Abstract
Background:
Adverse outcomes after unilateral vs bilateral breast reconstruction involve an unknown level of risk that warrants thorough investigation.
Methods:
To address this research need, PubMed, Ovid, Medline, EMBASE, and Scopus databases were searched through systematically from January 1, 1990, to January 1, 2019 to retrieve the relevant studies on the risk of postoperative complications after unilateral vs bilateral abdominal flap breast reconstruction. According to the pre-designed inclusion criteria, available data were extracted from the relevant studies, and then analyzed comparatively in order to identify the relative risk (RR) and 95% confidence intervals (CI) applying either a random or a fixed effects model.
Results:
Eventually, 20 studies involving 8122 female subjects met the inclusion criteria. It was found that unilateral reconstruction involved a significantly higher risk of flap loss (RR: 1.56, 95% CI: 1.21–2.00; P < .05) and fat necrosis (RR: 1.60, 95% CI: 1.23–2.09; P < .05) compared to bilateral reconstruction, while bilateral reconstruction involved a greater risk of abdominal hernia/bulge (RR: 1.67, 95% CI: 1.25–2.24; P < .05). The risk was found to be higher following bilateral free transverse rectus abdominis myocutaneous (fTRAM) flaps in comparison with deep inferior epigastric perforator (DIEP) flaps (RR: 2.62, 95% CI: 1.33–5.15; P < .05).
Conclusion:
The risk of postoperative flap complications in unilateral breast reconstruction is significantly higher than that in bilateral reconstruction. Contrarily, the abdominal complications were significantly higher in the bilateral group vs the unilateral group. Meanwhile, the risk of abdominal hernia/bulge complication after bilateral breast reconstruction was significantly higher with fTRAM vs DIEP. Therefore, DIEP flaps are recommended in priority for bilateral breast reconstruction, unless specifically contraindicated.
Keywords: abdominal flaps, adverse events, bilateral vs unilateral, breast reconstruction
1. Introduction
Breast reconstruction methods, including tissue expanders and implants,[1,2] may lead to adverse consequences. For instance, implants may require removal due to infection or other complication such as rupture or problematic capsular formation and, in addition, implants do not have an infinite lifespan and, therefore, require replacement at various stages throughout a patients life. Autologous tissue transplantation provides an excellent like-for-like reconstruction for most patients in that is it natural and durable, and is associated with documented higher patient satisfaction.[3,4] Abdominal flaps are most commonly used for breast reconstruction in autologous tissue transplantation because of their reliable blood supply and adequate tissue volume to reconstruction ratio.[5]
In order to reduce the risk of breast cancer in certain groups of patients with specific genetic mutations, preventative risk reducing mastectomy is increasingly accepted by doctors and patients.[6–11] Some related studies have shown that bilateral breast reconstruction with abdominal flaps is featured with reduced incidence of donor site morbidity, less postoperative pain and superior reconstructive outcomes, and therefore greatly improves patient satisfaction.[12–15] However, other studies have shown that bilateral breast reconstruction is deemed more complicated and carries a higher risk of complications than unilateral breast reconstruction.[32,35,38,40] The existing studies present controversial conclusions regarding the adverse outcomes and contain limited series reporting variable complication rates.
Previous meta-analysis have suggested that there is a higher risk of flap loss after bilateral deep inferior epigastric perforator (DIEP) flap in comparison with unilateral breast reconstruction,[16] but the included studies were limited and incomplete. With constant update from emerging research, a new meta-analysis was needed, which formed the drive for our study. In such a context, the present study aimed to perform a meta-analysis for comparing the recipient-site and donor-site complication rates following unilateral vs bilateral abdominal flap breast reconstruction based on the integrated data extracted from published research works in order to minimize deviation and enhance statistical accuracy.
2. Materials and methods
2.1. Search strategy
Referencing to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),[17] the meta-analysis was initiated with a systematic literature search using PubMed, Ovid, EMBASE, Medline, and Scopus databases from January 1, 1990, to January 1, 2019 to retrieve relevant studies evaluating the risk of postoperative complications after unilateral vs bilateral abdominal flap breast reconstruction. This study was approved by the Ethics Committee of Xiangya Hospital, Central South University. The keywords used in the literature search include:“breast reconstruction”, “DIEP”, ‘“TRAM” (transverse rectus abdominis myocutaneous), “SIEA” (superficial inferior epigastric artery), “unilateral” along with “bilateral”. No restriction was imposed, and the references and comments of each study were carefully reviewed.
2.2. Study selection
The preliminarily-retrieved studies were firstly reviewed by title, followed by abstract, and then full text to determine whether they had met the following inclusion criteria:
-
1.
Observational study comparing unilateral vs bilateral groups;
-
2.
Breast reconstruction performed with abdominal flap; and
-
3.
Postoperative recipient-site and donor-site complications included in the primary outcomes.
All the studies that contain only comments or summaries of meetings, or have no access to the full text were excluded.
2.3. Data extraction
Available information and results were extracted from each study meeting the inclusion criteria by 2 independent researchers. Disagreements were resolved through discussion. If the same experimental group appeared in more than 2 studies, data would be extracted from the latest and the most complete one.
The extracted data included the first author, country of origin, year of publication, mean age, group size, flap type, study design, device type, body mass index, and Newcastle Ottawa Scale (NOS) score.[18] The quality was evaluated for each study based on NOS from 3 perspectives: groups selection; comparability among various groups; ascertaining of exposure (case-control studies), or outcomes of interest (cohort studies). The primary results of interest were designed to be flap loss, fat necrosis, abdominal hernia/bulge, and vascular thrombosis complications requiring surgical revision or conservative treatments such as dressing change, skin graft, or local flap transposition.
2.4. Statistical analyses
In this meta-analysis, the incidences of postoperative complications after unilateral vs bilateral breast reconstruction were combined using dichotomous variables. Relative risk (RR) was chosen to represent dichotomous variables when measures were combined and the occurrence of events and total sample size of each group were known. The 95% confidence interval (CI) was recorded, with P < .05 being referred to as statistically significant.[19]
The heterogeneity of the combined results of observational studies was tested using Q statistics (P < .05 referred to as heterogeneous) and I2 statistics (I2 > 50% referred to as heterogeneous).[20] The random effects model was applied when the data combined from studies was significant heterogeneity, or else the fixed effects model was applied.[21] As the population characteristics, surgical proficiency, preoperative positioning methods, and related risk factors were inconsistent among several studies, we made a sensitivity analysis to elucidate the reason for heterogeneity. Then, a subgroup analysis of breast reconstructions was also performed using different abdominal flaps (DIEP, free TRAM (fTRAM), pedicled TRAM (pTRAM), unipedicled TRAM (unipTRAM), or “Others”) to explore the influence of covariate changes on RR.
