Abstract
Aphasia shows high incidence in stroke patients and seriously impairs language comprehension, verbal communication, and social activities. Therefore, screening aphasic patients during the acute phase of stroke is crucial for language recovery and rehabilitation. The present study developed a Chinese version of the Language Screening Test (CLAST) and validated it in post-stroke patients.
The CLAST was adapted from the Language Screening Test developed by Constance et al to incorporate Chinese cultural and linguistic specificities, and administered to 207 acute stroke patients and 89 stabilized aphasic or non-aphasic patients. Based on the Western Aphasia Battery (WAB) test, its reliability and validity were assessed. A cut-off for the CLAST in Chinese patients was determined by ROC curve analysis.
The CLAST comprised 5 subtests and 15 items, including 2 subscores, namely expression (8 points, assessing naming, repetition, and automatic speech) and receptive (7 points maximum, evaluating picture recognition, and verbal instructions) indexes. Analysis of the alternate-form reliability of the questionnaire showed a retest correlation coefficient of 0.945 (P < .001). Intraclass correlation coefficients of three rating teams were >0.98 (P < .001). Internal consistency analysis showed a Cronbach's alpha coefficient of 0.909 (P < .001). The non-aphasia group showed higher scores than the aphasia group (14.2 ± 1.3 vs 10.6 ± 3.8) (P < .01). The questionnaire showed good construct validity by factor analysis. ROC curve analysis showed high sensitivity and specificity for the CLAST, with a cut-off of 13.5.
The CLAST is suitable for Chinese post-stroke patients during the acute phase, with high reliability, validity, sensitivity, and specificity.
Keywords: language screening test, aphasia, post-stroke, Western Aphasia Battery test, validation
1. Introduction
Stroke has a high incidence in middle-aged and elderly individuals.[1–3] Due to stroke-induced damage to specific brain regions, about 15% to 38% of post-stroke cases are accompanied by aphasia,[4–7] an ailment that greatly impairs language comprehension, verbal communication, and social activities.[7–9] Aphasic patients have difficulties in recognizing and applying communication symbols without developing consciousness disorders, loss of senses (hearing or vision), ataxia, and muscle paralysis associated with pronunciation. Multiple aphasic individuals experience, even in the mildest disease form, severe social deficits.[10] Post-stroke aphasia considerably impacts personal and public life in patients, and gravely increases healthcare expenditures.[1,11,12] Therefore, early detection of stroke-induced aphasia and prompt intervention are extremely important. Studies have reported that intensive language therapy administered at the early stage may improve neurological function and increase the language recovery rate.[13–16] Meanwhile, recent findings indicate that during the acute-stroke phase, tools capable of identifying aphasia and assessing its severity could facilitate early rehabilitation and improve the prognosis of aphasic patients.[17,18]
For detecting post-stroke aphasia, an essential step consists of screening the patients before a comprehensive assessment of language ability. In China, despite the growing incidence of cerebral stroke in the population,[2] screening of aphasic patients is rarely researched, and few convenient and reliable screening scales are available. In addition, commonly used scales for diagnosing aphasia, including the Western Aphasia Battery (WAB), Boston Diagnostic Aphasia Evaluation (BDAE), and Aphasia Battery of Chinese (ABC), are comprehensive, time consuming and complex.[19,20] These scales involve speech assistance and language therapists, and are unsuitable for screening acute stroke patients at an early stage. Therefore, it is important to develop novel and efficient scales for detecting aphasia in acute stroke patients.
Constance and collaborators designed a brief language screening scale, termed the Language Screening Test (LAST), for evaluating patients with acute stroke,[18] which is easy to administer during the acute-stroke phase. We hypothesized that a Chinese version of the LAST will be efficient in detecting aphasia in Chinese stroke patients at an early stage. Therefore, the present study adapted the LAST to incorporate specificities of the Chinese language and culture, and developed a Chinese version of the Language Screening Test (CLAST).
2. Methods
2.1. Subjects
The patients were recruited in the Department of Neurology of the Affiliated Union Hospital, Fujian Medical University, from January to April 2018. Inclusion criteria for acute patients were:
-
1.
early stage of stroke onset (within 48 h);
-
2.
age over 45 years;
-
3.
