count and gender. With this scoring system, we were able to predict the survival of patients with SCAP, and the survival rate of patients with a lower overall score was significantly higher than that of patients with a higher overall score. In addition, the C-index indicated that this model has moderate prediction accuracy. The calibration plot matching the 45 line was used to further confirm the accuracy of the nomogram. Individual scores of patients with SCAP could be determined. Patients with high scores should be very closely monitored, receive nutrition supplementation, maintain a hemodynamic stability, and treated with platelet enhancement if necessary. This might improve the prognosis of patients with SCAP.
Some limitations of this study should be noted. This study is a single-center retrospective study based on a limited number of samples, and the results of this study should be verified in multicenter, large-sample studies. In addition, the GNRI-based nomogram was established based on a limited number of patients. Although the C-index and correction curve showed good predictive accuracy, it still should be verified in external samples obtained from multiple centers.
5. Conclusion
This study confirmed that the GNRI is an easy to obtain and effective prognostic indicator for elderly patients with SCAP. A GNRI-based nomogram can aid in developing individualized treatment plans for elderly patients with SCAP.
Author contributions
Conceptualization: Ping Yan.
Data curation: Lishuang Wei, Hailun Xie.
Formal analysis: Junkang Li, Rui Li, and Weijian Chen.
Methodology: Lanfang Huang, Xialin Li.
Writing – original draft: Lishuang Wei, Hailun Xie.
Writing – review & editing: Ping Yan.
Footnotes
Abbreviations: AUC = area under the curves, BMI = body mass index, CAP = community-acquired pneumonia, CIs = confidence intervals, GNRI = geriatric nutritional risk index, HAP = hospital-acquired pneumonia, IQR = inter quartile range, LMR = lymphocyte-to-monocyte ratio, MAP = mean arterial pressure, NLR = neutrophil-to-lymphocyte ratio, ORs = odds ratios, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic, SCAP = severe community-acquired pneumonia, SD = standard deviation.
How to cite this article: Wei L, Xie H, Li J, Li R, Chen W, Huang L, Li X, Yan P. The prognostic value of geriatric nutritional risk index in elderly patients with severe community-acquired pneumonia: A retrospective study. Medicine. 2020;99:37(e22217).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Sligl WI, Marrie TJ. Severe community-acquired pneumonia. Crit Care Clin 2013;29:563–601.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Confalonieri M, Kodric M, Santagiuliana M, et al. To use or not to use corticosteroids for pneumonia? A clinician's perspective. Monaldi Arch Chest Dis 2012;77:94–101.. [DOI] [PubMed] [Google Scholar]
- [3].Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, et al. The validity of Geriatric Nutrition Risk Index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin Nutr 2014;33:1108–16.. [DOI] [PubMed] [Google Scholar]
- [4].Mühlethaler R, Stuck AE, Minder CE, et al. The prognostic significance of protein-energy malnutrition in geriatric patients. Age Ageing 1995;24:193–7.. [DOI] [PubMed] [Google Scholar]
- [5].Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005;82:777–83.. [DOI] [PubMed] [Google Scholar]
- [6].Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44: suppl: S27–72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee JS, Choi HS, Ko YG, et al. Performance of the Geriatric Nutritional Risk Index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin Nutr 2013;32:843–8.. [DOI] [PubMed] [Google Scholar]
- [8].Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63.. [DOI] [PubMed] [Google Scholar]
- [9].Felblinger DM. Malnutrition, infection, and sepsis in acute and chronic illness. Crit Care Nurs Clin North Am 2003;15:71–8.. [DOI] [PubMed] [Google Scholar]
- [10].Kubo N, Sakurai K, Tamura T, et al. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus 2019;16:147–54.. [DOI] [PubMed] [Google Scholar]