We further carried out Beggs tests and funnel plots for evaluating the publication bias. All statistical analyses were executed in Review Manager 5.2 (RevMan, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) and STATA 12.0 software (Stata Corporation, College Station, TX).[22] As aforementioned, P < .05 was referred to as statistically significant, except where otherwise specified.
3. Results
3.1. Search results and study characteristics
There were 1514 studies retrieved in PubMed, Ovid, Medline, EMBASE, and Scopus databases initially. Figure 1 shows the inclusion process used in study selection, which identified 20 studies for final analysis. Table 1 presented the features[23–42] of included studies. All these studies were case-control in nature, including 3 prospective studies and 17 retrospective studies. Specifically, 8 studies involved DIEP breast reconstruction; 3 involved TRAM breast reconstruction; 6 involved DIEP and TRAM breast reconstruction; and 3 involved DIEP, TRAM, and SIEA. It is noteworthy that there are 4 studies,[25,26,35,36] in which results could be extracted only for recipient-site or donor-site complications. The NOS score was ranged from 5 to 8 among the included studies.
Figure 1.
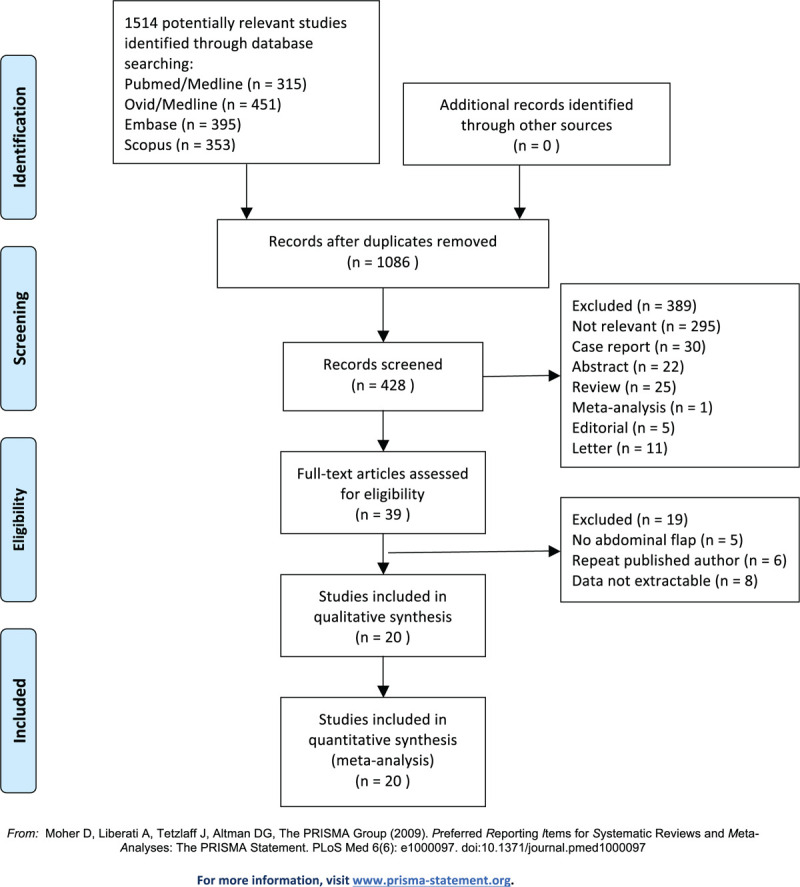
Flowchart for study selection.
Table 1.
Characteristics of the included studies.
3.2. Flap loss complications
Based on 16 observational studies involving 7828 flaps, the combined data for flap loss complications showed that patients who underwent unilateral abdominal flaps had significantly higher complications vs bilateral flaps (RR: 1.56, 95% CI: 1.21–2.00; P < .05). No indication of substantial heterogeneity was identified (P = .22; I2 = 20%), and analysis was carried out using a fixed effects model (Fig. 2). The results of sensitivity analysis on flap loss complications did not vary substantially, and RR was ranged from 1.33 (95% CI: 1.01–1.77; P = .04) to 1.70 (95% CI: 1.31–2.21; P < .05). There was no indication of substantial heterogeneity either (P = .17–0.47; I2 = 0%–24%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 3), and the Beggs rank correlation test did not reveal any publication bias (P = .970). In the subgroup analysis of flap loss complications (Fig. 2), statistically-significant differences were shown in the “Others” subgroup (RR: 1.68, 95% CI: 1.14–2.48; P < .05), but not in that of DIEP, fTRAM, pTRAM, and unipTRAM. There was no indication of significant heterogeneity in the subgroup analysis (P = .61; I2 = 0%).
Figure 2.
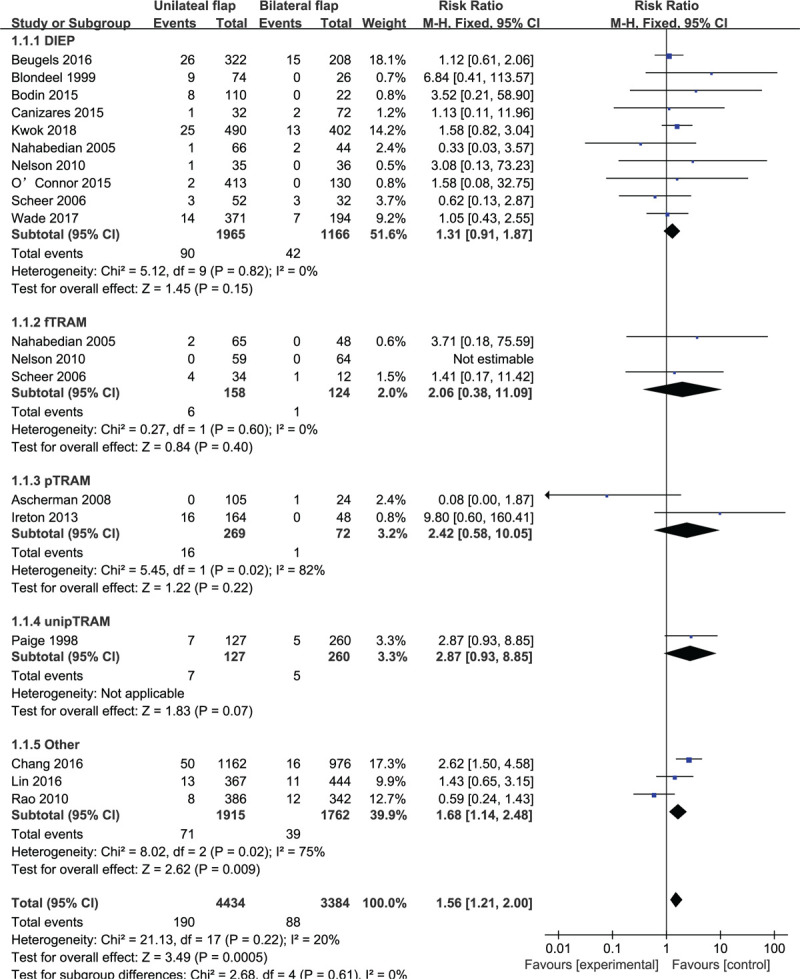
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: flap loss.