MRI-confirmed stroke;
-
4.
Chinese Han nationality;
-
5.
ability to understand and speak mandarin prior to stroke onset.
During the same period, “stable” stroke patients (not in the acute phase) were enrolled in the Department of Neurology, who were 7 days after stroke onset and were able to complete the entire WAB test. These patients were then classified as aphasic or non-aphasic according to WAB test results.
Exclusion criteria were:
-
1.
dementia or serious psychiatric disorders;
-
2.
loss of hearing or eyesight;
-
3.
altered consciousness;
-
4.
non-native mandarin speaker;
-
5.
unwillingness to cooperate.
The demographic data of all patients were sorted, and the National Institutes of Health Stroke Scale (NIHSS) scores of “acute” stroke patients were recorded. Informed consent was obtained from all participants in this study approved by the Ethics Committee of the Affiliated Union Hospital of Fujian Medical University.
2.2. Instruments
The present study employed two scales for patient assessment, including the WAB test and CLAST. The former is a standard aphasia scale currently employed for comprehensive aphasia evaluation in China. It evaluates several linguistic skills, including spontaneous speech, repeat, and naming.[21] The latter is composed of 5 subtests and a total of 15 items, including 2 subscores (an expression index with a score of 8, and a receptive index with a score of 7).
2.3. Development of the CLAST
The LAST is not copyrighted and free to use. We obtained authorization from Professor Constance (Service de Neurologie, Centre Hospitalo-Universitaire de Bicêtre), and made necessary changes to produce CLAST. In brief, two neurologists first translated the LAST into Chinese independently, and a reconciled version was summarized and modified by consensus. Then, an English teacher unaware of the original English LAST back-translated the reconciled version into English to assess consistency between the two versions. Next, the CLAST was modified according to the Chinese culture and linguistic specificities, for example, replacement of uncommon objectives by those familiar to Chinese patients and application of Chinese tongue twisters instead of English rhyming sentences. And the CLAST was revised based on comments provided by 7 neurologists (4 and 3 with senior and intermediate titles, respectively), who were invited to proofread the scale. The revised CLAST was finalized according to suggestions by 30 doctors or nurses who had tested the scale in stroke patients.
2.4. Scoring process
To avoid retest effects, we developed two parallel versions of CLAST, including CLAST-A and CLAST-B, in which each item was different but strictly matched. For example, the visual and verbal complexity of pictures as well as the rhythms of the words and sentences used for the repetition subtest were respectively matched in both versions. As week-day or alphabetic counting is relatively social-culturally influenced, counting of Arabic numerals from 1 to 10 was used in both versions.
Three examination pairs (two neurologists, a neurologist and a nurse, or a neurologist and a student) were given a 5-min explanation of the test administration process. Then, based on the random number table, patients 1 to 100 were evaluated by a neurologist and a nurse, 101 to 160 by two neurologists and 161 to 207 by a neurologist and a student, in parallel. “Acute” stroke patients were tested randomly via random number table method for CLAST-A or CLAST-B. Aphasic and non-aphasic “stable” patients were assessed by the WAB test and subsequently by CLAST-A and CLAST-B on the same day with about 3-min interval. The outcome was scored as 1 (correct answer) or 0 (imperfect answer or failure to answer). The maximum score was 15, corresponding to 15 items (Appendix 1).
2.5. Measurement indexes
The patients were assessed for gender, age, educational background, NIHSS score, CLAST score and WAB test results.
2.6. Statistical analysis
The data were analyzed with SPSS 16.0 (SPSS Inc, Chicago, IL). Epidata 3.02 was employed to collect patient information and establish a database. Descriptive analysis was conducted for measurement indexes. The Cronbach's alpha coefficient was adopted to assess the internal reliability of the CLAST. Intraclass correlation coefficients (ICCs) were determined to evaluate inter-examiner and alternate-form reliabilities of the CLAST. Exploratory factor analysis was performed to evaluate the construct validity of the CLAST. Independent samples t test was carried out to evaluate discriminant validity. A receiver operating characteristic (ROC) curve was generated to determine a cut-off point for the CLAST, as well as the test specificity and sensitivity. P values < .05 were considered statistically significant.