- [11].Tan P, Xie N, Ai J, et al. The prognostic significance of Albumin-to-Alkaline Phosphatase Ratio in upper tract urothelial carcinoma. Sci Rep 2018;8:12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang S, Xie H, Kuang J, et al. The Value of Geriatric Nutritional Risk Index in evaluating postoperative complication risk and long-term prognosis in elderly colorectal cancer patients. Cancer Manag Res 2020;12:165–75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shoji F, Matsubara T, Kozuma Y, et al. Preoperative Geriatric Nutritional Risk Index: a predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol 2017;26:483–8.. [DOI] [PubMed] [Google Scholar]
- [14].Gärtner S, Kraft M, Krüger J, et al. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr 2017;36:1048–53.. [DOI] [PubMed] [Google Scholar]
- [15].Cereda E, Pusani C, Limonta D, et al. The association of Geriatric Nutritional Risk Index and total lymphocyte count with short-term nutrition-related complications in institutionalised elderly. J Am Coll Nutr 2008;27:406–13.. [DOI] [PubMed] [Google Scholar]
- [16].Sullivan DH, Bopp MM, Roberson PK. Protein-energy undernutrition and life-threatening complications among the hospitalized elderly. J Gen Intern Med 2002;17:923–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Persson MD, Brismar KE, Katzarski KS, et al. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc 2002;50:1996–2002.. [DOI] [PubMed] [Google Scholar]
- [18].Corti MC, Guralnik JM, Salive ME, et al. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 1994;272:1036–42.. [PubMed] [Google Scholar]
- [19].Dey DK, Rothenberg E, Sundh V, et al. Body mass index, weight change and mortality in the elderly. A 15 y longitudinal population study of 70 y olds. Eur J Clin Nutr 2001;55:482–92.. [DOI] [PubMed] [Google Scholar]
- [20].Balkwill F. Tumour necrosis factor and cancer. Nature reviews. Cancer 2009;9:361–71.. [DOI] [PubMed] [Google Scholar]
- [21].Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nature reviews. Immunology 2015;15:362–74.. [DOI] [PubMed] [Google Scholar]
- [22].Jones CH, Smye SW, Newstead CG, et al. Extracellular fluid volume determined by bioelectric impedance and serum albumin in CAPD patients. Nephrol Dial Transplant 1998;13:393–7.. [DOI] [PubMed] [Google Scholar]
- [23].Archer CR, Recker M, Duffy E, et al. Intralocus sexual conflict can resolve the male-female health-survival paradox. Nat Commun 2018;9:5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98–104.. [DOI] [PubMed] [Google Scholar]
- [25].Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825–31.. [DOI] [PubMed] [Google Scholar]
- [26].Houwink AP, Rijkenberg S, Bosman RJ, et al. The association between lactate, mean arterial pressure, central venous oxygen saturation and peripheral temperature and mortality in severe sepsis: a retrospective cohort analysis. Crit Care 2016;20:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vlacha V, Feketea G. Thrombocytosis in pediatric patients is associated with severe lower respiratory tract inflammation. Arch Med Res 2006;37:755–9.. [DOI] [PubMed] [Google Scholar]
- [28].Mirsaeidi M, Peyrani P, Aliberti S, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest 2010;137:416–20.. [DOI] [PubMed] [Google Scholar]
- [29].Polat N, Yildiz A, Yuksel M, et al. Association of neutrophil-lymphocyte ratio with the presence and severity of rheumatic mitral valve stenosis. Clin Appl Thromb Hemost 2014;20:793–8.. [DOI] [PubMed] [Google Scholar]
- [30].Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2012;32:297–302.. [DOI] [PubMed] [Google Scholar]
- [31].de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One 2012;7:e46561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wesche DE, Lomas-Neira JL, Perl M, et al. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol 2005;78:325–37.. [DOI] [PubMed] [Google Scholar]
- [33].Jilma B, Blann A, Pernerstorfer T, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med 1999;159:857–63.. [DOI] [PubMed] [Google Scholar]
- [34].Akboga MK, Canpolat U, Yayla C, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology 2016;67:89–95.. [DOI] [PubMed] [Google Scholar]
- [35].Wang JL, Lu XY, Xu XH, et al. Predictive role of monocyte-to-lymphocyte ratio in patients with Klebsiella pneumonia infection: a single-center experience. Medicine (Baltimore) 2019;98:e17215. [DOI] [PMC free article] [PubMed] [Google Scholar]