Figure 3.
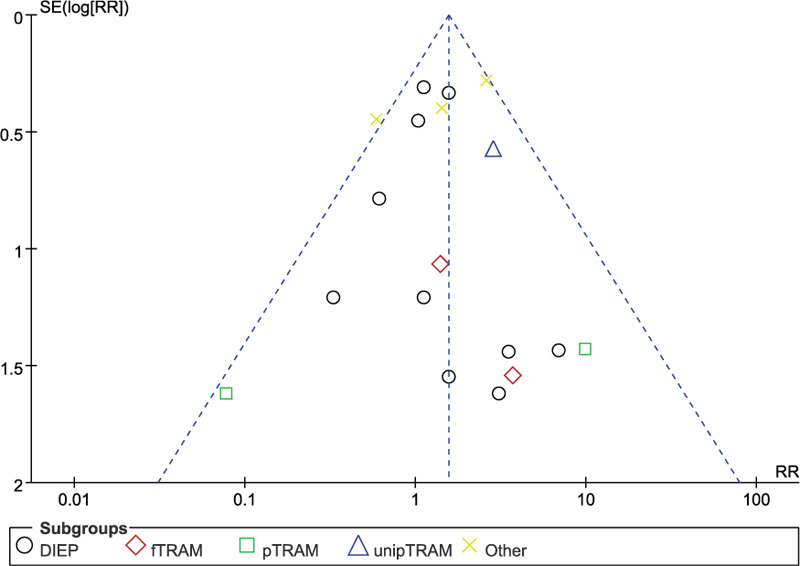
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: flap loss.
3.3. Fat necrosis complications
Based on 10 observational studies involving 2823 flaps, the combined data for fat necrosis complications showed that patients who underwent unilateral abdominal flaps had significantly higher complications vs bilateral flaps (RR: 1.60, 95% CI: 1.23–2.09; P < .05). No indication of substantial heterogeneity was identified (P = .41; I2 = 4%), and analysis was carried out using a fixed effects model (Fig. 4). The results of sensitivity analysis on fat necrosis complications did not vary substantially, and RR was ranged from 1.49 (95% CI: 1.12–1.99; P < .05) to 1.91 (95% CI: 1.40–2.59; P < .05). There was no indication of substantial heterogeneity either (P = .34–0.68; I2 = 0%–11%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 5), and the Beggs rank correlation test did not reveal any publication bias (P = 1.000). In the subgroup analysis of fat necrosis complications (Fig. 4), statistically significant differences were shown in the subgroups of DIEP and unipTRAM (RR: 1.66, 95% CI: 1.15–2.38; P < .05 and RR: 2.52, 95% CI: 1.25–5.08; P < .05, respectively), but not in that of fTRAM, pTRAM, and “Others”. There was no indication of significant heterogeneity in the subgroup analysis (P = .12; I2 = 45.1%).
Figure 4.
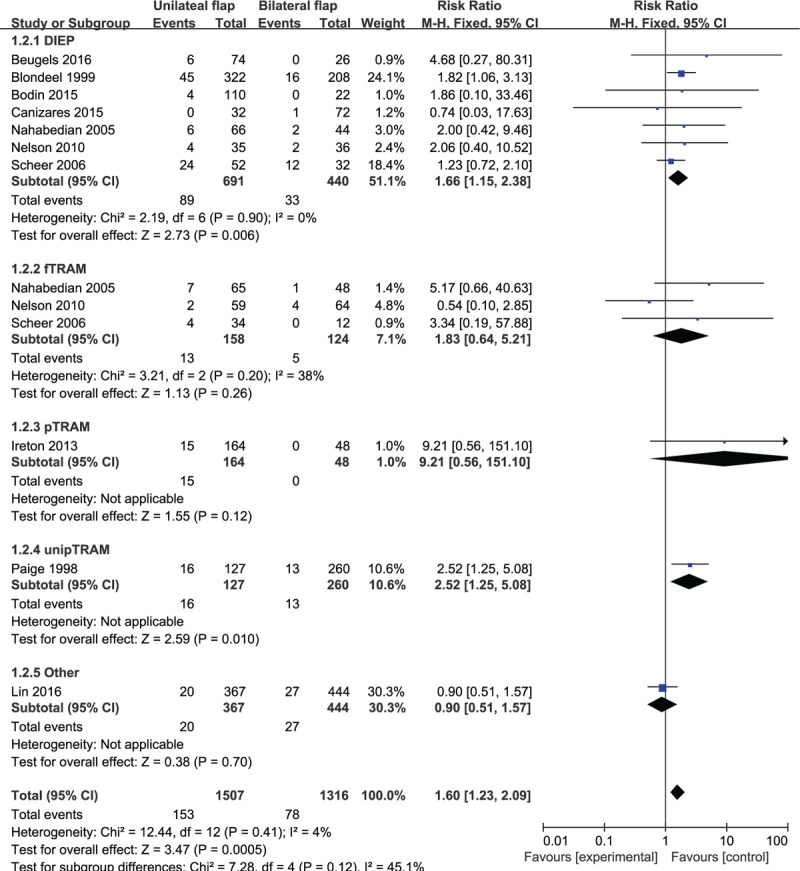
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: fat necrosis.
Figure 5.
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: fat necrosis.
Based on 3 observational studies involving 311 flaps, the combined data for unilateral fat necrosis complications did not suggest any significant difference between the subgroups of DIEP and fTRAM (RR: 2.14, 95% CI: 0.74–6.23; P > .05). Substantial heterogeneity was reflected (P = .08; I2 = 60%), and a random effects model was chosen for analysis (Fig. 6). The results of sensitivity analysis on fat necrosis complications varied substantially. After excluding the study by Nahabedian, et al,[35] the results showed a statistically significant difference (RR: 3.77, 95% CI: 1.64–8.68; P < .05), with no significant heterogeneity (P = .88; I2 = 0%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 7), and the Beggs rank correlation test did not reveal any publication bias (P = .604).
Figure 6.
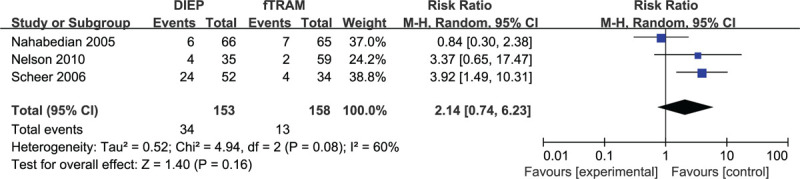
Forest plot of unilateral DIEP vs fTRAM breast reconstruction for the outcome: fat necrosis.