3. Results
3.1. Clinical and demographic features of patients
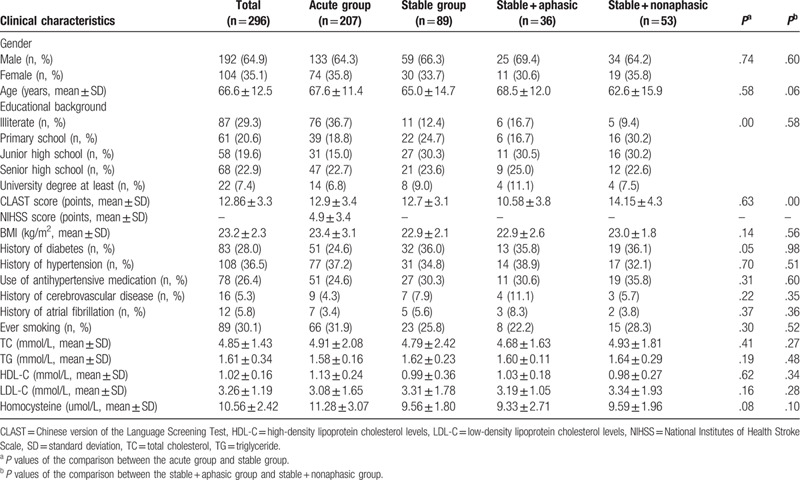
A total of 230 consecutive “acute” stroke patients admitted to the Department of Neurology for suspected acute stroke from January to April 2018 were included. Twenty-three patients were excluded (15 non-native Chinese speakers, 3 cases with a history of dementia, 2 cases of deafness or blindness, and 3 cases with altered consciousness). The remaining 207 “acute” stroke patients were enrolled in the assessment of internal validity and inter-examiner reliability, including 133 men and 74 women aged 64.0 ± 16.2 years with NIHSS scores of 4.9 ± 3.4. Ninety-two patients were enrolled in the “stable” group, including three who quit the study half way; therefore, 89 “stable” patients were evaluated (59 men and 30 women, aged 65.1 ± 14.7 years). The demographics and clinical characteristics of the study population are summarized in Table 1.
Table 1.
Clinical and demographic data of patients.
3.2. Test reliability
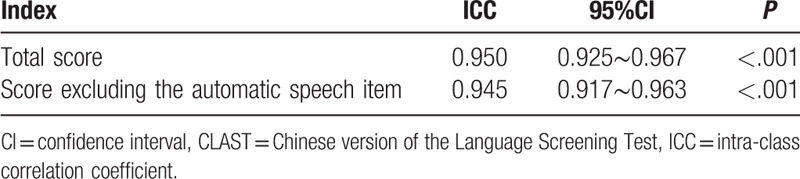
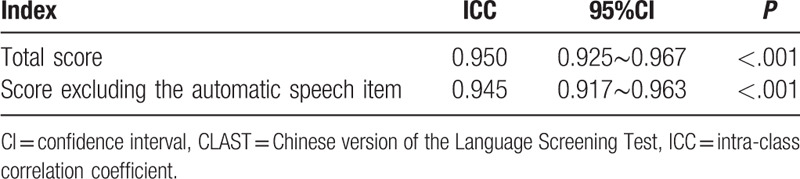
The alternate-form reliabilities of the two CLAST versions were analyzed. A comparison between CLAST-A and CLAST-B in the 89 “stable” stroke patients revealed that the two versions were strictly equivalent (ICC = 0.950). This result was not significantly changed (ICC = 0.945) when the automatic speech item was excluded from both versions (Table 2).
Table 2.
Comparison of CLAST-A and CLAST-B in “stable” stroke patients.