Figure 7.
Funnel plot of unilateral DIEP vs fTRAM breast reconstruction for the outcome: fat necrosis.
3.4. Vascular thrombosis complications
Based on 9 observational studies involving 2789 flaps, the combined data for vascular thrombosis complications showed no significant difference for unilateral vs bilateral flaps (RR: 0.81, 95% CI: 0.54–1.21; P = .31). No indication of substantial heterogeneity was identified (P = .78; I2 = 0%), and analysis was carried out using a fixed effects model (Fig. 8). The results of sensitivity analysis on vascular thrombosis complications did not vary substantially, and RR was ranged from 0.74 (95% CI: 0.49–1.12; P = .16) to 0.88 (95% CI: 0.58–1.33; P = .54). There was no indication of substantial heterogeneity either (P = .70–0.86; I2 = 0%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 9), and the Beggs rank correlation did not reveal any publication bias (P = 1.000). In the subgroup analysis of vascular thrombosis complications (Fig. 8), no statistically significant difference was reflected among the subgroups of DIEP, fTRAM, and “Others”. There was no indication of significant heterogeneity in the subgroup analysis (P = .94; I2 = 0%).
Figure 8.
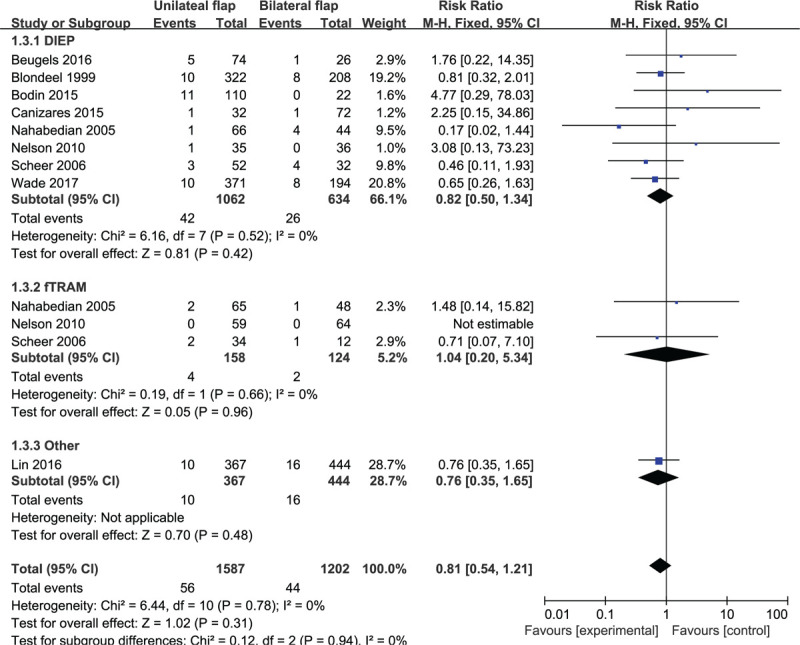
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: vascular thrombosis.
Figure 9.
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: vascular thrombosis.
3.5. Flap infection complications
Based on 8 observational studies involving 2958 flaps, the combined data for flap infection complications showed that patients who underwent unilateral abdominal flaps had significantly higher complications vs bilateral flaps (RR: 1.45, 95% CI: 1.02–2.06; P < .05). No indication of substantial heterogeneity was identified (P = .71; I2 = 0%), and analysis was carried out using a fixed effects model (Fig. 10). The results of sensitivity analysis on flap infection complications did not vary substantially, and RR was ranged from 1.30 (95% CI: 0.89–1.89; P = .17) to 1.84 (95% CI: 1.40–3.07; P < .05). There was no indication of substantial heterogeneity either (P = .62–0.86; I2 = 0%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 11), and the Beggs rank correlation test did not reveal any publication bias (P = .325). In the subgroup analysis of flap infection complications (Fig. 10), no statistically significant difference was reflected among the subgroups of DIEP, fTRAM, pTRAM, unipTRAM, and “Others”. There was no indication of significant heterogeneity in the subgroup analysis (P = .49; I2 = 0%).
Figure 10.
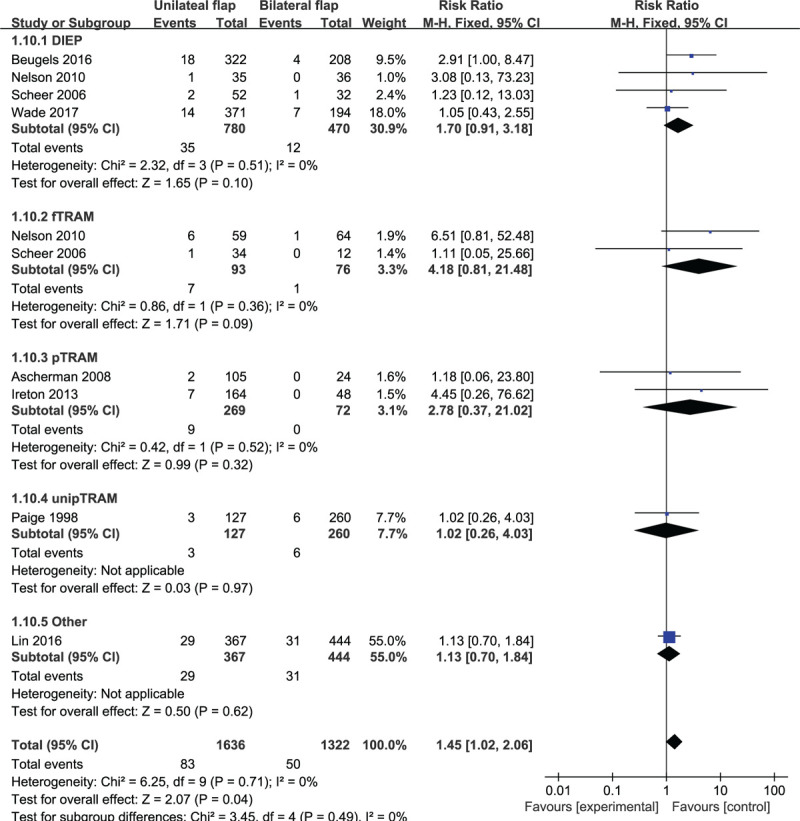
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: flap infection.
Figure 11.
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: flap infection.