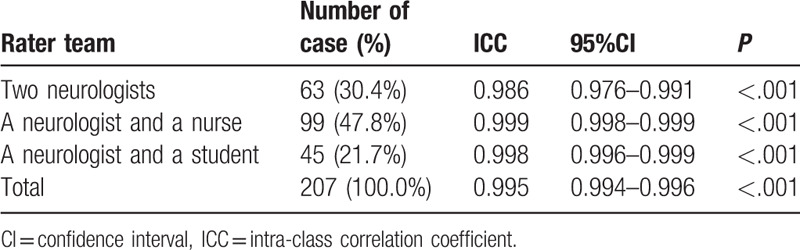
CLAST-A and CLAST-B were consistent, as shown in Table 2. Therefore, the two CLAST versions were analyzed together. The inter-examiner reliability in the 207 “acute” stroke patients was almost perfect (ICC = 0.995, P < .001) (Table 3). Specifically, the examiner pair consisting of two neurologists assessed 63 cases (30.4%). The one comprising a neurologist and a nurse evaluated 99 cases (47.8%), and the neurologist/student pair assessed 45 cases (21.7%). Inter-examiner reliabilities were, respectively, 0.986, 0.999, and 0.998 (all P < .001). ICCs were almost perfect despite the level difference between examiners.
Table 3.
ICC comparison of the three different examiner teams for the 207 “acute” stroke patients.
Together, the Cronbach's alpha coefficient of both questionnaires was 0.909, indicating excellent internal reliability.
3.3. Validity
This study determined the correlation coefficient between each item score and the score of each dimension to evaluate content validity. Correlation coefficients between various item scores of each expression index and the total score of the expression dimension were above 0.7 (P < .001); those between various item scores of the receptive index and the total score of the receptive dimension were above 0.6 (P < .001). These data indicated that the scale had high content validity.
The Kaiser–Meyer–Olkin (KMO) index of the sampling data constituted by the 15 items was 0.909 (>0.5) and the Bartlett's test of sphericity yielded χ2 = 1514.21 (P < .001), indicating that there were common factors in the correlation matrix in various factors, and construct validity was suitable for exploratory factor analysis. A principal component analysis produced 2 common factors, with a cumulative variance contribution rate of 54.90% (>40%), indicating good construct validity (Table 4). Moreover, taking the WAB test as the golden standard, 36 “stable” stroke patients were identified as aphasic and 53 as non-aphasic. We performed independent samples t test based on the CLAST scores of “stable” patients. The total score of aphasic patients was significantly lower than that of non-aphasic individuals (10.6 ± 3.8 vs 14.2 ± 1.3) (P < .01), demonstrating good discriminant validity (Table 5).
Table 4.
Results of factor analysis.
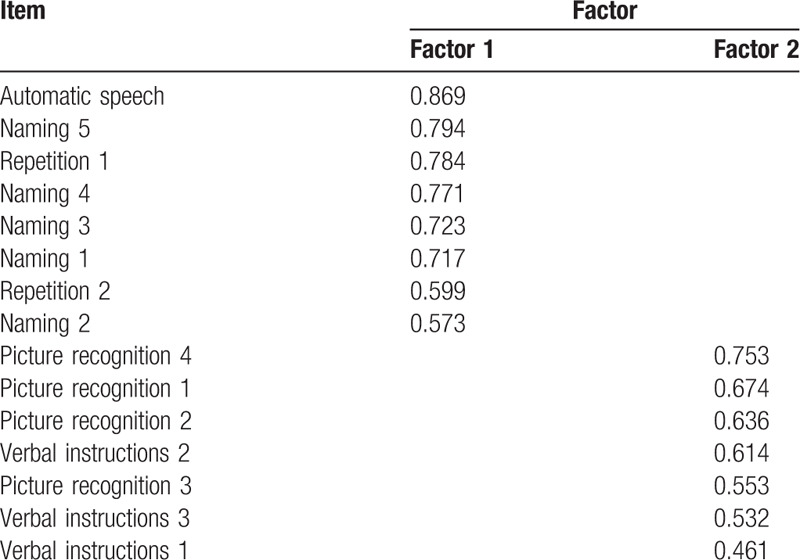
Table 5.
Discriminant validity of the CLAST in the 89 “stable” stroke patients.
3.4. Sensitivity and specificity of the CLAST
The area under the ROC curve was 0.850 (95%CI = 0.767–0.932, P < .05), indicating the CLAST could be used for aphasia diagnosis. With the WAB test as the golden standard, the CLAST had a sensitivity of 0.806 and a specificity of 0.774 for aphasia detection, with a cut-off point of 13.5 for the 89 “stable” stroke patients; a Youden index of 58% was obtained (Fig. 1). Based on the cut-off point of external validation (score ≤ 13), the CLAST detected language deficits in 35.3% of the 207 acute stroke patients.