3.6. Flap hematoma complications
Based on 7 observational studies involving 1195 flaps, the combined data for flap hematoma complications showed no significant difference for unilateral vs bilateral flaps (RR: 1.05, 95% CI: 0.61–1.83; P = .85). No indication of substantial heterogeneity was identified (P = .93; I2 = 0%), and analysis was carried out using a fixed effects model (Fig. 12). The results of sensitivity analysis on vascular thrombosis complications did not vary substantially, and RR was ranged from 0.96 (95% CI: 0.54–1.71; P = .88) to 1.11 (95% CI: 0.62–1.99; P = .73). There was no indication of substantial heterogeneity either (P = .86–0.97; I2 = 0%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 13), and the Beggs rank correlation did not reveal any publication bias (P = .453). In the subgroup analysis of vascular thrombosis complications (Fig. 12), no statistically significant difference was reflected among the subgroups of DIEP, fTRAM, and pTRAM. There was no indication of significant heterogeneity in the subgroup analysis (P = .92; I2 = 0%).
Figure 12.
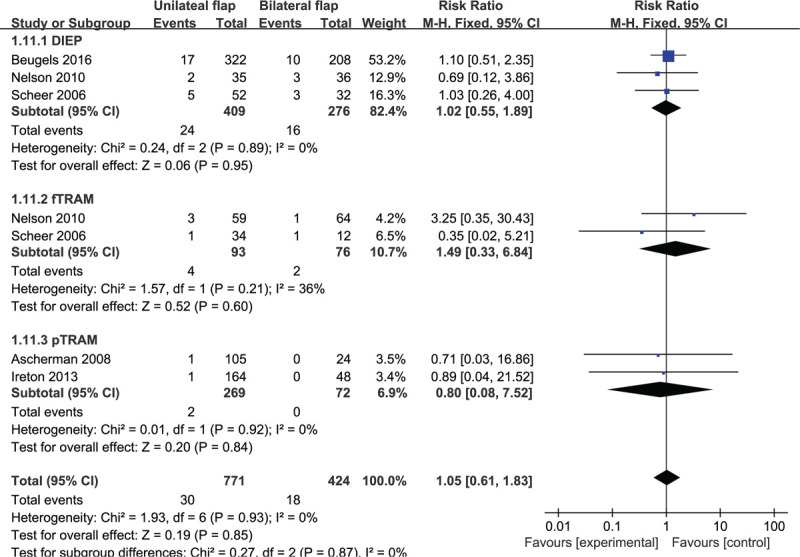
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: flap hematoma.
Figure 13.
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: flap hematoma.
3.7. Abdominal hernia/bulge complications
Based on 13 observational studies involving 3772 subjects, the combined data for abdominal hernia/bulge complications showed that patients who underwent bilateral abdominal flaps had significantly higher complications vs the unilateral group (RR: 1.67, 95% CI: 1.25–2.24; P < .05). No indication of substantial heterogeneity was identified (P = .51; I2 = 0%), and analysis was carried out using a fixed effects model (Fig. 14). The results of sensitivity analysis on abdominal hernia/bulge complications did not vary substantially, and RR was ranged from 1.59 (95% CI: 1.17–2.16; P < .05) to 1.81 (95% CI: 1.34–2.46; P < .05). There was no indication of substantial heterogeneity either (P = .44–0.74; I2 = 0%–1%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 15), and the Beggs rank correlation test did not reveal any publication bias (P = .787). In the subgroup analysis of abdominal hernia/bulge complications (Fig. 14), statistically significant differences were shown in the subgroups of fTRAM and pTRAM (RR: 1.75, 95% CI: 1.20–2.57; P < .05 and RR: 5.74, 95% CI: 1.32–24.93; P < .05, respectively), but not in that of DIEP and unipTRAM. There was no indication of significant heterogeneity in the subgroup analysis (P = .36; I2 = 7%).
Figure 14.
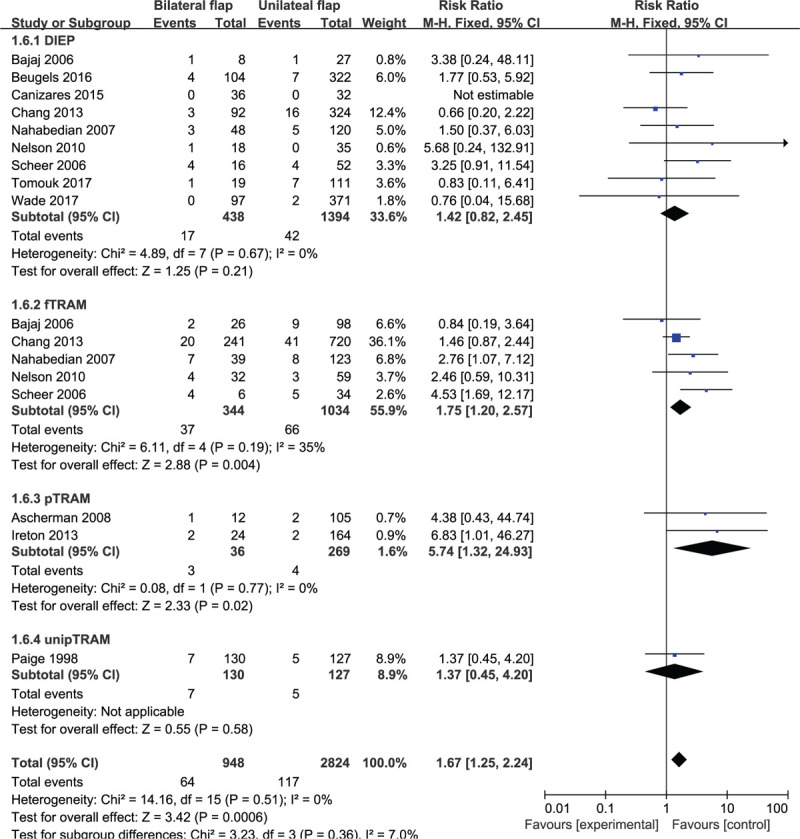
Forest plot of unilateral vs bilateral breast reconstruction for the outcome: abdominal hernia/bulge.
Figure 15.
Funnel plot of unilateral vs bilateral breast reconstruction for the outcome: abdominal hernia/bulge.
Based on 4 observational studies involving 492 subjects, the combined data for abdominal hernia/bulge complications showed that patients who underwent bilateral fTRAM had significantly higher complications vs the bilateral DIEP group (RR: 2.62, 95% CI: 1.33–5.15; P < .05). No indication of substantial heterogeneity was identified (P = 1.00; I2 = 0%), and analysis was carried out using a fixed effects model (Fig. 16). The results of sensitivity analysis on abdominal hernia/bulge complications did not vary substantially, and RR was ranged from 2.53 (95% CI: 1.14–5.61; P < .05) to 2.67 (95% CI: 1.20–5.92; P < .05). There was no indication of substantial heterogeneity either (P = .98–1.00; I2 = 0%). Meanwhile, the funnel plot did not suggest any substantial asymmetry (Fig. 17), and the Beggs rank correlation test did not reveal any publication bias (P = .497).