Figure 1.
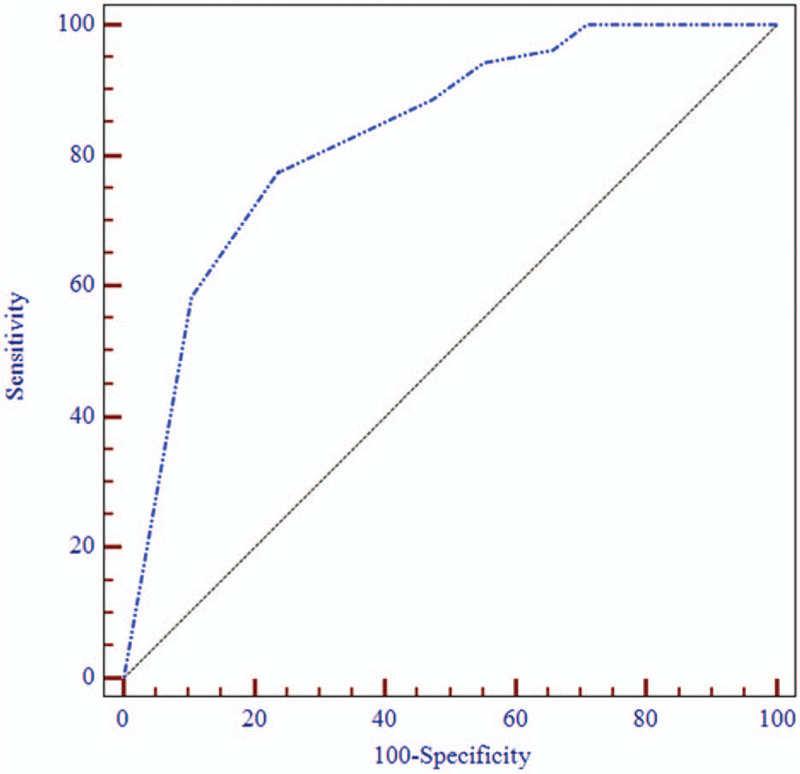
ROC curve of CLAST. The area under the curve (AUC) of CLAST for aphasia diagnosis in stroke patients was 0.850 (95%CI = 0.767–0.932, P < .001). With a cutoff of 13.5, a sensitivity of 80.6%, a specificity of 77.4% and a Youden index of 58% were obtained, indicating a high diagnostic value for CLAST in aphasia.
4. Discussion
The present study developed a CLAST for early identification of aphasic patients during the acute phase of stroke. The newly developed CLAST was shown to be suitable for Chinese post-stroke patients during the acute phase, exhibiting high reliability, validity, sensitivity, and specificity. The CLAST was adapted from the LAST designed by Constance et al[18] to incorporate Chinese cultural and linguistic specificities. It consisted of 5 subtests and a total of 15 items, including 2 subscores. Factor analysis indicated that this questionnaire had good construct validity. With a cut-off point of 13.5, the CLAST had high sensitivity and specificity. The language deficits detected by the CLAST was 35.3% in the 207 acute stroke patients, corroborating previous study (15–38%).[4–7] Therefore, we highly recommend this test for screening Chinese post-stroke patients during the acute phase.
It is widely considered that effective and efficient communication is vital to quality healthcare, as it impacts clinical assessment, health promotion and patient autonomy.[22] The sequelae of post-stroke aphasia are considerable, with implications at the social and personal levels.[23] Because of communication deficits, aphasic patients complain of difficulties in benefiting from available mental health services, participating in intervention programs, and/or finding support groups that could meet their needs.[24]
Despite controversies, timely and intensive speech and language rehabilitation in early aphasia has been recommended for maximizing recovery.[24] In addition, immediate post-stroke aphasia therapy improves communication outcomes,[25–27] which can persist for at least 12 months.[28] Therefore, detecting and evaluating aphasia in the acute phase represents a key concern. The current aphasia rating scales used in China require speech and language experts and take long.[19,29] As a result, they are not applicable to patients with acute stroke, as timely detection of aphasia post-acute stroke may facilitate language rehabilitation.
In various cultural backgrounds, languages and living habits differ. Therefore, the CLAST took into account the linguistic features and cultural characteristics of Chinese. For example, giraffe was changed into panda and fork into comb, because Chinese individuals, especially the elderly, are more familiar with the panda and comb. In the repetition portion, English rhyming sentences were replaced by Chinese tongue twisters like “si shi si zhi shi shi zi,” which are adopted in widely accepted Chinese screening scales such as Chinese version Montreal Cognitive Assessment (MoCA)[30] and ABC.
Next, we validated the CLAST in Chinese patients. The scale showed good alternate-form, inter-examiner, and internal reliabilities, and elevated discriminant, content and construct validities. Statistical analysis suggested that the CLAST had high reliability and effectiveness in assessing aphasic patients with great convenience and ease of application. Indeed, the scale contains only few items and can be performed at bedside within 2 min. It could complement other rating scales such as NIHSS for early evaluation of stroke patients. With a cut-off score ≤13 (from a maximum of 15), the CLAST was highly sensitive and specific to aphasia, indicating its significance in diagnosing aphasia early after acute stroke.
The current aphasia screening scales have multiple shortcomings:
-
1.
use of written language subtests, whose results are subject to hemiplegia and illiteracy[31–33];
-
2.
application of sophisticated visual materials, which are unsuitable for stroke cases complicated with neurovisual deficits[32,33];
-
3.
adoption of subtests, whose results are highly affected by attention/executive dysfunction[32,33]; and
-
4.
excessively lengthy administration.
What's more, these scales show low sensitivity, validity, and reliability.[13,32]
To the best of our knowledge, the CLAST developed in the current study is the first standardized aphasia screening test in China. It incorporates Chinese cultural and linguistic specificities and overcomes the aforementioned shortcomings by excluding the use of written and complex visual materials, as well as the evaluation of verbal executive functions in the questionnaire design. Meanwhile, the CLAST offers high sensitivity, validity, reliability, and great convenience for bedside administration by individuals without expertise in speech or linguistic therapy. Based on CLAST characteristics, we suggest that either version of the CLAST could be immediately adopted for evaluating acute stroke patients upon admission, with the other version employed after condition stabilization. Aphasia severity assessment by the CLAST could help determine whether more detailed aphasia rating scales are needed. The corresponding language function rehabilitation can be arranged based on the test results. The CLAST had acceptable psychometric values and could be used in clinical research for early diagnosis of aphasia.
The limitations of this study should be mentioned. First, the CLAST does not properly differentiate aphasia types, and requires further revisions to adapt to the requirements of different cultural regions and language specificities. In addition, its reliability, validity and cut-off point should be confirmed in larger multi-center studies.
In summary, the present study developed and validated the novel Chinese language screening scale for Chinese acute-stroke patients, which is valid, reliable, efficient, user friendly, and cost-effective. The CLAST could complement the available stroke rating scales such as the NIHSS for early evaluation of stroke patients.
Acknowledgments
We would like to thank Professor Hongzhi Huang of School of Foreign Languages, Fujian Medical University, for kind proofreading and polishing of this manuscript.
Author contributions
Conceptualization: Xiaodong Pan, Xiaochun Chen
Data curation: Mingyao Sun, Zhouwei Zhan, Bijuan Chen, Jiawei Xin
Formal analysis: Mingyao Sun, Zhouwei Zhan
Funding acquisition: Xiaodong Pan, Xiaochun Chen, Zhouwei Zhan, Bijuan Chen
Investigation: Mingyao Sun, Zhouwei Zhan, Erhan Yu, Lizhen Lin, Raoli He
Methodology: Mingyao Sun, Zhouwei Zhan, Xiaochun Chen, Xiaodong Pan
Project administration: Mingyao Sun, Zhouwei Zhan, Xiaochun Chen, Xiaodong Pan
Writing-original draft: Mingyao Sun, Zhouwei Zhan
Writing-review & editing: Mingyao Sun, Zhouwei Zhan, Xiaodong Pan, Xiaochun Chen.
Footnotes
Abbreviations: ABC = Aphasia Battery of Chinese, BDAE = Boston Diagnostic Aphasia Evaluation, CLAST = Chinese version of the Language Screening Test, ICCs = Intraclass correlation coefficients, KMO = Kaiser–Meyer–Olkin, LAST = Language Screening Test, MoCA = Montreal Cognitive Assessment., NIHSS = National Institutes of Health Stroke Scale, ROC = receiver operating characteristic, WAB = Western Aphasia Battery.