Figure 16.
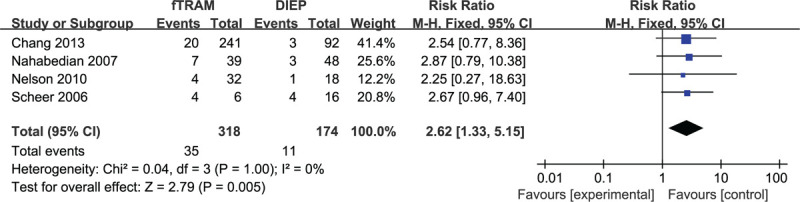
Forest plot of bilateral DIEP vs fTRAM breast reconstruction for the outcome: abdominal hernia/bulge.
Figure 17.
Funnel plot of bilateral DIEP vs fTRAM breast reconstruction for the outcome: abdominal hernia/bulge.
4. Discussion
In recent decades, an increasing number of unilateral breast cancer patients underwent contralateral breast prophylactic resections.[43,44] The increase in demand for bilateral breast reconstruction has been facilitated by improved genetic testing, as well as surgical and reconstruction techniques and outcomes.[45–49] However, the studies evaluating the risk of postoperative complications after unilateral vs bilateral breast reconstruction are controversial, especially in terms of complication percentage. The present meta-analysis further evaluated the risk of adverse outcomes after unilateral vs bilateral breast reconstruction.
The RR of flap loss for unilateral vs bilateral breast reconstruction in this study was 1.56 (95% CI: 1.21–2.00; P < .05), suggesting an increase of 1.56-fold in flap loss risk after unilateral breast reconstruction. The RR of fat necrosis for unilateral vs bilateral breast reconstruction was 1.60 (95% CI: 1.23–2.09; P < .05), suggesting an increase of 1.60-fold in the risk of developing fat necrosis after unilateral breast reconstruction. The RR of flap infection for unilateral vs bilateral breast reconstruction in this study was 1.45 (95% CI: 1.02–2.06; P < .05), suggesting an increase of 1.56-fold in infection risk after unilateral breast reconstruction. There was no significant difference between vascular crisis and subcutaneous hematoma. Postoperative flap loss is usually due to a combination of factors (infection, hematoma, postoperative management, etc.). Scheer et al[23] pointed out a possible reason for such a difference in RR, that is, large abdominal flaps were harvested in patients who underwent unilateral breast reconstruction to match the contralateral breast size, which increased the risk of flap loss and fat necrosis, especially in the distal part of the flap because of inadequate vascular support. During the bilateral breast reconstruction, covering the area of both breasts using abdominal flaps increases the scope of the flaps after dividing the original flap into 2 flaps of the same size, each with independent perforator blood vessels.
The RR of abdominal hernia/bulge between unilateral vs bilateral breast reconstruction was 1.67 (95% CI: 1.25–2.24; P < .05) in the present study, suggesting an increase of 1.67-fold in the risk of developing abdominal hernia/bulge after bilateral breast reconstruction. This is probably due to the fact that bilateral breast reconstruction requires a large abdominal flap, which therefore involves a larger degree of injury to the abdominal soft tissues. However, using computed tomographic angiography or Doppler ultrasound preoperatively to locate perforator blood vessels, supported by sophisticated surgical experience, can significantly reduce the degree of abdominal injury.
A subgroup analysis was also performed to evaluate breast reconstruction using different flaps. In the fat necrosis sub-analysis, statistically significant differences were shown in the subgroup of DIEP and unipTRAM (RR: 1.66, 95% CI: 1.15–2.38; P < .05 and RR: 2.52, 95% CI: 1.25, 5.08; P < .05, respectively), but not in that of fTRAM, pTRAM, and “Others”. Existing studies have shown that TRAM can provide adequate tissues for breast reconstruction and guarantee reliable blood vessel supply.[50] Blondeel et al[51] and Kroll[52] reported an increased prevalence of fat necrosis resulting from decreased venous drainage in DIEP flaps compared with TRAM flaps. The incidence of fat necrosis complications was not statistically different in the combined unilateral DIEP and fTRAM group (RR: 2.14, 95% CI: 0.74–6.23; P > .05). Although the risk of fat necrosis complications was significantly higher in unilateral breast reconstruction than the bilateral reconstruction, the risk of fat necrosis complications in the unilateral DIEP and fTRAM groups showed neither statistically significant difference nor substantial heterogeneity. Therefore, it can be concluded that the results of this study did not suggest a higher rate of fat necrosis in the DIEP vs fTRAM flap group.
In the abdominal hernia/bulge sub-analysis, statistically significant differences could be observed in the subgroups of fTRAM and pTRAM (RR: 1.75, 95% CI: 1.20–2.57; P < .05 and RR: 5.74, 95% CI: 1.32–24.93; P < .05, respectively), but not in that of DIEP and unipTRAM. Meanwhile, according to the combined results, the incidence of abdominal complications in the bilateral fTRAM group was significantly higher than that in the DIEP group (RR: 2.62, 95% CI: 1.33–5.15; P < .05). There was an increase of 2.62-fold in the risk of developing abdominal hernia/bulge after bilateral fTRAM breast reconstruction, which was significantly higher than that of DIEP. However, during the TRAM flap creation, abdominal wall injury is reportedly greater than the case of DIEP, which results in a greater risk of poor abdominal appearance and abdominal wall weakness complications.[53,54] Some related research also suggested that DIEP was advantageous over TRAM in protecting the structure and function of abdominal wall. DIEP was developed with the purpose of reducing the risk of postoperative complications after breast reconstruction using TRAM.[55,56] The meta-analysis by Wang, et als[57] indicated that TRAM patients were subject to a higher rate of postoperative complications than DIEP patients. Therefore, the relevant patient factors, such as comorbidities, must be carefully considered during the preparation for breast reconstruction in order to decrease the rate of complications and identify the procedures with the best chance for success.
The main advantage of this study is that, after performing the sensitivity analysis of various exclusion criteria, the association of postoperative risk with unilateral vs bilateral breast reconstruction was found to be statistically significant, and no substantial heterogeneity was observed. Furthermore, in the subgroup analyses stratified by different flap reconstruction methods, this association remained statistically significant. In addition, the funnel plots suggested no substantial asymmetry, and the Beggs rank correlation test did not reveal any publication bias. This meta-analysis included a total of 20 relevant studies, mainly in English language; the amount of studies and patients in each group were both increased compared to previous meta-analyses. Therefore, this study is comparatively powerful and specific in evaluating the risk of postoperative complications after unilateral vs bilateral breast reconstruction.