How to cite this article: Sun M, Zhan Z, Chen B, Xin J, Chen X, Yu E, Lin L, He R, Pan X. Development and application of a Chinese Version of the Language Screening Test (CLAST) in post-stroke patients. Medicine. 2020;99:37(e22165).
MS and ZZ contributed equally to this work.
This study was supported by grants from the National Natural Science Foundation of China (No. 81771179), Fujian Provincial Health-education joint research project (No. WKJ2016-2-08), Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2017Y9006), 2019 Financial Special Project of Fujian Province (No. 2019B023), Startup Fund for scientific research, Fujian Medical University(No. 2017XQ1208; No. 2017XQ1213), Ministry of Science and Technology of China, National Key R&D Program: Research on the Prevention and Control of Major Chronic Non-communicable Diseases (2016YFC1306404).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Tu W-J, Dong X, Zhao S-J, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke [J]. J Neuroendocrinol 2013;25:771–8.. [DOI] [PubMed] [Google Scholar]
- [2].Ren Y, Hu J, Lu B, et al. Prevalence and risk factors of hip fracture in a middle-aged and older Chinese population [J]. Bone 2019;122:143–9.. [DOI] [PubMed] [Google Scholar]
- [3].Słomka A, Świtońska M, Sinkiewicz W, et al. Haemostatic factors do not account for worse outcomes from ischaemic stroke in patients with higher C-reactive protein concentrations [J]. Ann Clin Biochem 2017;54:378–85.. [DOI] [PubMed] [Google Scholar]
- [4].Inatomi Y, Yonehara T, Omiya S, et al. Aphasia during the acute phase in ischemic stroke [J]. Cerebrovasc Dis 2008;25:316–23.. [DOI] [PubMed] [Google Scholar]
- [5].Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis [J]. Stroke 2006;37:1379–84.. [DOI] [PubMed] [Google Scholar]
- [6].Berthier ML. Poststroke aphasia [J]. Drugs Aging 2005;22:163–82.. [DOI] [PubMed] [Google Scholar]
- [7].Kauhanen M-L, Korpelainen JT, Hiltunen P, et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke [J]. Cerebrovasc Dis 2000;10:455–61.. [DOI] [PubMed] [Google Scholar]
- [8].Wade D, Hewer RL, David RM, et al. Aphasia after stroke: natural history and associated deficits [J]. J Neurol Neurosurg Psychiatry 1986;49:11–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee H, Lee Y, Choi H, et al. Community integration and quality of life in aphasia after stroke [J]. Yonsei Med J 2015;56:1694–702.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ellis C, Hardy RY, Lindrooth RC. Greater healthcare utilization and costs among Black persons compared to White persons with aphasia in the North Carolina stroke belt [J]. J Neurol Sci 2017;376:76–83.. [DOI] [PubMed] [Google Scholar]
- [11].Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association [J]. Circulation 2011;123:e18–209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rakesh N, Boiarsky D, Athar A, et al. Post-stroke rehabilitation: Factors predicting discharge to acute versus subacute rehabilitation facilities [J]. Medicine 2019;98:e15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Doesborgh S, Van de Sandt-Koenderman W, Dippel D, et al. Linguistic deficits in the acute phase of stroke [J]. J Neurol 2003;250:977–82.. [DOI] [PubMed] [Google Scholar]
- [14].Seni WJ, Waldowski K, Leśniak M, et al. Transcranial magnetic stimulation combined with speech and language training in early aphasia rehabilitation: a randomized double-blind controlled pilot study [J]. Top Stroke Rehabilit 2013;20:250–61.. [DOI] [PubMed] [Google Scholar]
- [15].Van der Meulen I, van de Sandt-Koenderman WME, Heijenbrok-Kal MH, et al. The efficacy and timing of melodic intonation therapy in subacute aphasia [J]. Neurorehabilit Neural Repair 2014;28:536–44.. [DOI] [PubMed] [Google Scholar]
- [16].Simic T, Rochon E, Greco E, et al. Baseline executive control ability and its relationship to language therapy improvements in post-stroke aphasia: a systematic review [J]. Neuropsychol Rehabilit 2019;29:395–439.. [DOI] [PubMed] [Google Scholar]