The limitations of this meta-analysis are as follows. First, it was unable to extract data from the original studies regarding the preoperative method used to locate perforators, and uncontrolled confounding factors including comorbidities, age, smoking history, breast reconstruction time, and body mass index may also lead to potential problems, especially considering that obesity may be associated with postoperative complications. Second, future studies should evaluate the postoperative follow-up and compare the longer-term differences in complications between unilateral and bilateral groups, including abdominal aesthetics and patient satisfaction.
5. Conclusions
This meta-analysis has shown that the risk of postoperative flap complications in unilateral breast reconstruction is significantly higher than that in bilateral reconstruction. Contrarily, the abdominal complications were significantly higher in the bilateral group vs the unilateral group. Meanwhile, the risk of abdominal hernia/bulge complication after bilateral breast reconstruction was significantly higher with fTRAM vs DIEP. Therefore, DIEP flaps are recommended as the preferred method for bilateral breast reconstruction, unless clearly contraindicated.
Author contributions
Data curation: Xiaoyang Pang, Jiri Cao, Zheming Cao.
Formal analysis: Jiri Cao, Zheming Cao.
Funding acquisition: Panfeng Wu.
Investigation: Xiaoyang Pang, Wei Du.
Methodology: Jiri Cao, Wei Du, Zheming Cao.
Resources: Xiaoyang Pang, Wei Du, Zheming Cao.
Software: Jiri Cao, Zheming Cao.
Supervision: Panfeng Wu.
Validation: Panfeng Wu.
Writing – original draft: Xiaoyang Pang, Zheming Cao.
Writing – review & editing: Zheming Cao, Panfeng Wu.
Footnotes
Abbreviations: CI = confidence intervals, DIEP = deep inferior epigastric artery perforator, fTRAM = free transverse abdominal myocutaneous, NOS = Newcastle Ottawa Scale, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, pTRAM = pedicled transverse rectus abdominis myocutaneous, RR = relative risk, SGAP = superior gluteal artery perforator, SIEA = superficial inferior epigastric artery perforator, upTRAM = unipedicled transverse rectus abdominis myocutaneous.
How to cite this article: Cao Z, Cao J, Pang X, Du W, Wu P. A comparative study for the rate of adverse outcomes in unilateral and bilateral abdominal flap breast reconstruction: a meta-analysis. Medicine. 2020;99:37(e22096).
ZC and JC contributed equally to this work.
This publication was funded in part by the Natural Science Foundation of Hunan Province (2018JJ2640).
The authors declare that they have no conflict of interests.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Alderman A, Gutowski K, Ahuja A, et al. ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg 2014;134:648e–55e.. [DOI] [PubMed] [Google Scholar]
- [2].Wenzel CG, Wacholtz WF, Janssen DA, et al. Weight measurement and volumetric displacement of breast implants and tissue expanders. Clin Plast Surg 2015;42:481–91.. [DOI] [PubMed] [Google Scholar]
- [3].Colakoglu S, Khansa I, Curtis MS, et al. Impact of complications on patient satisfaction in breast reconstruction. Plast Reconstr Surg 2011;127:1428–36.. [DOI] [PubMed] [Google Scholar]
- [4].Vega SJ, Bossert RP, Serletti JM. Improving outcomes in bilateral breast reconstruction using autogenous tissue. Ann Plast Surg 2006;56:487–90.. [DOI] [PubMed] [Google Scholar]
- [5].Kroll SS. Bilateral breast reconstruction. Clin Plast Surg 1998;25:251–8.. [PubMed] [Google Scholar]
- [6].Arrington AK, Jarosek SL, Virnig BA, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol 2009;16:2697–704.. [DOI] [PubMed] [Google Scholar]
- [7].Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203–9.. [DOI] [PubMed] [Google Scholar]
- [8].King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol 2011;29:2158–64.. [DOI] [PubMed] [Google Scholar]
- [9].Han E, Johnson N, Glissmeyer M, et al. Increasing incidence of bilateral mastectomies: the patient perspective. Am J Surg 2011;201:615–8.. [DOI] [PubMed] [Google Scholar]
- [10].Stucky CC, Gray RJ, Wasif N, et al. Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol 2010;17:S330–7.. [DOI] [PubMed] [Google Scholar]
- [11].Yao K, Stewart AK, Winchester DJ, et al. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the national cancer data base, 1998-2007. Ann Surg Oncol 2010;17:2554–62.. [DOI] [PubMed] [Google Scholar]
- [12].Wu LC, Bajaj A, Chang DW, et al. Comparison of donor-site morbidity of SIEA, DIEP, and muscle-sparing TRAM flaps for breast reconstruction. Plast Reconstr Surg 2008;122:702–9.. [DOI] [PubMed] [Google Scholar]
- [13].Guerra AB, Metzinger SE, Bidros RS, et al. Bilateral breast reconstruction with the deep inferior epigastric perforator (DIEP) flap: an experience with 280 flaps. Aesth Plast Surg 2004;52:246–52.. [DOI] [PubMed] [Google Scholar]
- [14].Drazan L, Vesely J, Hyza P, et al. Bilateral breast reconstruction with DIEP flaps: 4 years experience. J Plast Reconstr Aesthet Surg 2008;61:1309–15.. [DOI] [PubMed] [Google Scholar]
- [15].Craft RO, Colakoglu S, Curtis MS, et al. Patient satisfaction in unilateral and bilateral breast reconstruction. Plast Reconstr Surg 2011;127:1417–24.. [DOI] [PubMed] [Google Scholar]
- [16].Wormald JC, Wade RG, Figus A. The increased risk of adverse outcomes in bilateral deep inferior epigastric artery perforator flap breast reconstruction compared to unilateral reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2014;67:143–56.. [DOI] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med 2009;151:W-65–94.. [DOI] [PubMed] [Google Scholar]
- [18].Wells GA, Shea BJ, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric 2014;18:727–34.. [Google Scholar]
- [19].Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ 1997;315:1533–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dersimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scheer AS, Novak CB, Neligan PC, et al. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg 2006;56:355–8.. [DOI] [PubMed] [Google Scholar]
- [24].Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg 2006;117:737–46.. [DOI] [PubMed] [Google Scholar]
- [25].Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg 2013;132:1383–91.. [DOI] [PubMed] [Google Scholar]
- [26].Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive evaluation of risk factors and management of impending flap loss in 2138 breast free flaps. Ann Plast Surg 2016;77:67–71.. [DOI] [PubMed] [Google Scholar]