- [17].Laska AC, Bartfai A, Hellblom A, et al. Clinical and prognostic properties of standardized and functional aphasia assessments [J]. J Rehabilit Med 2007;39:387–92.. [DOI] [PubMed] [Google Scholar]
- [18].Flamand-Roze C, Falissard B, Roze E, et al. Validation of a new language screening tool for patients with acute stroke: the Language Screening Test (LAST) [J]. Stroke 2011;42:1224–9.. [DOI] [PubMed] [Google Scholar]
- [19].Wu J-B, Lyu Z-H, Liu X-J, et al. Development and standardization of a new cognitive assessment test battery for Chinese aphasic patients: a preliminary study [J]. Chin Med J 2017;130:2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang H, Tian S, Flamand-Roze C, et al. A Chinese version of the Language Screening Test (CLAST) for early-stage stroke patients [J]. PLoS One 2018;13: e0196646-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu Z-Z, Jiang S-J, Sheng B, et al. Relationship between linguistic functions and cognitive functions in a clinical study of Chinese patients with post-stroke aphasia [J]. Chin Med J 2013;126:1252–6.. [PubMed] [Google Scholar]
- [22].Thompson J, Mckeever M. The impact of stroke aphasia on health and well being and appropriate nursing interventions: an exploration using the Theory of Human Scale Development [J]. J Clin Nursing 2014;23:410–20.. [DOI] [PubMed] [Google Scholar]
- [23].Tippett DC. Update in aphasia research [J]. Curr Neurol Neurosci Rep 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Worrall L, Ryan B, Hudson K, et al. Reducing the psychosocial impact of aphasia on mood and quality of life in people with aphasia and the impact of caregiving in family members through the Aphasia Action Success Knowledge (Aphasia ASK) program: study protocol for a randomized controlled trial [J]. Trials 2016;17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Godecke E, Hird K, Lalor EE, et al. Very early poststroke aphasia therapy: a pilot randomized controlled efficacy trial [J]. Int J Stroke 2011;7:635–44.. [DOI] [PubMed] [Google Scholar]
- [26].Godecke E, Ciccone NA, Granger AS, et al. A comparison of aphasia therapy outcomes before and after a Very Early Rehabilitation programme following stroke [J]. Int J Lang Commun Disord 2014;49:149–61.. [DOI] [PubMed] [Google Scholar]
- [27].Code C. Multifactorial Processes in Recovery from Aphasia: Developing the Foundations for a Multileveled Framework [J]. Brain Lang 2001;77:25–44.. [DOI] [PubMed] [Google Scholar]
- [28].Bakheit AM, Shaw S, Carrington S, et al. The rate and extent of improvement with therapy from the different types of aphasia in the first year after stroke [J]. Clin Rehabilit 2007;21:941–9.. [DOI] [PubMed] [Google Scholar]
- [29].Yang H, Tian S, Flamand-Roze C, et al. A Chinese version of the Language Screening Test (CLAST) for early-stage stroke patients: Correction [J]; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang L, Chen KL, Lin BY, et al. Chinese version of Montreal Cognitive Assessment Basic for discrimination among different severities of Alzheimer's disease [J]. Neuropsychiatr Dis Treatm 2018;14:2133–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sabe L, Courtis MJ, Saavedra MM, et al. Development and validation of a short battery of tests for the assessment of aphasia: ’bedside assessment of language’. Its use in a rehabilitation centre [J]. RevNeurol 2008;46:454–60.. [PubMed] [Google Scholar]
- [32].Nakase-Thompson R, Manning E, Sherer M, et al. Brief assessment of severe language impairments: Initial validation of the Mississippi aphasia screening test [J]. Brain Injury 2005;19:685–91.. [DOI] [PubMed] [Google Scholar]
- [33].Enderby PM, Wood VA, Wade DT, et al. The Frenchay Aphasia Screening Test: a short, simple test for aphasia appropriate for non-specialists [J]. Int Rehabilit Med 1987;8:166–70.. [DOI] [PubMed] [Google Scholar]