- [27].Fitzgerald OE, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg 2016;5:93–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bodin F, Dissaux C, Lutz JC, et al. The DIEP flap breast reconstruction: starting from scratch in a university hospital. Ann Chir Plast Esthet 2015;60:171–8.. [DOI] [PubMed] [Google Scholar]
- [29].Lin IC, Nelson JA, Wu LC, et al. Assessing surgical and medical complications in bilateral abdomen-based free flap breast reconstructions compared with unilateral free flap breast reconstructions. Ann Plast Surg 2016;77:61–6.. [DOI] [PubMed] [Google Scholar]
- [30].Beugels J, Hoekstra LT, Tuinder SM, et al. Complications in unilateral versus bilateral deep inferior epigastric artery perforator flap breast reconstructions: a multicentre study. J Plast Reconstr Aesthet Surg 2016;69:1291–8.. [DOI] [PubMed] [Google Scholar]
- [31].Ascherman JA, Seruya M, Bartsich SA. Abdominal wall morbidity following unilateral and bilateral breast reconstruction with pedicled TRAM flaps: an outcomes analysis of 117 consecutive patients. Plast Reconstr Surg 2008;121:1–8.. [DOI] [PubMed] [Google Scholar]
- [32].Nelson JA, Guo Y, Sonnad SS, et al. A Comparison between DIEP and muscle-sparing free TRAM flaps in breast reconstruction: a single surgeon's recent experience. Plast Reconstr Surg 2010;126:1428–35.. [DOI] [PubMed] [Google Scholar]
- [33].Ireton JE, Kluft JA, Ascherman JA. Unilateral and bilateral breast reconstruction with pedicled TRAM flaps: an outcomes analysis of 188 cnsecutive patients. Plast Reconstr Surg 2013;1:1–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kwok AC, Edwards K, Donato DP, et al. Operative time and flap failure in unilateral and bilateral free flap breast reconstruction. J Reconstr Microsurg 2018. [DOI] [PubMed] [Google Scholar]
- [35].Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM Flap: is there a difference? Plast Reconstr Surg 2005;115:436–44.. [DOI] [PubMed] [Google Scholar]
- [36].Nahabedian MY. Secondary operations of the anterior abdominal wall following microvascular breast reconstruction with the TRAM and DIEP flaps. Plast Reconstr Surg 2008;120:365–72.. [DOI] [PubMed] [Google Scholar]
- [37].Canizares O, Mayo J, Soto E, et al. Optimizing efficiency in deep inferior epigastric perforator flap breast reconstruction. Ann Plast Surg 2015;75:186–92.. [DOI] [PubMed] [Google Scholar]
- [38].Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104–11.. [DOI] [PubMed] [Google Scholar]
- [39].Paige KT, Bried JT, Jones G. A comparison of morbidity from bilateral, unipedicled and unilateral, unipedicled TRAM flap breast reconstructions. Plast Reconstr Surg 1998;101:1819–27.. [DOI] [PubMed] [Google Scholar]
- [40].Wade RG, Razzano S, Sassoon EM, et al. Complications in DIEP flap breast reconstruction after mastectomy for breast cancer: a prospective cohort study comparing unilateral versus bilateral reconstructions. Ann Surg Oncol 2017;24:1–0.. [DOI] [PubMed] [Google Scholar]
- [41].Rao SS, Parikh PM, Goldstein JA, et al. Unilateral failures in bilateral microvascular breast reconstruction. Plast Reconstr Surg 2010;126:17–25.. [DOI] [PubMed] [Google Scholar]
- [42].Tomouk T, Mohan AT, Azizi A, et al. Donor site morbidity in DIEP free flap breast reconstructions: a comparison of unilateral, bilateral, and bipedicled surgical procedure types. J Plast Reconstr Aesthet Surg 2017;70:1505–13.. [DOI] [PubMed] [Google Scholar]
- [43].Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg 2011;128:1025–33.. [DOI] [PubMed] [Google Scholar]
- [44].Boughey JC, Hoskin TL, Hartmann LC, et al. Impact of reconstruction and reoperation on long-term patient-reported satisfaction after contralateral prophylactic mastectomy. Ann Surg Oncol 2015;22:401–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 2009;27:1362–7.. [DOI] [PubMed] [Google Scholar]
- [46].Cubitt J, Barber Z, Khan AA, et al. Breast reconstruction with deep inferior epigastric perforator flaps. Ann R Coll Surg Engl 2012;94:552–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nelson JA, Tchou J, Domchek S, et al. Breast reconstruction in bilateral prophylactic mastectomy patients: factors that influence decision making. J Plast Reconstr Aesthet Surg 2012;65:1481–9.. [DOI] [PubMed] [Google Scholar]
- [48].Hofer SO, Damen TH, Mureau MA, et al. A critical review of perioperative complications in 175 free deep inferior epigastric perforator flap breast reconstructions. Ann Plast Surg 2007;59:137–42.. [DOI] [PubMed] [Google Scholar]
- [49].Andree C, Langer S, Seidenstuecker K, et al. A single center prospective study of bilateral breast reconstruction with free abdominal flaps: a critical analyses of 144 patients. Med Sci Monit 2013;19:467–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mizgala CL, Hartrampf CR, Bennet GK. Assessment of the abdominal wall after pedicled TRAM flap surgery: 5 to 7 year follow-up of 150 consecutive patients. Plast Reconstr Surg 1994;93:988–1004.. [DOI] [PubMed] [Google Scholar]
- [51].Kroll SS. Fat necrosis in free transverse rectus abdominus myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:576–83.. [DOI] [PubMed] [Google Scholar]
- [52].Blondeel PN, Arnstein M, Verstrate K, et al. Venous congestion and blood flow in free transverse rectus abdominus myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:1295–9.. [DOI] [PubMed] [Google Scholar]
- [53].Nahabedian MY, Dooley W, Singh N, et al. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: the role of muscle preservation. Plast Reconstr Surg 2002;109:91–101.. [DOI] [PubMed] [Google Scholar]
- [54].Nahabedian MY, Manson PN. Contour abnormalities of the abdomen after transverse rectus abdominis muscle flap breast reconstruction: a multifactorial analysis. Plast Reconstr Surg 2002;109:81–7.. [DOI] [PubMed] [Google Scholar]
- [55].Kässmann C, Peek A, Luxenhofer F, et al. Myosonographic evaluation of rectus abdominis muscle function after DIEP flap breast reconstruction. Handchir Mikrochir Plast Chir 2002;34:386–94.. [DOI] [PubMed] [Google Scholar]
- [56].Lee SJ, Lim J, Tan WT, et al. Changes in the local morphology of the rectus abdominis muscle following the DIEP flap: an ultrasonographic study. Br J Plast Surg 2004;57:398–405.. [DOI] [PubMed] [Google Scholar]
- [57].Wang XL, Liu LB, Song FM, et al. Meta-analysis of the safety and factors contributing to complications of MS-TRAM, DIEP, and SIEA flaps for breast reconstruction. Aesth Plast Surg 2014;38:681–91.. [DOI] [PubMed] [Google Scholar]


